Abstract
Objective
The purpose was to compare four strategies for stimulus presentation in terms of their efficiency when generating a speech-evoked cortical acoustic change complex (ACC) in adults and children.
Design
Ten normally hearing adults (ages 22 to 31 years) and nine normally hearing children (ages six to nine years) served as participants. The ACC was elicited using a 75 dB SPL synthetic vowel containing 1000 Hz changes of second formant frequency, creating a change of perceived vowel between /u/ and /i/. The ACC was recorded from Cz using four stimulus formats:
interrupted presentation of a one-second stimulus containing a single change from /u/ to /i/ using a two-second inter-onset interval.
interrupted presentation of the same stimulus using a one-second inter-onset interval.
interrupted presentation of a 1.5-second stimulus containing a change from /u/ to /i/ followed by a reverse change from /i/ to /u/, using a two-second inter-onset interval.
continuous presentation of a stimulus alternating between /u/ and /i/ using a one-second repetition interval.
ACC magnitude was expressed as the standard devation of the voltage waveform within a window believed to span the ACC. Noise magnitude was estimated from the variances at each sampling point in the same window. Efficiency was expressed in terms of the ACC-to-noise magnitude ratio divided by testing time.
Results
ACC magnitude was not significantly different for the two directions of second formant change. Reducing inter-onset interval from two seconds to one second increased efficiency by a factor close to two. Combining data from the two directions of change increased efficiency further, by a factor approximating the square root of two.
Conclusion
Continuous alternating stimulus presentation is more efficient than interrupted stimulus presentation in eliciting the ACC. The benefits of eliminating silent periods and doubling the number of acoustic changes presented in a given time period, are not seriously offset by a reduction in rms response amplitude, at least in young adults and in children as young as 6 years old.
Keywords: Acoustic change complex, ACC, P1-N1-P2, cortical, auditory, SNR, efficiency
Introduction
The Acoustic Change Complex (ACC) is a cortical auditory evoked potential (P1-N1-P2) elicited by a change within an ongoing sound stimulus (Martin and Boothroyd, 1999). The ACC has been obtained in response to intensity, frequency, and phase modulations in sustained tones (e.g., Arlinger, Elberling, Bak, Kofoed, Lebech and Saermark, 1982; Clynes, 1969; Dimitrijevic, Michalewski, Zeng, Pratt, and Starr, 2008; Harris, Mills and Dubno, 2007; Jerger & Jerger, 1970; Lenhardt, 1971; Naatanen & Picton, 1987; Ross, Tremblay and Picton, 2007; Ruhm, 1970; Ruhm and Jansen, 1969; Spoor, Timmer, & Odenthal, 1969; Yingling & Nethercut, 1983). It has also been obtained in response to spectral and intensity changes within speech or speech-like stimuli (Hari, 1991; Imaizumi, Mori, Kiritani, & Yumoto, 1996; Kaukoranta, Hari, & Lounasmaa, 1987; Ostroff, Martin, & Boothroyd, 1998; Martin and Boothroyd, 1999; 2000; Tremblay, Friesen, Martin and Wright, 2003). The ACC indicates the encoding of potentially discriminable information at the level of the auditory cortex (Martin and Boothroyd, 1999; 2000; Ostroff, Martin and Boothroyd, 1998; Ostroff, reference note 1).
There are several findings that suggest that the ACC may be a potentially useful measure for the clinical assessment of speech perception capacity. First, the ACC shows good agreement with behavioral measures of intensity discrimination (∼3 dB) (Martin and Boothroyd, 2000), frequency discrimination (∼10 Hz) (Martin, 2007; Ostroff, reference note 1), and the upper frequency limit for the detection of 180 degree phase shifts in an amplitude modulated tone (∼50 Hz) (Ross, Tremblay and Picton, 2007). Second, the ACC shows excellent test-retest reliability at the individual participant level in adults (Tremblay, Friesen, Martin and Wright, 2003). Ongoing research in our laboratory extends this conclusion to children. Third, the ACC can be elicited in individuals with sensorineural hearing loss with and without hearing aids and cochlear implants (Billings, Tremblay, Souza and Binns, 2007; Brown, Etler, He, O’Brien, Erenberg, Kim, Dhuldhoya, and Abbas, 2008; Friesen and Tremblay, 2006; Jerger and Jerger, 1970; Martin, 2007; Martin, Tremblay and Stapells, 2007; Tremblay, Billings, Friesen and Souza, 2006).
