Abstract
Neural stem cells persist in the adult mammalian forebrain and are a potential source of neurons for repair after brain injury. The two main areas of persistent neurogenesis, the subventricular zone (SVZ)-olfactory bulb pathway and hippocampal dentate gyrus, are stimulated by brain insults such as stroke or trauma. Here we focus on the effects of focal cerebral ischemia on SVZ neural progenitor cells in experimental stroke, and the influence of mechanical injury on adult hippocampal neurogenesis in models of traumatic brain injury (TBI). Stroke potently stimulates forebrain SVZ cell proliferation and neurogenesis. SVZ neuroblasts are induced to migrate to the injured striatum, and to a lesser extent to the peri-infarct cortex. Controversy exists as to the types of neurons that are generated in the injured striatum, and whether adult-born neurons contribute to functional restoration remains uncertain. Advances in understanding the regulation of SVZ neurogenesis in general, and stroke-induced neurogenesis in particular, may lead to improved integration and survival of adult-born neurons at sites of injury. Dentate gyrus cell proliferation and neurogenesis similarly increase after experimental TBI. However, pre-existing neuroblasts in the dentate gyrus are vulnerable to traumatic insults, which appear to stimulate neural stem cells in the SGZ to proliferate and replace them, leading to increased numbers of new granule cells. Interventions that stimulate hippocampal neurogenesis appear to improve cognitive recovery after experimental TBI. Transgenic methods to conditionally label or ablate neural stem cells are beginning to further address critical questions regarding underlying mechanisms and function significance of neurogenesis after stroke or TBI. Future therapies should be aimed at directing appropriate neuronal replacement after ischemic or traumatic injury while suppressing aberrant integration that may contribute to co-morbidities such as epilepsy or cognitive impairment.
First proposed nearly a century ago (Allen, 1912), the persistence of neural stem cells and neurogenesis in the adult mammalian central nervous system (CNS) is now accepted. This change in dogma is based largely upon evidence accumulated over the past four decades indicating that neural stem cells populate two main areas, the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus, where they give rise to neurons throughout adulthood. Adult neurogenesis is found in these forebrain regions in all mammalian species examined, including humans (Curtis et al., 2007; Eriksson et al., 1998a), and may serve to replace cells damaged by brain insults. Whether they do replace dying or diseased cells, and if so to what extent, are among the questions upon which current research is intensely focused. In this review, we will briefly describe the normal pathways of adult forebrain neurogenesis and then discuss how neurogenesis is altered in models of ischemic stroke and traumatic brain injury (TBI). For the former, we focus on studies of the SVZ, and for the latter on the hippocampal dentate gyrus.
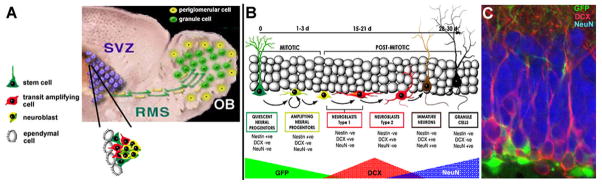
The SVZ neural stem cell is a nestin- and glial fibrillary acidic protein (GFAP)-immunoreactive radial glia-like cell that probably arises from embryonic radial glia (Merkle et al., 2004). SVZ neural stem cells give rise to neuroblasts that migrate in chains to the olfactory bulb through the rostral migratory stream (Fig 1A; (Corotto et al., 1993; Lois and Alvarez-Buylla, 1994; Lois et al., 1996). Once they reach the bulb, the migrating neuroblasts detach from the chains, disperse and differentiate into granule and periglomerular neurons (Altman, 1969; Corotto et al., 1993; Lois and Alvarez-Buylla, 1994). A portion of adult-born granular and periglomerular interneurons survive long-term (Winner et al., 2002) and appear to integrate into bulb circuitry (Carlen et al., 2002; Carleton et al., 2003; Livneh et al., 2009). Studies using conditional transgenic reporter mice and ablation strategies show that olfactory granule and periglomerular cells are continuously added to the bulb to both increase total cell numbers over time in these layers as well as replace pre-existing cells (Imayoshi et al., 2008; Lagace et al., 2007). The function of persistent olfactory bulb neurogenesis is largely unknown. Increasing evidence supports a role for the new neurons in olfactory memory and odor discrimination (Gheusi et al., 2000; Petreanu and Alvarez-Buylla, 2002; Rochefort et al., 2002); however, the use of transgenic ablation strategies has failed to yield deficits in some of these measures (Imayoshi et al., 2008).
Figure 1.
Regions of persistent neurogenesis in the adult. A, The subventricular zone (SVZ)-olfactory bulb (OB) pathway. Radial glia-like neural stem cells in the SVZ give rise to rapidly dividing transit amplifying cells and then neuroblasts. All these cells are closely apposed in the SVZ niche that includes ependymal cells and endothelial cells (not shown). The SVZ-derived neuroblasts migrate tangentially to the bulb in neuronophilic chains via the rostral migratory stream (RMS). B, Stages of neurogenesis in the dentate subgranular zone (SGZ). GFP refers to reporter expression in a nestin-GFP mouse line. C, Section through the dentate granule cell layer of an adult nestin-GFP mouse showing GFP-expressing progenitors (green), doublecortin immunolabeled neuroblasts (DCX, red) and NeuN immunoreactive mature dentate granule cells (blue). The dentate hilus is at the very bottom of the image.
