Abstract
The high incidence of neurodevelopmental disability in premature infants requires continued efforts at understanding the underlying microstructural changes in the brain that cause this perturbation in normal development. Magnetic resonance imaging (MRI) methods offer great potential to fulfill this need. Serial MR imaging and the application of newer analysis techniques such as, diffusion tensor imaging (DTI), volumetric MR analysis, cortical surface analysis, functional connectivity (fcMRI) and diffusion tractography, provide important insights into the trajectory of brain development in the premature infant and the impact of injury on this developmental trajectory. While some of these imaging techniques are currently available in the research setting only, other measures such as DTI and brain metric measures can be used clinically. MR imaging also has enormous potential to be used as a surrogate, short-term outcome measure in clinical studies evaluating new therapeutic interventions of neuroprotection of the developing brain. In this article we review the current status of these advanced MR imaging techniques.
Introduction
Five to 15% of prematurely-born children develop cerebral palsy, with an additional 30 to 50% displaying impaired academic achievement and/or behavioral disorders requiring additional educational resources. 1-6 Establishing the nature, timing, and location of the cerebral lesions predisposing premature infants to these long-term neurodevelopmental difficulties is an important step towards understanding and applying potentially beneficial neuroprotective strategies in the neonatal intensive care unit. In addition, prognostic information before discharge from the hospital would be of high clinical utility by assisting in defining the need for intervention strategies, such as continuing therapy services. MR imaging plays an important role in achieving both of these goals, serving to 1) accurately define the nature of cerebral injury in this population for research purposes and 2) identify the imaging features that predict adverse neurodevelopmental outcome.
In addition to defining cerebral injury evident on conventional (T1- and T2-weighted) MRI, recently developed MR-based neuroimaging approaches such as diffusion tensor imaging (DTI), functional connectivity MRI (fcMRI), volumetric analysis and surface based morphometry (SBM) are now providing insight into alterations in structural and functional brain maturation associated with premature birth that were not detectable with other neuroimaging methods. With current conventional neuroimaging techniques, severely abnormal findings are moderately accurate for predicting an adverse outcome, particularly in relation to motor outcomes such as cerebral palsy.7 However, there is a less robust relationship between the findings of these conventional techniques and cognitive outcome in the premature infant, even when images are obtained later in life. 8 Newer MR imaging methods offer higher sensitivity to more subtle structural and functional alterations. Thus, they have the potential to provide a better understanding of the anatomic substrate for the increased susceptibility for cognitive difficulties in this high-risk population.
MR Imaging and the Preterm Infant
Practical issues with MR imaging
Obtaining an MRI scan in the preterm infant in a safe manner is a unique challenge to both the neonatology and radiology staff. Compared to ultrasound, MR has the disadvantage of requiring that the infant be transported from the NICU to the scanner for study. The risk to the infant associated with transport out of the NICU has been ameliorated by the availability of MR-compatible, purpose-built transport isolettes. These devices include an MRI compatible ventilator, support equipment for the infant, and the RF coil necessary for MR studies. The entire isolette can be placed in the MR scanner for imaging. Once placed in such an isolette in the NICU, an infant can be taken to the scanner and left undisturbed until he/she returns to the NICU after the scan. These isolettes are now commercially available.
While many centers perform MR studies with sedation, our center has a long experience in obtaining quality MR imaging in unsedated infants. 9 It is important to allow preparation time in the intensive care unit prior to taking the infant to the scanner. Infants should be fed between 30-45 minutes prior to the scan. The MR compatible pulse-oximetry probe should then be placed with confirmed adequate signal prior to wrapping. Ear protection (Minimuffs, Natus, San Carlos, CA) should also be placed to minimize distraction from noise. Patients should be wrapped snugly in 1-2 sheets and a vac-fix device, placed in the transport isolette, and allowed to settle for a period of time. Using this approach, infants are often sleeping by the time the transport team arrives at the MRI scanner and remain sleeping during the scan, resulting in quality scans with very little motion artifact.
Conventional MR Imaging
MR imaging of the premature infant brain has been available for approximately 20 years and remains an area of active research. The earliest studies used conventional MR imaging and focused on studies obtained after discharge from the hospital on patients for whom periventricular leukomalacia (PVL) had been diagnosed by cranial ultrasound (CUS) during the perinatal period. Areas of abnormal signal intensity in periventricular white matter, ventriculomegaly, varying degrees of cerebral atrophy, thinning of the corpus callosum, and delayed myelination have been described in children as old as five years.10,11 These findings are common in prematurely-born children12,13 and suggest that MR can be used to detect the sequelae of PVL in older children. However, MR abnormalities consistent with PVL detected in prematurely-born children at ages eight14 and 15-17 years 8 show only moderate correlation with cognitive and motor deficits. In contrast, abnormalities of visual pathways detected on MR images obtained in late infancy show strong correlation with visual impairment.15
Conventional MR studies obtained at term equivalent postmenstrual age (PMA), near the time of discharge of the premature infant from the hospital, have prognostic significance (Fig 1). The presence of parenchymal lesions had a sensitivity of 100% and specificity of 79% for motor abnormality in a study of 51 infants, 11 of whom showed neurologic deficits at follow up.16 The parenchymal lesions consisted of hemorrhage, changes consistent with PVL, infarction, and reduction in white matter volume. In a study of 167 very preterm infants (≤ 30 weeks gestational age), moderate to severe cerebral white matter injury on MRI obtained at term equivalent PMA, whether cystic or diffuse in nature, was strongly predictive of cognitive delay (OR 3.6; 95% CI, 1.5 to 8.7), motor delay (OR 10.3; 95% CI, 3.5 to 30.8), cerebral palsy (OR 9.6; 95% CI, 3.2 to 28.3), and neurosensory impairment (OR 4.2; 95% CI, 1.6 to 11.3) at two years of age.7 In this study, white matter injury was quantified from a composite scoring system that incorporated white-matter signal abnormalities, periventricular white matter volume, presence of cystic abnormalities, ventricular dilatation, and thinning of the corpus callosum.