The ACC is likely a simple change detection response (Hillyard and Picton, 1978; Picton, Alain, Otten, Ritter, and Achim, 2000) that results from the activation of new neural elements together with the deactivation of others (Martin and Boothroyd, 1999; 2000). The factors that influence the elicitation of the onset P1-N1-P2 complex appear to similarly influence the ACC P1-N1-P2 complex. Several excellent reviews of the P1-N1-P2 complex have been published which discuss component generators as well as participant, stimulus, and response factors influencing its elicitation (e.g., Crowley and Colrain, 2004; Hyde, 1997; Martin, Tremblay and Stapells, 2007; Picton, Alain, Woods, John, Scherg, Valdes-Sosa, Bosch-Bayard, and Trujillo, 1999; Stapells, 2002).
It should be noted that ACC differs from mismatch negativity (MMN), which is another evoked potential that indexes the neural processing of acoustic change (for review, see Kujala, Tervaniemi and Schroger, 2007; Naatanen 1990; 1992; 2003; Naatanen, Gaillard, and Mantysalo, 1978; Picton, Alain, Otten, Ritter and Achim, 2000). For ACC, the response of interest is elicited by an acoustic change within a sound stimulus. For MMN, the response is elicited by an acoustic difference between different stimuli or stimulus patterns. While not typical, it is possible to elicit both responses using a single paradigm. For example, one study used a continuous tonal stimulus that contained an occasional frequency increment (which served as the deviant). Both the N1 component of the ACC as well as the mismatch negativity were elicited by this paradigm (Lavikainen, Huotilainen, Ilmoniemi, Simola, and Naatanen, 1995). Most studies of ACC have not elicited MMN, because the acoustic change within the stimulus occurred with high probability (that is, there was no deviant). An important advantage of the ACC paradigm over MMN is that every trial contributes to the response (Martin and Boothroyd, 1999; 2000), and this has important implications for the signal-to-noise ratio of the averaged waveforms as well as the time needed to establish an identifiable response.
Clinical behavioral tests of speech perception capacity are often inappropriate for infants, young children and young children with hearing loss (Boothroyd, 1991; 2005; Tyler, 1993). With the mandating of universal newborn hearing screening, children are being identified with hearing loss at younger ages. As a result, there is a growing need for objective tests of suprathreshold function to inform decisions about sensory and habilitative management. A practical electrophysiologic test of auditory speech discrimination capacity could help meet this need.
Before the ACC can be used clinically with young children, however, there are two important considerations. First, the P1-N1-P2 complex elicited by sound onset (a change from silence to sound) shows significant changes in morphology with maturation (e.g., Kraus, McGee, Carrell, Sharma, Micco, and Nicol, 1993; Morr, Shafer, Kreuzer, and Kurtzberg, 2002; Ponton, Don, Eggermont, and Kwong, 1997; Ponton, Eggermont, Khosla, Kwong and Don, 2002; Ponton, Eggermont, Kwong, and Don, 2000). These changes are dependent on stimulus rate (Ceponiene, Rinne and Naatanen, 2002; Gilley, Sharma, Dorman, and Martin, 2005; Gomes, Dunn, Ritter, Kurtzberg, Brattson, Kreuzer and Vaughan, 2001). After infancy, children show a large, relatively late P1, followed by a broad, slow negativity (N2) (Ponton et al., 2000; Sharma, Kraus, McGee et al., 1997). The P1 occurs at roughly 100 ms after stimulus onset in children, whereas in adults it occurs with a latency of approximately 50 ms. P1-N2 waveform pattern in childhood shows large changes with maturation and the largest change is the emergence of N1. In part, because of the increased neural refractoriness in children, the N1 component is only observed when stimuli are presented at very slow rates. Inter-onset intervals needed to elicit N1 are around 800 ms for 7 to 9 year olds and can be as high as 3 or 4 seconds for younger children (Ceponiene et al., 2002; Gilley et al., 2005; Gomes et al., 2001; Gomes, Sussman, Ritter, Kurtzberg, Cowan and Vaughan, 1999; Wunderlich, Cone-Wesson, and Shepherd, 2006). It is unclear to what extent refractoriness will be an issue for the ACC since the acoustic change of interest typically occurs soon after stimulus onset.