The SGZ of the dentate gyrus is the other anatomically discrete location where neural precursors persist throughout life. The rodent dentate gyrus develops mostly during the postnatal period and the reservoir of SGZ progenitors persists well into adulthood (Pozniak and Pleasure, 2006). As in the SVZ, the primary progenitor or neural stem-like cell in the SGZ is a nestin- and GFAP-expressing, radial glia-like astrocyte (Fig. 1B; (Seri et al., 2001)). These progenitors give rise almost exclusively to granular neurons within the most basal layers of the dentate gyrus (Duan et al., 2008). The dentate gyrus receives input from the cortex and other brain areas where it is integrated and transmitted to the rest of the hippocampus. Its precursors, which integrate throughout the lifespan, generate neurons that make and receive functional synapses (Eriksson et al., 1998b; Faulkner et al., 2008; Palmer et al., 2000; Song et al., 2002; Toni et al., 2008; van Praag et al., 2002). Enhanced neurogenesis that occurs in the hippocampus is also known to facilitate long-term potentiation and stimulate learning and memory (Farmer et al., 2004; Imayoshi et al., 2008; van Praag et al., 1999; Wang et al., 2005). Ablation of adult-born dentate granule cells impairs certain forms of hippocampus-dependent learning (Clelland et al., 2009; Dupret et al., 2008; Imayoshi et al., 2008), further suggesting that these cells play a role in this function. In addition, adult-born dentate granule cells may be involved in regulating anxiety-associated behavior (Revest et al., 2009).
Despite the tremendous interest in neural stem cell biology, there is little mechanistic insight into stem cell survival following common conditions induced by trauma or other brain insults. Recently, many paradigms of brain injury, including TBI, seizures, stroke, hypoxia-ischemia, and neurodegenerative diseases, implicate neural stem cells in the remodeling that occurs following such injuries (Arvidsson et al., 2002; Jin et al., 2001; Kernie et al., 2001a; Miles and Kernie, 2008; Parent et al., 2002; Parent et al., 1997; Zhang et al., 2001). The physiologic relevance of this proliferation remains unknown, but it may in part explain some of the spontaneous recovery that occurs in all of these disease states. Alternatively, aberrant neurogenesis after injury could contribute to ongoing morbidity that impairs functional recovery. In the following sections, we describe the current knowledge and outstanding research questions in the field of injury-induced neurogenesis. We first focus on experimental stroke and the SVZ, and then shift to TBI models and dentate granule cell neurogenesis.
Stroke-induced neurogenesis
Stroke is a leading cause of morbidity and mortality, yet no regenerative therapies currently exist. Focal ischemic stroke is the most common form and involves a discrete area of necrotic brain tissue surrounded by hypoperfused tissue at risk known as the ischemic penumbra. Treatments aimed at salvaging the penumbra using neuroprotective strategies have received considerable attention without substantial success, but the idea of improving functional recovery by replacing lost neurons remains relatively unexplored. The persistence of neural stem cells in the adult brain, as well as advances in stem cell biology, raise the possibility of using endogenous or transplantable neural stem cells to replace neurons lost after ischemic insults as regenerative therapies. Here we focus on endogenous SVZ neural progenitors and stroke, and describe current knowledge of how stroke influences neurogenesis, the potential underlying mechanisms, and current challenges for the field.
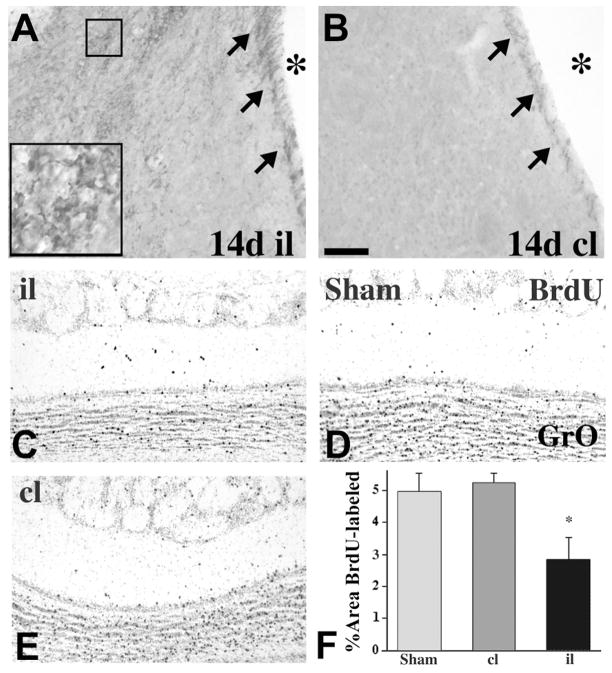
Studies of experimental stroke in rodents over the past decade indicate that focal ischemia potently stimulates forebrain SVZ cell proliferation and neurogenesis (Arvidsson et al., 2002; Jin et al., 2001; Parent et al., 2002; Zhang et al., 2001). The striatal SVZ expands and contains increased numbers of neuroblasts identified by immunolabeling for doublecortin (Fig. 2A, B), neuron-specific β-tubulin or polysialylated neural cell adhesion molecule. Most of these studies involve the classic transient middle cerebral artery occlusion (tMCAO) stroke model. Although initial studies suggested that the increase in SVZ neurogenesis after stroke is transient (Arvidsson et al., 2002; Parent et al., 2002), more recent work indicates that it persists for at least four months after ischemia (Thored et al., 2006).