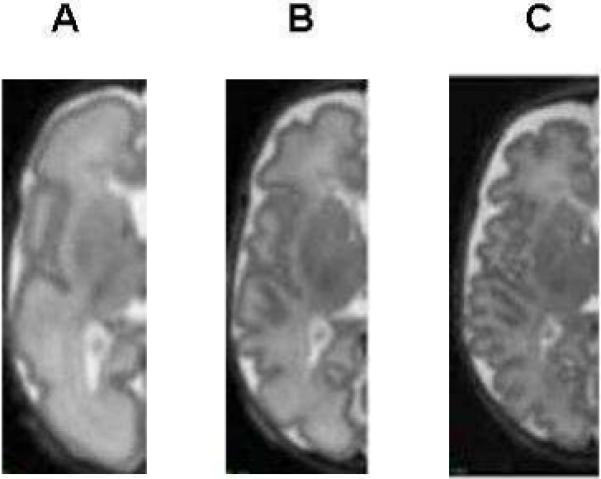
Fig 1.
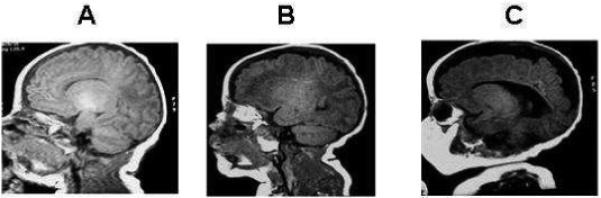
Conventional T1 weighted images in three premature infants imaged at term. Panel A shows normal white matter maturation; Panel B shows moderate white matter injury with punctate lesions in the periventricular regions and Panel C shows severe white matter injury with a severe reduction in white matter volume and ventriculomegaly.
The clinical significance of more subtle white matter abnormalities such as diffuse excessive high signal intensities (DEHSI) remains more controversial. These high signal intensity lesions of the white matter extend from the immediate periventricular region into the adjacent periventricular and subcortical regions and are commonly detected on T2-weighted imaging. DEHSI is present in the majority of preterm infants imaged at term equivalent and absent in healthy term controls. Debate exists as to whether it represents a delayed form of maturation or true pathology. Recent studies have shown that these white matter lesions correspond to diffuse quantitative abnormalities in diffusion measurements throughout the white matter, implicating axonal and/or oligodendrocyte injury.17,18 Several studies have now linked these lesions with lower developmental quotients at 18-24 months. 19,20
There has also been focus on abnormalities of the posterior limb of the internal capsule (PLIC) and outcome. In preterm infants with intraventricular hemorrhage and unilateral hemorrhagic parenchymal involvement, signal abnormalities in the PLIC on MR studies obtained at term equivalent PMA strongly predicted future hemiplegia.21 It is likely that changes in the MR properties of the PLIC reflect the effects of Wallerian degeneration of the corticospinal motor system as a consequence of grey and/or white matter injury.22,23 Thus, MR signal changes in the PLIC may reflect established and more widespread injury.
Diffusion MR imaging
MR images can be obtained in which contrast is dependent on water displacement (diffusion tensor imaging or DTI). Although the technical aspects of DTI are discussed in detail elsewhere in this edition, briefly, with this method, water displacements on the order of 10 μm can be detected. The physical constant characterizing this water motion is called the “apparent” diffusion coefficient (ADC) in recognition of the fact that water displacements in tissue are influenced by factors other than simple Brownian motion, such as restrictions due to cell membranes.24 Quantitative ADC values (in units of μm2/ms) can be measured, simplifying image interpretation. Water displacements in the brain are influenced by a wide range of complex and poorly-understood factors, including overall tissue water content, the relative volume fractions of the intra- and extracellular spaces, the energy status of cells, and tissue microstructure (e.g., the degree of myelination). Thus, diffusion-based MR imaging provides remarkably rich information about cerebral structure and its disruption. For example, diffusion-based MR images provide one of the earliest indicators of tissue injury, showing changes many hours to days before abnormalities are detectable with other forms of imaging. As outlined below, it has also been shown to be a useful technique for evaluating the microstructural state of white matter and developing cortex.25
Anisotropy refers to the condition in which water ADC values differ depending upon the direction along which they are measured. In myelinated white matter, for example, water molecular displacements are smaller perpendicular to fibers than parallel to them because motion perpendicular to fibers requires that water molecules pass through or around layers of myelin membrane, whereas motion parallel to them does not.24 Thus, water apparent diffusion in mature white matter is highly anisotropic, and this anisotropy reflects tissue microstructure. Two parameters related to diffusion anisotropy are particularly useful for microstructural characterization: the degree of anisotropy of water motion (e.g., relative anisotropy (RA) or fractional (FA) anisotropy) and the direction along which water apparent diffusion is greatest (the direction of the major eigenvector of the diffusion tensor). RA values are relatively high for myelinated white matter due to the fact that ADC values for diffusion perpendicular fibers are on the order of three times smaller than ADC values parallel to fibers. For white matter, the direction along which water ADC values are greatest represents the primary orientation of myelinated fibers with an image voxel (remember that water displacements are greatest parallel to fibers). This information can be used for a form of “tract tracing” in which neural connections can be inferred by following the major eigenvectors from brain region to brain region.26 Whereas anisotropy increases during development for white matter, primarily influenced by myelination, anisotropy values for developing cerebral cortex show the opposite pattern. Early in development, grey matter anisotropy is high, and the direction along which water ADC values are greatest reflects the radial organization of developing cortex .27 As the cortex matures, its anisotropy disappears due mainly to the appearance of interneurons and elaboration of dendrites from pyramidal cells (Fig 2). This effect has been used to demonstrate regionally heterogeneous rates of cortical development in baboons. 28
Fig 2.