A second consideration important for the clinical application of the ACC is that young children, and even many adults, cannot participate for long test sessions. There is, therefore, need maximize efficiency defined, for present purposes as the ACC-to-noise amplitude ratio divided by the testing time. Efficiency is the focus of the present study. To date, the ACC has been implemented using stimuli containing a single change with fairly high inter-onset interval and a period of silence between stimuli. The silent period could potentially be eliminated. The result would be a continuous alternating stimulus. In addition to reducing overall testing time, the presence of two changes in the alternating stimulus would double the opportunity to elicit the ACC within each repetition cycle. The potential drawback of continuous alternating presentation, however, is that neurons responsible for generating the ACC may become refractory resulting in small response amplitudes (Naatanen and Picton, 1987), perhaps offsetting the benefits of reducing test time and doubling changes.
While cortical auditory evoked potentials have been obtained in response to interrupted (Martin and Boothroyd, 1999) and continuous alternating stimuli in the past (Arlinger, Jerlvall, Ahren, and Holmgren, 1976; Kohn, Lifshitz, and Litchfield, 1978; 1980; Spoor, Timer, and Odenthal, 1969), to our knowledge, there has been no direct comparison of these presentation strategies in terms of efficiency, no comparison using speech stimuli, and no comparison of the methods in children.
The purpose of the present study was to compare the efficiencies of several strategies of stimulus presentation when eliciting the ACC in adults and children.
Methods
Participants
Event related potentials (ERPs) were recorded from ten adults (five men, five women, ages 22 to 31 years. Mean = 25, s.d. = 2.6) and nine children (four boys, five girls, 6 to 9 years. Mean = 8, s.d. = 1.3). Data from a tenth child were eliminated as this participant was subsequently diagnosed with attention deficit disorder. All participants had normal hearing sensitivity from 250 to 8000 Hz bilaterally (ANSI, 1996) along with normal middle ear function (type A tympanograms and present acoustic reflexes at 1000 Hz bilaterally). Participants also had no history of neurological or learning problems. Participation was by informed consent for the adults and parental consent along with child assent for the children.
Stimulus
The basic stimulus was a synthetic vowel that contained 1000 Hz changes of second formant frequency. The stimulus was synthesized using a Klatt synthesizer and the following parameters: Fo = 100 Hz, F1 = 400 Hz, F2 = 1000 or 2000 Hz; F3 = 3000 Hz, and F4 = 4000 Hz. The transition between the lower and upper values of F2 occupied 40 msec. Perceptually, the change was from /u/ to /i/ or from /i/ to /u/.