Figure 2.
Stroke-induced neurogenesis. A, B, Doublecortin immunostaining at 14 days after tMCAO shows increased SVZ and striatal neurogenesis of the ipsilateral (il) hemisphere compared to contralateral (cl). The asterisks denote the lateral ventricle. C–E, Coronal sections of BrdU-immunoreactive olfactory neurons in the granular layer (GrO) of rats after tMCAO either ipsilateral (il, C) or contralateral (cl, D) to the infarct, or from a sham-operated control (E) at 28 days after surgery. (D) Quantification of GrO BrdU labeling shows significantly decreased BrdU-immunoreactivity il to the stroke after 28 days. *, p < 0.05 vs. cl and sham control. Scale bar, 50 μm for A and B; 150 μm for C–E.
Potential mediators of stroke-induced cell proliferation and neurogenesis are beginning to be identified. Several groups have found that Notch signaling, particularly through Notch1, stimulates SVZ cell proliferation and neurogenesis after stroke (Androutsellis-Theotokis et al., 2006; Wang et al., 2009), although conflicting results have been found (Carlen et al., 2009). Infusion of various growth factors or reagents that stimulate growth factor expression also increases stroke-induced SVZ neurogenesis (Chen et al., 2004; Jin et al., 2002a; Jin et al., 2002b; Kolb et al., 2007; Leker et al., 2007; Sun et al., 2003; Teramoto et al., 2003; Wang et al., 2004). Conversely, decreased growth factor expression via inhibitors or in knockout mice impairs SVZ neurogenesis after stroke (Chen et al., 2005; Tsai et al., 2006; Yan et al., 2006). Other signaling pathways that appear important for stroke-induced SVZ neurogenesis include retinoid (Plane et al., 2008), bone morphogenetic protein (Chou et al., 2006), tumor necrosis factor-alpha (TNF-α) (Iosif et al., 2008) and sonic hedgehog (Sims et al., 2009) pathways.
The SVZ neuroblasts are normally destined to migrate to the olfactory bulb via chain-like formations in the rostral migratory stream. After focal ischemia, however, many of them migrate in chains toward the ischemic striatum (Arvidsson et al., 2002; Jin et al., 2003; Ohab et al., 2006; Parent et al., 2002; Yamashita et al., 2006). This redirected migration occurs at the expense of olfactory bulb migration as fewer neuroblasts reach the ipsilateral bulb (Fig. 2C–F). Several molecular factors that direct this ectopic migration to peri-infarct regions have been identified. These include matrix metalloproteases (Lee et al., 2006; Liu et al., 2009b) and chemokine/chemokine receptor interactions (Ohab et al., 2006; Robin et al., 2006; Thored et al., 2006; Yan et al., 2007). In addition, the vasculature appears to play an important role in the migration of neuroblasts to regions of ischemic damage. Many neuroblasts are found in close proximity to blood vessels in the ischemic striatum (Ohab et al., 2006; Thored et al., 2007; Yamashita et al., 2006), and infusion of pro- or anti-angiogenic factors stimulate and inhibit neuroblast migration to peri-infarct regions, respectively (Ohab et al., 2006). Interestingly, a population of neural progenitor cells that inhabit more caudal aspects of the SVZ underlying the corpus callosum appears to generate neuroblasts that migrate to the injured hippocampus after more global ischemic insults that damage the hippocampal pyramidal cell layer (Nakatomi et al., 2002). Thus, the population of SVZ progenitors that may potentially serve as a source of cells for neuronal replacement after injury is likely dispersed throughout the forebrain periventricular regions.
What is the fate of neuroblasts that migrate to the injured striatum after focal ischemia? Although a large number of neuroblasts reach regions of striatal damage after stroke, few of them differentiate into mature neurons. Most adult-born neurons appear to die (Arvidsson et al., 2002; Parent et al., 2002), perhaps from a failure to integrate or due to the inflammatory milieu. In support of the latter idea, treatment with the anti-inflammatory agent indomethacin to suppress inflammation and microglial activation stimulates the accumulation of newborn cells in the injured striatum following 2-hour MCAO in adult rats (Hoehn et al., 2005). Furthermore, signaling through the TNF-α receptor-1 suppresses stroke-induced SVZ neurogenesis (Iosif et al., 2008). Although inhibiting inflammation and microglia might prove useful, this approach may be complicated by recent findings of positive chronic effects of microglia on stroke-induced neurogenesis (Thored et al., 2009). Importantly, the migration of SVZ neuroblasts to the injured striatum persists for up to a year after ischemia (Thored et al., 2007), suggesting that the SVZ may serve as a constant reservoir of new neurons that offers a long time window for therapeutic manipulations.