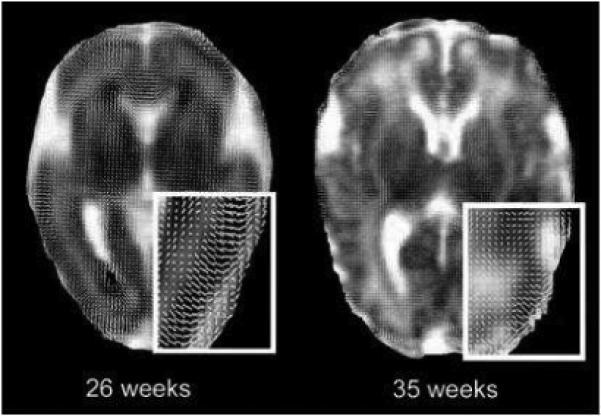
Diffusion whisker plots overlaid on ADC images from infants of 26 and 35 weeks GA. The line segments are the projection of the major eigenvector onto the plane of the image and represent the orientation of the major eigenvectors. The insets with white borders are magnifications of the parieto-occipital regions of the images. Note that the major axes are oriented radially in cortex at 26 weeks GA. By 35 weeks GA, this feature is much less evident. In both images, organization of whiter matter is visible in the genu of the corpus callosum. The dark areas at the occipital horn of the lateral ventricles of the image from 26 weeks GA is due to small intraventricular hemorrhages layering dependently (with permission McKinstry et al. Oxford University Press)27.
Diffusion MR imaging in brain development and injury
Parameters of diffusion have been studied extensively in the preterm neonate. While the majority of studies have been based upon data obtained at term equivalent PMA, a few studies have evaluated serial diffusion imaging in premature infants. For example, parameters of the cerebral white matter were directly related to gestational age and there was an increase in FA values and a decrease in ADC values with increasing maturation.29,30 There was also a statistically significant correlation between the FA of the PLIC and gestational age (GA).31 As noted above, this reflects the normal maturation of cerebral white matter with increasing myelination of axonal fibers as they pass through the PLIC.
Early diffusion MR imaging in preterm neonates is capable of detecting alterations in the normal maturation of cerebral white matter in infants with white matter injury (WMI). In a serial MR study, Miler et al. demonstrated that ADC decreased significantly with age in all brain regions in newborns classified as normal and those with minimal white matter injury, while ADC increased with age or failed to decline in widespread areas of white matter in newborns with moderate white matter injury. Further, anisotropy increased with age in all white matter regions in newborns classified as normal. Anisotropy did not increase in the frontal white matter in those with minimal white matter injury and in widespread white matter areas in those with moderate white matter injury. 32
MR studies at term equivalent PMA in preterm neonates demonstrate significant differences in MR diffusion parameters when compared to term infants. In a study of relatively healthy preterm infants imaged at term equivalent, the central white matter (centrum semiovale, frontal white matter and the genu of the corpus callosum) showed significantly lower FA values when compared to term control infants.33 Infants born at less than or equal to 28 weeks gestational age displayed additional reductions in FA in the external capsule, the posterior aspect of the posterior limb of the internal capsule and the isthmus and middle portion of the body of the corpus callosum.33 In another study of 111 preterm infants, measures showed significant region-specific changes in ADC, and FA in preterm infants with extensive white matter signal abnormality. Analysis of axial and radial diffusivity (see below for discussion) suggested that disruption of premyelinating oligodendroglia was the predominant correlate of WMI. 34
Abnormal diffusion imaging parameters at term equivalent PMA have been associated with poor neurodevelopmental outcome. In the presence of a normal conventional MRI, an isolated increase in ADC values in central white matter in preterm infants at term equivalent age correlated with a lower developmental quotient at two years corrected age.35 One small study found that decreases in anisotropy in the internal capsule and occipital white matter correlated with poor motor outcome at two years corrected age.36 Furthermore, alterations in white matter anisotropy have been described in infants with PVL and those who go on to develop spastic cerebral palsy. Abnormalities of diffusion parameters of the PLIC also correlate with outcome. For preterm infants imaged at term equivalent PMA, low values for diffusion anisotropy in the PLIC significantly correlated with cerebral palsy.37,38
These data suggest that the central white matter in the preterm infant is uniquely vulnerable to injury, and the degree of gestational immaturity is directly related to this vulnerability. 30,32 In a provocative study, Als at al. demonstrated that institution of the Newborn Individualized Developmental Care and Assessment Program (NIDCAP) program in a small cohort of patients resulted in increased anisotropy in the internal capsule along with improved behavioral testing compared to premature controls at nine months corrected age.39 This demonstrates the potential for the application of anisotropy measures as a biomarker to evaluate the short term impact of potential neuroprotective interventions.
Diffusion MR tractography
As described briefly above, DTI tractography is a noninvasive method by which to follow white matter tracts on the basis of the orientation of the major eigenvector of the diffusion tensor. 40 This method can be used to identify specific white matter tracts throughout their course. For example, it is possible to follow the motor fibers from a seed point in the white matter underlying motor cortex through the internal capsule and into the brainstem. Once a tract has been thus defined, it is relatively straightforward to collect other diffusion parameters, such as ADC and anisotropy, for the tract (Fig 3). In many instances, these parameters are taken as an indication of the “health” of the tract, with high anisotropy values in particular associated with healthy white matter. Analysis of DTI data also provides estimates of ADC values parallel to fibers (parallel diffusivity) and perpendicular to them (axial diffusivity). It has been shown that disruption of axons leads to a decrease in parallel diffusivity, possibly because the axon pathways along which water molecules diffuse are disrupted. Conversely, loss of myelin is associated with an increase in axial diffusivity, possibly because there are fewer intact myelin membranes to hinder water displacements perpendicular to fiber tracts. 41 It is notable that either a reduction in parallel diffusivity or an increase in axial diffusivity leads to a decrease in overall anisotropy. Thus differentiation between injury to axons and myelin requires assessment of more than fractional or relative anisotropy values.
Fig 3.