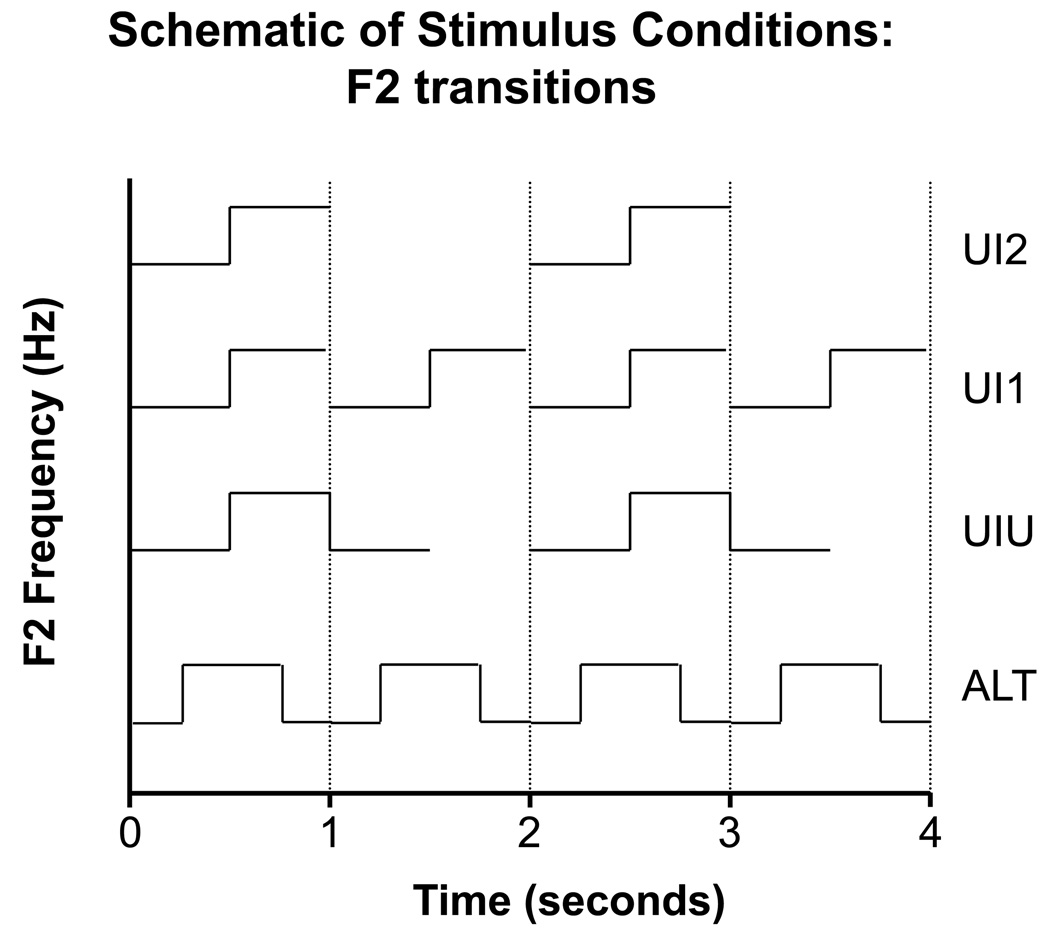
There were 4 different stimulus presentation strategies:
UI2. The stimulus lasted 1 second and had a change from /u/ to /i/ at midpoint. Inter-onset interval was two seconds, giving a one second silent period between successive stimuli. This was the stimulus strategy used in much of the previous work by the authors.
UI1. This stimulus strategy used the same stimulus but with an inter-onset interval of one second. The offset of /i/ was trimmed slightly to give a five ms silent period between successive stimuli. This strategy cut testing time in half but retained only one change per stimulus cycle.
UIU. This stimulus strategy used a 1500 ms duration stimulus containing a change from /u/ to /i/ at 500 ms and a return to /u/ at 1000 ms. Inter-onset interval was 2 seconds, giving a silent period of 500 ms between successive stimuli. This strategy doubled the number of changes within a stimulus but did not reduce testing time.
ALT. The stimulus alternated between 500 ms of /u/ and 500 ms of /i/. This stimulus strategy eliminated the silent periods and provided two changes within the one-second repetition cycle.
A schematic of the four stimulus strategies is shown in Figure 1.
Figure 1.
A schematic of second formant frequency (F2) in each strategy is shown as a function of time (in seconds). The vertical axis indicates the change in F2 frequency from 1000 to 2000 Hz.
The four stimulus strategies were presented in separate blocks and the order was randomized across participants. Each strategy involved 500 trials presented in separate blocks. Stimuli were calibrated to 75 dB SPL and presented using Adobe Audition software via a loudspeaker placed 1 meter in front of participants at a 0 degree azimuth.