In most stroke models, many of the surviving cells differentiate into neurons, but the precise nature of the neurons that persist long-term (at least months) in the striatum is controversial. Two groups first reported that most neurons expressed markers of striatal medium spiny neurons, including DARPP-32 and calbindin, after tMCAO in adult rats (Arvidsson et al., 2002; Parent et al., 2002). Subsequently, another group using tMCAO in adult mice along with epidermal growth factor infusions found mostly parvalbumin-expressing interneurons were generated after stroke (Teramoto et al., 2003). All of these studies used bromodeoxyuridine (BrdU) administration to label progenitors at early time points after ischemia and determine their phenotypes after varying survival durations. More recently, Liu and colleagues used retroviral reporters to label SVZ progenitors prior to inducing stroke in adult rats and found that the adult-born neurons exclusively differentiated into calretinin-expressing interneurons (Liu et al., 2009a). The reasons for these disparate findings are not entirely clear. Additional studies using retroviral reporter injections or transgenic approaches to permanently label adult-born neurons are therefore necessary to resolve this issue.
A critical question revolves around the functional significance of stroke-induced neurogenesis. At present, it is not clear whether the limited numbers of surviving adult-born neurons replace lost cells by integrating appropriately, and whether this improves recovery. Some evidence supports the integration of a small portion of adult-born neurons that migrate to the injured striatum after stroke (Yamashita et al., 2006), but whether these cells make appropriate connections remains uncertain. Several approaches should help to answer these questions in the near future. First, the use of newer retroviral reporter constructs (e.g., using synapse-specific promoters) should allow a more in depth analysis of the integration of adult-born neurons that survive after stroke. This analysis should include combinations of electron microscopy and slice electrophysiology to assess both the structural and functional integration of the maturing neurons. Manipulations that are directed at increasing neurogenesis have been shown to improve functional outcome (e.g., (Leker et al., 2007; Ohab et al., 2006; Wang et al., 2004)), but interventions that are more specific to neurogenesis, particularly the survival of adult-born neurons after stroke, are needed to more convincingly link improved behavioral recovery with increased neurogenesis. Finally, more specific means to ablate adult-born neurons have been developed recently (Garcia et al., 2004; Imayoshi et al., 2008; Revest et al., 2009; Singer et al., 2009; Yu et al., 2008a). These methods should allow a determination of whether the absence of neurogenesis after stroke impairs recovery. If stroke-induced neurogenesis indeed contributes to stroke recovery, then focused efforts are needed to direct and augment this process for the development of regenerative therapies for cerebral ischemia.
Neurogenesis following traumatic brain injury
Significant self-recovery occurs following all but the most severe episodes of TBI (Anderson et al., 2000; Demeurisse, 2000; Ewing-Cobbs et al., 2003). The mechanisms underlying this remain unclear, though injury-induced neurogenesis is one compelling potential contributor to post-injury recovery (Chen et al., 2003; Chirumamilla et al., 2002; Eriksson et al., 1998a; Richardson et al., 2007). As described above, the two most well studied and validated reservoirs for neural stem and progenitor cells in mammals are the SVZ of the lateral ventricles and the SGZ of the dentate gyrus (Alvarez-Buylla et al., 2001; Seaberg and van der Kooy, 2002). Normally, the postnatal SVZ contributes progenitors to the rostral migratory stream to support ongoing olfactory neurogenesis, while the SGZ of the dentate gyrus provides new granular neurons throughout life (Alvarez-Buylla et al., 2001; Temple and Qian, 1995). Following TBI, progenitor cells in each of these areas become activated, though it is still unclear whether this activation results in stable and productive neurogenesis (Richardson et al., 2007). Since the dentate gyrus is a key component to the hippocampus, where the basis for much of learning and memory reside, it is a particularly compelling target for studying the potential implications of injury-induced neurogenesis and is the focus of this part of the review.
Evidence for long-lasting hippocampal neurogenesis after traumatic cortical injury has been accumulating since first described in 2001 (Dash et al., 2001; Kernie et al., 2001b). The dentate gyrus itself develops mostly during the postnatal period and the reservoir of subgranular zone progenitors persists well into adulthood (Pozniak and Pleasure, 2006). These progenitors develop almost exclusively into granular neurons within the most basal layers of the dentate gyrus and their functions remain largely unknown (Duan et al., 2008). After injury, however, even in adult animals, it is clear that progenitors are activated and increased neurogenesis follows whereby new neurons are found in the outer layers of the dentate gyrus, where normally this would occur only during early development (Chen et al., 2003; Chirumamilla et al., 2002; Dash et al., 2001; Ramaswamy et al., 2005; Richardson et al., 2007; Urrea et al., 2007; Yu et al., 2008b). Most of the evidence to support these findings is based on BrdU (5-bromo-2-deoxyuridine) incorporation into dividing cells that when followed over time express markers suggesting mature and stable neurogenesis. There are a variety of problems with using cell cycle markers such as BrdU to quantify this effect (Gould and Gross, 2002). For example, since BrdU is a thymidine analogue that incorporates into dividing cells, it may be a marker of cells undergoing DNA repair or alternatively may mark dying cells attempting to repair themselves. In addition, BrdU only labels cells dividing at the time that BrdU or other thymidine analogues are administered and therefore only provides a temporally limited glimpse at the full contributions of the progenitor population to injury-induced neurogenesis.