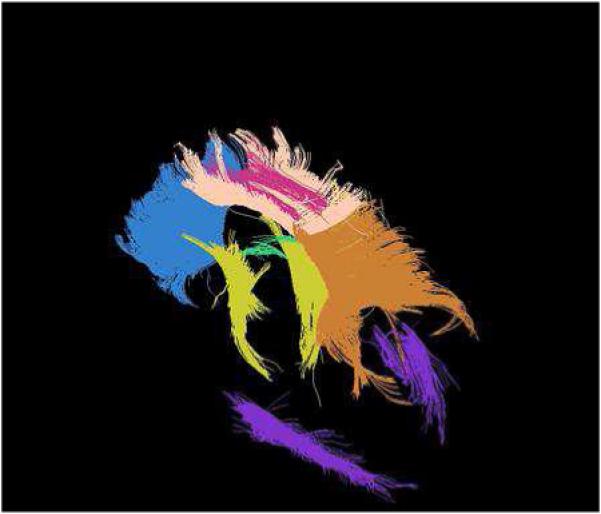
DTI MR tractography. The colors depict various white matter tracts as follows: Blue - Corpus Callosum genu: Skin color - Corpus Callosum body: Orange color - Corpus Callosum splenium: Green color – Fornix; Pink color - Cingulum;, Yellow color - Corticospinal Tract; and Purple color - Optic Radiations. (Image provided courtesy of Dr. Joshua Shimony, MD; PhD.)
The feasibility of tractography in neonates was demonstrated in a small group of 26-40 week gestation infants. 42,43 In a study of fifteen infants with congenital hemiparesis and seventeen age matched controls, Glenn et al demonstrated that the clinical severity of hemiparesis correlated with asymmetry in fractional anisotropy, radial diffusivity, and ADC. With increasing severity of hemiparesis, fractional anisotropy decreased and radial diffusivity and ADC increased in the affected pyramidal tract compared with controls. 44 The validity of this MR tool was further established by a study showing a marked disruption of thalamo-cortical connections in an infant with significant white matter injury compared to infants without WMI. 45 In this case, seed points were placed in the thalamus and tracts were followed to cortex. The significance of thalamo-cortical tract injury was demonstrated in a study of former premature infants with cerebral palsy who were imaged at age five years. Using DTI tractography, it was evident that injury to the posterior thalamic radiation correlated with reduced contralateral touch threshold, proprioception, and motor severity, whereas injury to the corticospinal tract did not correlate with motor or sensory outcome measures. 46 Thus this mode of anisotropy analysis provides detail regarding WMI, the most common site of injury in the developing premature brain.
Volumetric measurements through MR Imaging
Conventional MR images can be evaluated for tissue volumes (Fig 4). IOverall, prematurity and white matter injury are associated with reduced brain tissue volume and increased cerebrospinal fluid (CSF) volumes.47,48 Studies of former premature infants when they reach adolescence show reductions in overall brain volume and grey matter volume with an increase in lateral ventricular volume.49 The presence of signal abnormalities in white matter of former premature infants evaluated at age 15 years was associated with reduced white matter volume.50 Further, increased lateral ventricular size in this population was associated with cognitive and motor deficits.51 The volumes of sensori-motor and mid-temporal cortices was associated positively with full-scale, verbal, and performance IQ scores. 52 Thus, alterations in cerebral volumes have significant clinical correlates (i.e., size matters).
Fig 4.
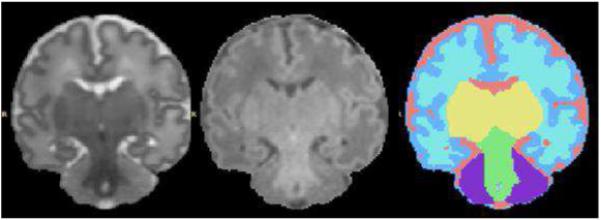
Image segmentation for quantitative volumetric analysis. The image on the left is a coronal T2-weighted image. The image in the center is the corresponding T1-weighted image. The image on the right is the segmentation map derived from these MR images. In this segmentation map components of the brain can be identified as follows: CSF (pink), unmyelinated white matter (taupe), cortical grey matter (blue), basal ganglia (yellow), myelinated white matter (green), and cerebellum (purple).
Few studies have been obtained in which volumes are reported for premature infants imaged at term equivalent PMA, and there are limited published studies that include clinical follow up. Nevertheless, a variety of MR abnormalities have been described. In infants with prior evidence of PVL by either CUS or MR, there is a marked reduction in cerebral cortical grey matter volume as compared with either preterm infants without PVL or term control infants. This change is associated with a significant decrease in total myelinated white matter volume and a complementary increase in total cerebrospinal fluid volume.53 Further, studies indicate that gyral development is markedly immature in infants with evidence of white matter injury.54 Thus, while there has been a strong focus on white matter injury in premature infants, these studies provide evidence for abnormalities in the development of cortical grey matter. It is not yet known whether these grey matter changes are a consequence of white matter injury or occur independently. While volumetric approaches hold much promise in the research setting, their integration into clinical care remains limited because of their complexity. Generating volumetric measurements is typically not fully automated and typically requires hours of operator input. Nevertheless the measures provide valuable insights into the impact of prematurity on the developing brain.
Brain Metrics
Simplified measurements of brain size on conventional MRI data offer a useful alternative technique for quantifying regional abnormalities. Using measurements obtained from fetal MRIs as a reference55,56 clinicians can take simple, one-dimensional measurements of specific areas of the brain (frontal lobes, cerebellum, sulcal distance) for comparison to gestational norms (Fig 5). Application of these metrics to a large group of preterm (at term equivalent PMA) and term control infants demonstrated that the bifrontal, biparietal and transverse cerebellar diameters were reduced (−11.6 %; CI= −13.8 to −9.3%; −12%, −14 to −9.8% and −8.7%, −10.5 to −7% respectively) and the left ventricle diameter was increased (+22.3%; 2.9 to 41.6%) in preterm infants (p<0.01). The biparietal diameter was weakly related to immaturity at birth, although the difference in biparietal diameter of preterm infants at term PMA versus term born infants was more striking. This narrowing of the biparietal diameter can be interpreted in relation to the scaphocephalic head shape characteristic of preterm infants at term PMA. There was no difference in the frontal-occipital distance, suggesting that the scaphocephaly is characterized by a narrowing of the head without a compensatory increase in length. There were strong correlations between the bifrontal and biparietal measures and total brain tissue volume, while the size of the ventricles and inter-hemispheric measure correlated with CSF volume. Intra-observer reliability was high (interclass correlation ICC coefficient >0.7), while inter-observer agreement was acceptable for tissue measures (ICC >0.6) but lower for fluid measures (ICC <0.4).57 These measures represent a reliable and easily applicable method to quantify brain growth and assess brain atrophy in this at-risk population. Neurodevelopmental correlates of these brain metrics are still awaited.