There are two major reasons why the extent and relevance of injury-induced neurogenesis in the hippocampus has not become more clearly delineated. One is that tracing of dentate gyrus progenitors has been limited to labeling with thymidine analogues such as BrdU. The second is that many of the genetic and histologic markers that these progenitors express are also found in reactive astrocytes (Eng and Ghirnikar, 1994; Myer et al., 2006; Ridet et al., 1997). Since reactive astrocytosis is a hallmark of TBI, it becomes difficult to differentiate between stem/progenitor cells within the dentate gyrus and astrocytes that have become activated and express some of the same markers. Thus, expression of nestin, Sox 2, vimentin, and glial fibrillary associated protein (GFAP) are not specific for neural stem and progenitor cells but may also be expressed in astrocytes following injury (Ridet et al., 1997; Yu et al., 2008b).
Recently, genetic tools have become available that, at least in part, attenuate some of these limitations. By directing expression of a variety of transgenes specifically in neural stem and progenitors in mice, more clearly defining the implications of injury-induced neurogenesis is more straightforward. One of the most commonly used markers for stem and progenitor cells is expression of the intermediate filament protein nestin. However, nestin expression is not limited to neural stem and progenitor cells, but is also found in developing muscle, endothelial cells, and reactive astrocytes (Lendahl et al., 1990; Lin et al., 1995). The genetic enhancer elements that regulate this expression have been defined in mice, and the second intron of the nestin gene directs expression exclusively in neural stem and progenitor cells (Figure 1B, C) (Zimmerman et al., 1994). Many investigators have taken advantage of this specificity to make transgenic mice that express a variety of genes exclusively in the progenitor population (Kawaguchi et al., 2001; Mignone et al., 2004; Yu et al., 2005). It has also been recently demonstrated that reporter transgenes under the control of the nestin promoter are not expressed in reactive astrocytes after injury, making it a compelling model for the study of injury-induced neurogenesis (Miles and Kernie, 2008; Shi et al., 2007; Yu et al., 2008b).
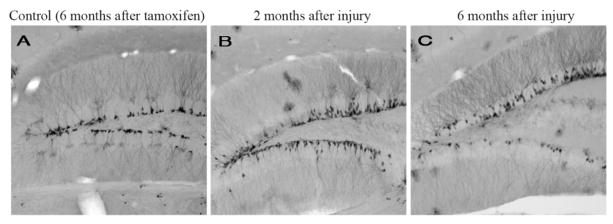
Using this kind of transgenic approach, a few issues have emerged regarding TBI-induced hippocampal neurogenesis. First, it is the early, nestin-expressing progenitors that are activated by the injury, whereas the later doublecortin-expressing committed neuroblasts appear especially vulnerable (Miles and Kernie, 2008; Yu et al., 2008b). Later, the doublecortin-expressing cells within the dentate reemerge and are the likely contributors to stable neurogeneis (Yu et al., 2008b). Finally, tamoxifen-inducible systems allow for the ultimate fate labeling of these activated progenitors, and it does appear that stable neurogenesis persists over time (Figure 3) (Lagace et al., 2007; Li et al., 2008).
Figure 3.
YFP-expressing cells in the dentate gyrus following tamoxifen-mediated cre recombination in controls and after injury. In control animals, YFP-expressing progenitors and neurons remain confined to the most basal layers of the dentate gyrus (A). In the injured dentate gyrus both 2 and 6 months after tamoxifen injection followed by injury, there are stably incorporated neurons throughout the granular layer that exhibit extensive dendritic arborizations (B, C).
Since it is reasonably well established that hippocampal progenitors are activated by injury and result in increased numbers of new neurons within the dentate gyrus, ongoing studies can now be directed at relevance and mechanism. First, it needs to be established whether injury-induced neurogenesis is an adaptive response. There are three possibilities for its ultimate relevance. First, the generation of new neurons might be beneficial and contribute to recovery of learning and memory and possibly other functions impaired by brain injury. Second, neurogenesis may contribute to TBI-related morbidity such as temporal lobe epilepsy, which occurs relatively commonly following moderate and severe TBI. Finally, this reservoir of progenitors may be nothing more than a developmental remnant that is incapable of providing functionally relevant neurons into the sophisticated hippocampal circuitry.
In order to test these various possibilities, a number of strategies have emerged. One is to ablate neurogenesis at the time of injury to determine whether this then impairs recovery. Strategies to do this include systemic or local administration of anti-mitotic agents such as Ara-C that are known to affect dividing neural progenitors (Doetsch et al., 1999; Lau et al., 2009). Another is to perform cranial irradiation directed to neurogenic zones such as the SVZ and dentate gyrus in order to more selectively impair neurogenesis (Hellstrom et al., 2008; Naylor et al., 2008). Problems with these approaches include both their lack of specificity and potentially toxic side effects that lead to unwanted immune activation that may affect other mediators of recovery or damage not related to neurogenesis.