Fig 5.
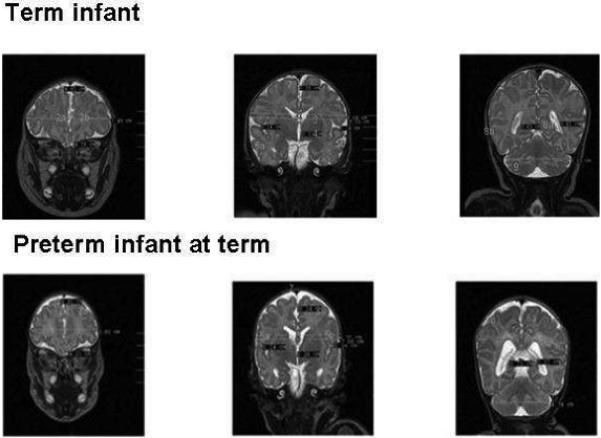
Standard neonatal brain metric measures in a premature and a term infant. Panel A shows measures in a term infant while Panel B demonstrates a former 30 week infant at term equivalent age. 1: bifrontal diameter; 2a and b: frontal height; 3:biparietal diameter; 4: bone biparietal diameter; 5a,b: cranio-caudal interopercular distance; 6: lateral diameter of the 3rd ventricle; 7: interhemispheric distance; 8a,b: lateral ventricle diameter; 9: transverse cerebellar diameter.
Surface-based analyses of cerebral cortex
In adults, studies of cortical folding patterns and their variability across individuals and groups have been greatly aided by surface-based approaches. Surface-based analysis (cortical cartography) offers inherent advantages in terms of visualization (easier visualization of cortex buried in sulci), quantitative analysis (determination of cortical surface area), and comparison across individuals (surface-based registration as an alternative to conventional volume-based registration)58-62. Semi-automated methods for segmentation, surface-based analysis and visualization have been critical to the success of these efforts (Fig 6). The power of this approach has been demonstrated in a recent study of Williams Syndrome63 which revealed statistically-significant cortical folding abnormalities compared to control subjects. In premature infants with WMI on MR imaging, cortical folding is delayed when compared to gestationally matched preterm infants without WMI. 64 Further, norms for sulcal and gyral development for infants from 30 to 40 weeks corrected gestational age have been developed and used to identify unique features of altered cortical folding in a cohort of twins, infants with intrauterine growth retardation (IUGR) and normal controls.65 Twins demonstrated a delayed but harmonious maturation, with reduced surface and sulcation index (the ratio of the brain “hull” surface area to the cortical surface area) compared to singletons, whereas the gyrification of IUGR newborns was discordant to the normal developmental trajectory, with a more pronounced reduction of surface area in relation to the sulcation index compared to normal newborns. Furthermore, these structural measurements of the brain at birth were predictors of outcome at term equivalent age evaluated with the assessment of preterm infant’s behaviour (APIB). 65,66 These studies illustrate the power of this approach in defining alterations of cerebral cortical development.
Fig 6.
Serial MR imaging of grey matter gyral maturation in a premature infant on T2 weighted MR images. The image on the left shows immature gyral folding in the left hemisphere of a preterm infant at 30 weeks corrected GA (born at 28 weeks). The center image demonstrates the pattern of folding at 34 weeks and the image on the right shows more mature folding with secondary gyri more consistent with a corrected GA of 38 weeks in the same infant.
Functional MR imaging and Connectivity
Functional MRI (fMRI) offers a novel way to bridge the gap between injury seen on MRI in the preterm population and the deficiencies seen in neurodevelopmental follow up. This imaging technique is covered elsewhere in this edition. Briefly, fMRI can be used to detect neural activation through its sensitivity to local oxyhemoglobin and deoxyhemoglobin levels Neural activation is associated with a local reduction in deoxyhemoglobin level, which leads to an increase in signal intensity in the blood-oxygenation-level-dependent (BOLD) imaging method used for fMRI.67 This method is commonly used in studies in which the subjects are asked to perform a task. BOLD images obtained with the subject resting and performing the task are subtracted to identify regions of activation associated with the task. Clearly, such an experimental paradigm is impractical for infants. However, a modification of fMRI, known as “functional connectivity” MRI (fcMRI), is applicable to infants. With this approach, spontaneous fluctuations in the BOLD signal during the resting state are measured (Fig 7). It has been shown that these spontaneous fluctuations have similar phase for regions of brain that are functionally connected. 68 In essence, this method shows neural networks. For example, if a seed point is placed in the leg motor area, fcMRI shows a phase correlation with BOLD signal arising from the contralateral leg motor area, which is known to be connected via the corpus callosum.
Fig 7.