Recently, genetically engineered mice have become available that can regulate neurogenesis in a more direct and temporally controlled manner. One strategy is to inducibly express diptheria toxin or its receptor in progenitor cells that can be ablated in a temporally controlled manner (Durieux et al., 2009; Luquet et al., 2005). One confounder with this approach is that all progenitors are ablated and not just dividing ones and therefore the pool becomes depleted and cannot be reactivated. An alternative approach that has been used in traumatic brain injury is a transgenic mouse that expresses the herpes simplex virus thymidine kinase (HSV-TK) under the control of the GFAP promoter. This allows for the inducible ablation of dividing cells that express GFAP, which includes early type 1 hippocampal progenitor cells as well as dividing reactive astrocytes (Morshead et al., 2003; Saxe et al., 2006; Sofroniew et al., 1999). Recently, a more specific modified version of HSV-TK has been shown in nestin-expressing progenitor cells to inducibly ablate early progenitors after TBI. These inducible approaches show promise as to determining the relevance of endogenous progenitor activation following injury (Yu et al., 2008b).
Whether endogenous TBI-induced neurogenesis proves to be adaptive or not, it also needs to be determined whether enhancing this process can improve outcome. Several studies have shown that a variety of pharmacologic agents associated with increasing neurogenesis lead to improved outcomes following TBI. These include exogenously administered or endogenously produced molecules such as estrogen, erythropoietin, and basic fibroblast growth factor (bFGF), as well as administration of drugs developed for other purposes such as statins and antidepressants (Encinas et al., 2006; Garcia-Segura et al., 2001; Lu et al., 2007; Sun et al., 2009; Wu et al., 2008; Xiong et al., 2008; Yoshimura et al., 2001). All of these molecules and drugs have effects on the brain that are independent of their promotion of hippocampal neurogenesis and it therefore becomes difficult to attribute improvements in behavior to their effects on neurogenesis in the dentate gyrus.
In order to demonstrate that functional recovery requires progenitor cell activation, more cell-specific genetic assays need to be used. These can include but are not limited to either selectively ablating tumor suppressor genes known to play roles in neurogenesis such as PTEN (phosphatase and tensin homolog deleted on chromosome 10) in the progenitor population at the time of injury, or activating known regulators of growth such as inducible overexpression of activated forms of the epidermal growth factor receptor (Gregorian et al., 2009; Holland et al., 1998). Although, these genetic models may not provide direct therapeutic targets, they will be able to answer the critical question of whether it is hippocampal neurogenesis itself that leads to improved outcomes.
Finally, although injury-induced neurogenesis occurs both in response to injury and selectively to cells that are adjacent to a more vulnerable population, the mechanisms underlying these observations need more mechanistic investigation. The injured brain releases numerous extracellular proteins and ions that may play roles in regulating neurogenesis. Two of the best studied of these, KCl and glutamate, have both been implicated in enhancement of proliferation in immature cells while at the same time directing toxicity in more mature cell types (Mattson, 2008; Shi et al., 2007). In addition, the injured brain activates both astroctyes and microglia, which are both known to secrete a variety of growth factors as well as immune modulators that may effect progenitor proliferation and survival (Bessis et al., 2007; Myer et al., 2006). Also, the progenitor cells themselves make physical contact with the vasculature so circulating factors such as cytokines and growth factors that increase after injury may also direct some of these effects (Mignone et al., 2004). Thus, the mechanisms underlying TBI-induced neurogenesis are likely not straightforward nor easily worked out, and therefore remain compelling targets to study.
Amid all the optimism surrounding the potential of injury-induced neurogenesis, there remain a variety of significant concerns. Post-traumatic epilepsy is a fairly common morbidity associated with both stroke and TBI (Diaz-Arrastia et al., 2009). One postulated mechanism for this is that aberrant neurogenesis serves as the epileptic focus (Parent and Murphy, 2008). Clearly, any strategy aimed at enhancing neurogenesis might result in this and other unwanted side effects. In addition, since all strategies aimed towards enhancing neurogenesis would increase cell growth, it remains a possibility that increasing proliferation could result in potentially unwanted tumor development. Therefore, although enhancing neurogenesis following stroke or TBI remains a compelling and potentially field-changing strategy towards improving recovery, many issues regarding specificity, mechanism, and potential toxicity need to be more thoroughly investigated before meaningful clinical interventions can occur.