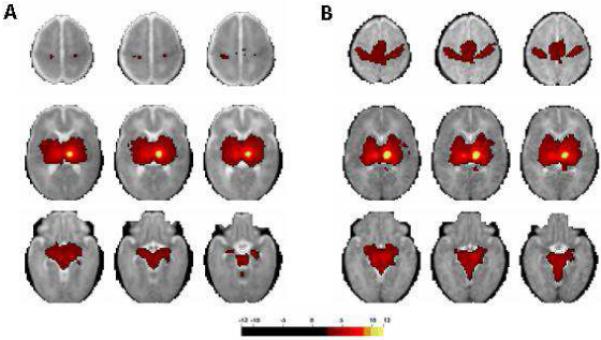
fcMRI maps generated using thalamic seed. The images on the left (A) are the average of 28 preterm infants with minimal injury on conventional MRI who underwent fcMRI at term equivalent PMA. The images on the right (B) are the average of 10 term control infants who underwent fcMRI during the first week of life. The colored areas represent regions in which a significant correlation was found between spontaneous fluctuations of the local BOLD signal and those of the thalamic seed. Note the marked difference in thalamo-cortical connectivity between these two data sets. (Images provided courtesy of Dr. Chris Smyser)
Fransson et.al., using fcMRI. have demonstrated resting-state networks in the infant brain that encompass the primary visual cortex; bilateral sensorimotor areas; bilateral auditory cortex; a network including the precuneus area, lateral parietal cortex, and the cerebellum; and an anterior network that incorporates the medial and dorsolateral prefrontal cortex. This suggests that resting-state networks driven by spontaneous signal fluctuations are already present in the infant brain. 69 In another study on 85 children including 35 neonates, fcMRI showed connectivity in the sensorimotor and visual areas. The percent of the brain showing connectivity increased with maturation. In addition, the sensorimotor connectivity preceded that of the visual cortex from 2 weeks - 1 year, but by 2 years of age they were comparable. 70
Conventional fMRI has been used to compare sensory, language, memory, and executive function between premature infants and term controls in subjects ranging from term equivalent PMA to early adolescence.71,72 The technology offers fascinating clues as to how the premature brain adapts its neural networks following injury. Premature infants studied at age 12 years have different patterns of activation when compared to their term counterparts in aspects of language processing, though they do not show any associated differences in performance scores.72 Rushe et al 73 studied 6 preterm infants with corpus callosal thinning and compared their phonological processing skills at 12 years of age with 6 term controls. Their hypothesis was that the children with a history of corpus callosal thinning would display incomplete lateralization of language function to the left hemisphere. BOLD signal response did indeed differ between the two groups, with activation in the left peristriate cortex, left cerebellum and right precuneus regions being reduced in the preterm infants along with an apparently compensatory increase in activation in the right precentral gyrus and superior frontal cortex. Despite these differences, there was no difference in the task performance score between the two groups. These variations in brain functional networks are not isolated to language function. Nosarti et al. 74 found similar alterations in activation in a population of preterm males tested at age 16 years compared to term controls in executive function tasks such as attention and inhibition. Again, despite these differences on fMRI, both the preterm and term controls tested similarly in respect overall graded task performance. In a recent study of 22 preterm infants without brain injury on cranial ultrasound and 26 term born controls, Schafer et al. noted that children born prematurely and those born at term had no difference in performance on a simple lexical semantic processing task and activated similar areas. Connectivity analysis, however, suggested that the PT subjects relied upon different neural pathways for lexical semantic processing when compared to term controls. 75 These studies suggest that the development of compensatory neural networks in response to injury in the premature brain that seem to preserve overall function through plasticity. In the future, fMRI and fcMRI may provide the key to understanding to why some preterm infants are able to compensate for environmental stresses and preserve overall function while others develop significant neurodevelopmental delay.
Future directions in MR imaging the developing brain
The use of MRI to evaluate the brain of premature infants is still a moving target in the sense that advanced DTI, fcMRI, surface morphometry and volumetric methods are still under active investigation. However, MRI clearly has an important role to play in evaluating premature infants today. It is rapidly being accepted as a biomarker for brain injury in the newborn with reliable sensitivity and specificity. Serial MR imaging and the application of newer analysis techniques provide an insight into the trajectory of brain development in the premature infant and the impact of injury on this developmental trajectory. MRI also has enormous potential to be used as a surrogate, short term outcome measure in clinical studies evaluating new therapeutic interventions of neuroprotection of the developing brain. With rapid advances in automation and analysis not only will the global impact of any intervention become identified early, but any regional differences in tissue structure, volume, and connectivity (both anatomic and functional) will be available. In addition, the plasticity of the developing brain could be tracked using fMRI and fcMRI as premature infants develop, possibly revealing alternative compensatory neural pathways that arise during development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990’s. Early Hum Dev. 1999;53(3):193–218. doi: 10.1016/s0378-3782(98)00052-8. [DOI] [PubMed] [Google Scholar]
- 2.Hack M, Flannery DJ, Schluchter M, et al. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346(3):149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 3.Hack M, Taylor HG, Klein N, et al. Functional limitations and special health care needs of 10- to 14-year-old children weighing less than 750 grams at birth. Pediatrics. 2000;106(3):554–560. doi: 10.1542/peds.106.3.554. [DOI] [PubMed] [Google Scholar]
- 4.Saigal S, Szatmari P, Rosenbaum P, et al. Cognitive abilities and school performance of extremely low birth weight children and matched term control children at age 8 years: a regional study. J Pediatr. 1991;118(5):751–760. doi: 10.1016/s0022-3476(05)80043-5. [DOI] [PubMed] [Google Scholar]
- 5.Whitaker AH, Van Rossem R, Feldman JF, et al. Psychiatric outcomes in low-birth-weight children at age 6 years: relation to neonatal cranial ultrasound abnormalities. Arch Gen Psychiatry. 1997;54(9):847–856. doi: 10.1001/archpsyc.1997.01830210091012. [DOI] [PubMed] [Google Scholar]
- 6.Whitfield MF, Grunau RV, Holsti L. Extremely premature (< or = 800 g) schoolchildren: multiple areas of hidden disability. Arch Dis Child Fetal Neonatal Ed. 1997;77(2):F85–90. doi: 10.1136/fn.77.2.f85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodward LJ, Anderson PJ, Austin NC, et al. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355(7):685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 8.Cooke RW, Abernethy LJ. Cranial magnetic resonance imaging and school performance in very low birth weight infants in adolescence. Arch Dis Child Fetal Neonatal Ed. 1999;81(2):F116–121. doi: 10.1136/fn.81.2.f116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathur AM, Neil JJ, McKinstry RC, et al. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 2008;38(3):260–264. doi: 10.1007/s00247-007-0705-9. [DOI] [PubMed] [Google Scholar]
- 10.De Vries LS, Connell JA, Dubowitz LM, et al. Neurological, electrophysiological and MRI abnormalities in infants with extensive cystic leukomalacia. Neuropediatrics. 1987;18(2):61–66. doi: 10.1055/s-2008-1052453. [DOI] [PubMed] [Google Scholar]
- 11.Flodmark O, Lupton B, Li D, et al. MR imaging of periventricular leukomalacia in childhood. AJR Am J Roentgenol. 1989;152(3):583–590. doi: 10.2214/ajr.152.3.583. [DOI] [PubMed] [Google Scholar]
- 12.Barkovich AJ, Truwit CL. Brain damage from perinatal asphyxia: correlation of MR findings with gestational age. AJNR Am J Neuroradiol. 1990;11(6):1087–1096. [PMC free article] [PubMed] [Google Scholar]
- 13.Truwit CL, Barkovich AJ, Koch TK, et al. Cerebral palsy: MR findings in 40 patients. AJNR Am J Neuroradiol. 1992;13(1):67–78. [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen P, Paakko E, Vainionpaa L, et al. Magnetic resonance imaging of periventricular leukomalacia and its clinical correlation in children. Ann Neurol. 1997;41(6):754–761. doi: 10.1002/ana.410410611. [DOI] [PubMed] [Google Scholar]
- 15.Lanzi G, Fazzi E, Uggetti C, et al. Cerebral visual impairment in periventricular leukomalacia. Neuropediatrics. 1998;29(3):145–150. doi: 10.1055/s-2007-973551. [DOI] [PubMed] [Google Scholar]
- 16.Valkama AM, Paakko EL, Vainionpaa LK, et al. Magnetic resonance imaging at term and neuromotor outcome in preterm infants. Acta Paediatr. 2000;89(3):348–355. [PubMed] [Google Scholar]
- 17.Counsell SJ, Allsop JM, Harrison MC, et al. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003;112(1 Pt 1):1–7. doi: 10.1542/peds.112.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Counsell SJ, Shen Y, Boardman JP, et al. Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics. 2006;117(2):376–386. doi: 10.1542/peds.2005-0820. [DOI] [PubMed] [Google Scholar]
- 19.Dyet LE, Kennea N, Counsell SJ, et al. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118(2):536–548. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 20.Domizio S, Barbante E, Puglielli C, et al. Excessively high magnetic resonance signal in preterm infants and neuropsychobehavioural follow-up at 2 years. Int J Immunopathol Pharmacol. 2005;18(2):365–375. doi: 10.1177/039463200501800218. [DOI] [PubMed] [Google Scholar]
- 21.De Vries LS, Groenendaal F, van Haastert IC, et al. Asymmetrical myelination of the posterior limb of the internal capsule in infants with periventricular haemorrhagic infarction: an early predictor of hemiplegia. Neuropediatrics. 1999;30(6):314–319. doi: 10.1055/s-2007-973511. [DOI] [PubMed] [Google Scholar]
- 22.Mazumdar A, Mukherjee P, Miller JH, et al. Diffusion-weighted imaging of acute corticospinal tract injury preceding Wallerian degeneration in the maturing human brain. AJNR Am J Neuroradiol. 2003;24(6):1057–1066. [PMC free article] [PubMed] [Google Scholar]
- 23.Neil JJ, Inder TE. Detection of wallerian degeneration in a newborn by diffusion magnetic resonance imaging (MRI) J Child Neurol. 2006;21(2):115–118. doi: 10.1177/08830738060210021501. [DOI] [PubMed] [Google Scholar]
- 24.Neil JJ. Diffusion imaging concepts for clinicians. J Magn Reson Imaging. 2008;27(1):1–7. doi: 10.1002/jmri.21087. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee P, McKinstry RC. Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin N Am. 2006;16(1):19–43. vii. doi: 10.1016/j.nic.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002;15(7-8):468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 27.McKinstry RC, Mathur A, Miller JH, et al. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cereb Cortex. 2002;12(12):1237–1243. doi: 10.1093/cercor/12.12.1237. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke CD, Van Essen DC, Inder TE, et al. Microstructural changes of the baboon cerebral cortex during gestational development reflected in magnetic resonance imaging diffusion anisotropy. J Neurosci. 2007;27(46):12506–12515. doi: 10.1523/JNEUROSCI.3063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huppi PS, Maier SE, Peled S, et al. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44(4):584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Berman JI, Mukherjee P, Partridge SC, et al. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005;27(4):862–871. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Dudink J, Lequin M, van Pul C, et al. Fractional anisotropy in white matter tracts of very-low-birth-weight infants. Pediatr Radiol. 2007;37(12):1216–1223. doi: 10.1007/s00247-007-0626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller SP, Vigneron DB, Henry RG, et al. Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn Reson Imaging. 2002;16(6):621–632. doi: 10.1002/jmri.10205. [DOI] [PubMed] [Google Scholar]
- 33.Anjari M, Srinivasan L, Allsop JM, et al. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. Neuroimage. 2007;35(3):1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 34.Cheong JL, Thompson DK, Wang HX, et al. Abnormal white matter signal on MR imaging is related to abnormal tissue microstructure. AJNR Am J Neuroradiol. 2009;30(3):623–628. doi: 10.3174/ajnr.A1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnan ML, Dyet LE, Boardman JP, et al. Relationship between white matter apparent diffusion coefficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics. 2007;120(3):e604–609. doi: 10.1542/peds.2006-3054. [DOI] [PubMed] [Google Scholar]
- 36.Drobyshevsky A, Bregman J, Storey P, et al. Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Dev Neurosci. 2007;29(4-5):289–301. doi: 10.1159/000105470. [DOI] [PubMed] [Google Scholar]
- 37.Arzoumanian Y, Mirmiran M, Barnes PD, et al. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am J Neuroradiol. 2003;24(8):1646–1653. [PMC free article] [PubMed] [Google Scholar]
- 38.Rose J, Mirmiran M, Butler EE, et al. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol. 2007;49(10):745–750. doi: 10.1111/j.1469-8749.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- 39.Als H, Duffy FH, McAnulty GB, et al. Early experience alters brain function and structure. Pediatrics. 2004;113(4):846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- 40.Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998;39(6):928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- 41.Song SK, Sun SW, Ju WK, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Zhai G, Lin W, Wilber KP, et al. Comparisons of regional white matter diffusion in healthy neonates and adults performed with a 3.0-T head-only MR imaging unit. Radiology. 2003;229(3):673–681. doi: 10.1148/radiol.2293021462. [DOI] [PubMed] [Google Scholar]