References
- Allen E. The cessation of mitosis in the central nervous system of the albino rat. J Comp Neurol. 1912;22:547–568. [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, et al. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–93. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Anderson VA, et al. Recovery of memory function following traumatic brain injury in pre-school children. Brain Inj. 2000;14:679–92. doi: 10.1080/026990500413704. [DOI] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–6. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. NatMed. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Bessis A, et al. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–8. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- Carlen M, et al. Functional integration of adult-born neurons. Curr Biol. 2002;12:606–8. doi: 10.1016/s0960-9822(02)00771-6. [DOI] [PubMed] [Google Scholar]
- Carlen M, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–67. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- Carleton A, et al. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–18. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain Res. 2004;1005:21–8. doi: 10.1016/j.brainres.2003.11.080. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–75. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XH, et al. Neurogenesis and glial proliferation persist for at least one year in the subventricular zone following brain trauma in rats. Journal of Neurotrauma. 2003;20:623–32. doi: 10.1089/089771503322144545. [DOI] [PubMed] [Google Scholar]
- Chirumamilla S, et al. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J Neurotrauma. 2002;19:693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- Chou J, et al. Neuroregenerative effects of BMP7 after stroke in rats. J Neurol Sci. 2006;240:21–9. doi: 10.1016/j.jns.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–3. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corotto FS, et al. Neurogenesis persists in the subependymal layer of the adult mouse brain. Neurosci Lett. 1993;149:111–4. doi: 10.1016/0304-3940(93)90748-a. [DOI] [PubMed] [Google Scholar]
- Curtis MA, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–9. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Dash PK, et al. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–9. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Demeurisse G. Mechanisms of functional restoration after brain injury. Acta Neurol Belg. 2000;100:77–83. [PubMed] [Google Scholar]
- Diaz-Arrastia R, et al. Posttraumatic epilepsy: the endophenotypes of a human model of epileptogenesis. Epilepsia. 2009;50(Suppl 2):14–20. doi: 10.1111/j.1528-1167.2008.02006.x. [DOI] [PubMed] [Google Scholar]
- Doetsch F, et al. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96:11619–24. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, et al. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18:108–15. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux PF, et al. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–5. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- Encinas JM, et al. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4:229–37. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998a;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998b;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, et al. Early brain injury in children: development and reorganization of cognitive function. Dev Neuropsychol. 2003;24:669–704. doi: 10.1080/87565641.2003.9651915. [DOI] [PubMed] [Google Scholar]
- Farmer J, et al. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Faulkner RL, et al. Development of hippocampal mossy fiber synaptic outputs by new neurons in the adult brain. Proc Natl Acad Sci U S A. 2008;105:14157–62. doi: 10.1073/pnas.0806658105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, et al. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Garcia AD, et al. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–41. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Gheusi G, et al. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci U S A. 2000;97:1823–8. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Gross CG. Neurogenesis in adult mammals: some progress and problems. J Neurosci. 2002;22:619–23. doi: 10.1523/JNEUROSCI.22-03-00619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorian C, et al. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci. 2009;29:1874–86. doi: 10.1523/JNEUROSCI.3095-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom NA, et al. Differential recovery of neural stem cells in the subventricular zone and dentate gyrus after ionizing radiation. Stem Cells. 2008 doi: 10.1634/stemcells.2008-0732. [DOI] [PubMed] [Google Scholar]
- Hoehn BD, et al. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–24. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- Holland EC, et al. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–85. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Iosif RE, et al. Suppression of stroke-induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J Cereb Blood Flow Metab. 2008;28:1574–87. doi: 10.1038/jcbfm.2008.47. [DOI] [PubMed] [Google Scholar]
- Jin K, et al. Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Invest. 2002a;110:311–319. doi: 10.1172/JCI15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, et al. Heparin-binding epidermal growth factor-like growth factor: hypoxia-inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J Neurosci. 2002b;22:5365–5373. doi: 10.1523/JNEUROSCI.22-13-05365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, et al. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–89. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Kawaguchi A, et al. Nestin-EGFP transgenic mice: visualization of the self-renewal and multipotency of CNS stem cells. Mol Cell Neurosci. 2001;17:259–73. doi: 10.1006/mcne.2000.0925. [DOI] [PubMed] [Google Scholar]
- Kernie SG, et al. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res. 2001a;66:317–26. doi: 10.1002/jnr.10013. [DOI] [PubMed] [Google Scholar]
- Kernie SG, et al. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res. 2001b;66:317–26. doi: 10.1002/jnr.10013. [DOI] [PubMed] [Google Scholar]
- Kolb B, et al. Growth factor-stimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats. J Cereb Blood Flow Metab. 2007;27:983–97. doi: 10.1038/sj.jcbfm.9600402. [DOI] [PubMed] [Google Scholar]
- Lagace DC, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–9. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BW, et al. Intracerebroventricular infusion of cytosine-arabinoside causes prepulse inhibition disruption. Neuroreport. 2009;20:371–7. doi: 10.1097/WNR.0b013e328324edcd. [DOI] [PubMed] [Google Scholar]
- Lee SR, et al. Involvement of matrix metalloproteinase in neuroblast cell migration from the subventricular zone after stroke. J Neurosci. 2006;26:3491–5. doi: 10.1523/JNEUROSCI.4085-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leker RR, et al. Long-lasting regeneration after ischemia in the cerebral cortex. Stroke. 2007;38:153–61. doi: 10.1161/01.STR.0000252156.65953.a9. [DOI] [PubMed] [Google Scholar]
- Lendahl U, et al. CNS stem cells express a new class ofintermediate filament protein. Cell. 1990;60:585–95. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RC, et al. Re-expression of the intermediate filament nestin in reactive astrocytes. Neurobiol Dis. 1995;2:79–85. doi: 10.1006/nbdi.1995.0008. [DOI] [PubMed] [Google Scholar]