- 43.Yoo SS, Park HJ, Soul JS, et al. In vivo visualization of white matter fiber tracts of preterm- and term-infant brains with diffusion tensor magnetic resonance imaging. Invest Radiol. 2005;40(2):110–115. doi: 10.1097/01.rli.0000149491.69201.cb. [DOI] [PubMed] [Google Scholar]
- 44.Glenn OA, Ludeman NA, Berman JI, et al. Diffusion tensor MR imaging tractography of the pyramidal tracts correlates with clinical motor function in children with congenital hemiparesis. AJNR Am J Neuroradiol. 2007;28(9):1796–1802. doi: 10.3174/ajnr.A0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Counsell SJ, Dyet LE, Larkman DJ, et al. Thalamo-cortical connectivity in children born preterm mapped using probabilistic magnetic resonance tractography. Neuroimage. 2007;34(3):896–904. doi: 10.1016/j.neuroimage.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 46.Hoon AH, Jr, Stashinko EE, Nagae LM, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 2009 doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inder TE, Warfield SK, Wang H, et al. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115(2):286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 48.Thompson DK, Warfield SK, Carlin JB, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130(Pt 3):667–677. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- 49.Nosarti C, Al-Asady MH, Frangou S, et al. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125(Pt 7):1616–1623. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 50.Panigrahy A, Barnes PD, Robertson RL, et al. Volumetric brain differences in children with periventricular T2-signal hyperintensities: a grouping by gestational age at birth. AJR Am J Roentgenol. 2001;177(3):695–702. doi: 10.2214/ajr.177.3.1770695. [DOI] [PubMed] [Google Scholar]
- 51.Melhem ER, Hoon AH, Jr., Ferrucci JT, Jr., et al. Periventricular leukomalacia: relationship between lateral ventricular volume on brain MR images and severity of cognitive and motor impairment. Radiology. 2000;214(1):199–204. doi: 10.1148/radiology.214.1.r00dc35199. [DOI] [PubMed] [Google Scholar]
- 52.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284(15):1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 53.Inder TE, Huppi PS, Warfield S, et al. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol. 1999;46(5):755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 54.Inder TE, Wells SJ, Mogridge NB, et al. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003;143(2):171–179. doi: 10.1067/S0022-3476(03)00357-3. [DOI] [PubMed] [Google Scholar]
- 55.Garel C. The role of MRI in the evaluation of the fetal brain with an emphasis on biometry, gyration and parenchyma. Pediatr Radiol. 2004;34(9):694–699. doi: 10.1007/s00247-004-1249-x. [DOI] [PubMed] [Google Scholar]
- 56.Garel C. MRI of the fetal brain. Springer-Verlag; Berlin: 2004. [Google Scholar]
- 57.Nguyen The Tich S, Anderson PJ, Shimony JS, et al. A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR Am J Neuroradiol. 2009;30(1):125–131. doi: 10.3174/ajnr.A1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drury HA, Van Essen DC, Anderson CH, et al. Computerized mappings of the cerebral cortex: a multiresolution flattening method and a surface-based coordinate system. J Cogn Neurosci. 1996;8(1):1–28. doi: 10.1162/jocn.1996.8.1.1. [DOI] [PubMed] [Google Scholar]
- 59.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 60.Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28(3):635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 61.Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29(3):148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Essen DC. Towards a quantitative, probabilistic neuroanatomy of cerebral cortex. Cortex. 2004;40(1):211–212. doi: 10.1016/s0010-9452(08)70954-7. [DOI] [PubMed] [Google Scholar]
- 63.Van Essen DC, Dierker D, Snyder AZ, et al. Symmetry of cortical folding abnormalities in Williams syndrome revealed by surface-based analyses. J Neurosci. 2006;26(20):5470–5483. doi: 10.1523/JNEUROSCI.4154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramenghi LA, Fumagalli M, Righini A, et al. Magnetic resonance imaging assessment of brain maturation in preterm neonates with punctate white matter lesions. Neuroradiology. 2007;49(2):161–167. doi: 10.1007/s00234-006-0176-y. [DOI] [PubMed] [Google Scholar]
- 65.Dubois J, Benders M, Borradori-Tolsa C, et al. Primary cortical folding in the human newborn: an early marker of later functional development. Brain. 2008;131(Pt 8):2028–2041. doi: 10.1093/brain/awn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dubois J, Benders M, Cachia A, et al. Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex. 2008;18(6):1444–1454. doi: 10.1093/cercor/bhm180. [DOI] [PubMed] [Google Scholar]
- 67.Babiloni C, Pizzella V, Gratta CD, et al. Fundamentals of electroencefalography, magnetoencefalography, and functional magnetic resonance imaging. Int Rev Neurobiol. 2009;86:67–80. doi: 10.1016/S0074-7742(09)86005-4. [DOI] [PubMed] [Google Scholar]
- 68.Fair DA, Cohen AL, Dosenbach NU, et al. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fransson P, Skiold B, Horsch S, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A. 2007;104(39):15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin W, Zhu Q, Gao W, et al. Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. AJNR Am J Neuroradiol. 2008;29(10):1883–1889. doi: 10.3174/ajnr.A1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peterson BS, Vohr B, Kane MJ, et al. A functional magnetic resonance imaging study of language processing and its cognitive correlates in prematurely born children. Pediatrics. 2002;110(6):1153–1162. doi: 10.1542/peds.110.6.1153. [DOI] [PubMed] [Google Scholar]
- 72.Ment LR, Peterson BS, Meltzer JA, et al. A functional magnetic resonance imaging study of the long-term influences of early indomethacin exposure on language processing in the brains of prematurely born children. Pediatrics. 2006;118(3):961–970. doi: 10.1542/peds.2005-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rushe TM, Temple CM, Rifkin L, et al. Lateralisation of language function in young adults born very preterm. Arch Dis Child Fetal Neonatal Ed. 2004;89(2):F112–118. doi: 10.1136/adc.2001.005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nosarti C, Rubia K, Smith AB, et al. Altered functional neuroanatomy of response inhibition in adolescent males who were born very preterm. Dev Med Child Neurol. 2006;48(4):265–271. doi: 10.1017/S0012162206000582. [DOI] [PubMed] [Google Scholar]
- 75.Schafer RJ, Lacadie C, Vohr B, et al. Alterations in functional connectivity for language in prematurely born adolescents. Brain. 2009;132(Pt 3):661–670. doi: 10.1093/brain/awn353. [DOI] [PMC free article] [PubMed] [Google Scholar]