- Liu F, et al. Brain injury does not alter the intrinsic differentiation potential of adult neuroblasts. J Neurosci. 2009a;29:5075–87. doi: 10.1523/JNEUROSCI.0201-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, et al. Angiopoietin 2 mediates the differentiation and migration of neural progenitor cells in the subventricular zone after stroke. J Biol Chem. 2009b;284:22680–9. doi: 10.1074/jbc.M109.006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Y, et al. Sensory input enhances synaptogenesis of adult-born neurons. J Neurosci. 2009;29:86–97. doi: 10.1523/JNEUROSCI.4105-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–8. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lois C, et al. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- Lu D, et al. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24:1132–46. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, et al. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–5. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, et al. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–32. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignone JL, et al. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–24. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Miles DK, Kernie SG. Hypoxic-ischemic brain injury activates early hippocampal stem/progenitor cells to replace vulnerable neuroblasts. Hippocampus. 2008;18:793–806. doi: 10.1002/hipo.20439. [DOI] [PubMed] [Google Scholar]
- Morshead CM, et al. The ablation of glial fibrillary acidic protein-positive cells from the adult central nervous system results in the loss of forebrain neural stem cells but not retinal stem cells. Eur J Neurosci. 2003;18:76–84. doi: 10.1046/j.1460-9568.2003.02727.x. [DOI] [PubMed] [Google Scholar]
- Myer DJ, et al. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–72. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Nakatomi H, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–41. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Naylor AS, et al. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl AcadSci U S A. 2008;105:14632–7. doi: 10.1073/pnas.0711128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohab JJ, et al. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–16. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, et al. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Parent JM, Murphy GG. Mechanisms and functional significance of aberrant seizure-induced hippocampal neurogenesis. Epilepsia. 2008;49(Suppl 5):19–25. doi: 10.1111/j.1528-1167.2008.01634.x. [DOI] [PubMed] [Google Scholar]
- Parent JM, et al. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–13. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Parent JM, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–38. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106–13. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plane JM, et al. Retinoic acid and environmental enrichment alter subventricular zone and striatal neurogenesis after stroke. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozniak CD, Pleasure SJ. Genetic control of hippocampal neurogenesis. Genome Biol. 2006;7:207. doi: 10.1186/gb-2006-7-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, et al. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053:38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Revest JM, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Richardson RM, et al. Neurogenesis after traumatic brain injury. Neurosurg Clin N Am. 2007;18:169–81, xi. doi: 10.1016/j.nec.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Ridet JL, et al. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–7. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Robin AM, et al. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–34. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- Rochefort C, et al. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–89. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–93. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, et al. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–60. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, et al. Injury-induced neurogenesis in Bax-deficient mice: evidence for regulation by voltage-gated potassium channels. Eur J Neurosci. 2007;25:3499–512. doi: 10.1111/j.1460-9568.2007.05624.x. [DOI] [PubMed] [Google Scholar]
- Sims JR, et al. Sonic Hedgehog Regulates Ischemia/Hypoxia-Induced Neural Progenitor Proliferation. Stroke. 2009 doi: 10.1161/STROKEAHA.109.561951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BH, et al. Conditional ablation and recovery of forebrain neurogenesis in the mouse. J Comp Neurol. 2009;514:567–82. doi: 10.1002/cne.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, et al. Genetically-targeted and conditionally-regulated ablation of astroglial cells in the central, enteric and peripheral nervous systemsin adult transgenic mice. Brain Res. 1999;835:91–5. doi: 10.1016/s0006-8993(99)01639-x. [DOI] [PubMed] [Google Scholar]
- Song H, et al. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Sun D, et al. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp Neurol. 2009;216:56–65. doi: 10.1016/j.expneurol.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–51. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple S, Qian X. bFGF, neurotrophins, and the control or cortical neurogenesis. Neuron. 1995;15:249–52. doi: 10.1016/0896-6273(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Teramoto T, et al. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. Journal of Clinical Investigation. 2003;111:1125–32. doi: 10.1172/JCI17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–47. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Thored P, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–49. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- Thored P, et al. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–9. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- Toni N, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–7. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, et al. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–74. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrea C, et al. Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor Neurol Neurosci. 2007;25:65–76. [PubMed] [Google Scholar]
- van Praag H, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–4. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–7. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wang S, et al. Electrophysiological correlates of neural plasticity compensating for ischemia-induced damage in the hippocampus. Exp Brain Res. 2005 doi: 10.1007/s00221-005-2296-8. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. Involvement of Notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, et al. Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur J Neurosci. 2002;16:1681–9. doi: 10.1046/j.1460-9568.2002.02238.x. [DOI] [PubMed] [Google Scholar]
- Wu H, et al. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma. 2008;25:130–9. doi: 10.1089/neu.2007.0369. [DOI] [PubMed] [Google Scholar]
- Xiong Y, et al. Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J Neurosurg. 2008;109:510–21. doi: 10.3171/JNS/2008/109/9/0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–36. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YP, et al. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1213–24. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- Yan YP, et al. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci. 2006;24:45–54. doi: 10.1111/j.1460-9568.2006.04872.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, et al. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A. 2001;98:5874–9. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T-S, et al. Temporally regulated expression of Cre recombinase in neural stem cells. Genesis. 2005;41:147–53. doi: 10.1002/gene.20110. [DOI] [PubMed] [Google Scholar]
- Yu TS, et al. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J Neurosci. 2008a;28:12901–12. doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, et al. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J Neurosci. 2008b doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, et al. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zimmerman L, et al. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]