Procedure
Participants were tested in a sound treated and electrically shielded booth. They were seated in a recliner and watched a captioned video of their choice with the soundtrack turned off. Participants were instructed to ignore the stimuli presented to them and to remain as quiet and still as possible. Breaks were provided as needed.
Evoked Potential Recordings
All evoked potentials for this study were recorded using the Neuroscan SCAN system and 32 channel SynAmp amplifier. Electrodes were placed according to the International 10–20 system (Jasper, 1958) using an electrode cap. An electrode on the nose served as a reference while an electrode between Fz and FCz served as ground. Vertical eye movements and eyeblinks were monitored via electrodes immediately above and below the right eye. All impedances were maintained below 5000 ohms.
Data Processing
During data acquisition, the continuous electroencephalogram (EEG) was digitized (sampling rate = 1000 Hz), amplified (gain = 1000), and band-pass filtered (0.15 to 100 Hz). An eyeblink reduction algorithm was applied to the continuous EEG (Semlitch, Anderer, Schuster, and Presslich, 1986). Epochs of 1600 ms, which included a 100 ms prestimulus period, were extracted from the continuous EEG. Epochs in which voltage changes exceeded 100 µV were rejected from further analysis. Each epoch was baseline corrected and then data were averaged within subject group and strategy.
Data Analyses
ACC amplitude
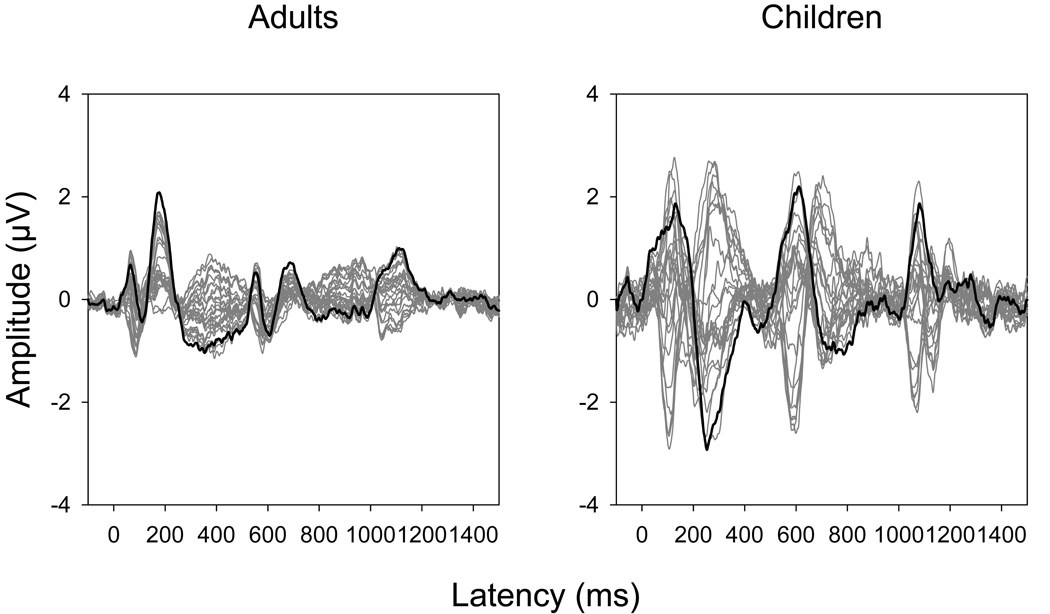
All amplitude measures were taken from Cz using response latency windows spanning the ACC of interest. The ACC is typically maximal near the vertex (at or lateral to Cz and FCz). Butterfly plots of the waveforms obtained at each electrode site in the UI2 condition are shown in Figure 2 for adults and children, with the response from Cz shown in black. The response windows were determined using the grand mean waveforms. The windows for the change from /u/ to /i/ were from 500 to 750 ms relative to stimulus onset for all stimulus strategies with the exception of the ALT strategy in which the window was from 250 to 500 ms relative to the sampling trigger which was placed at the midpoint of the steady state portion of /u/ since there was no true “onset”). The change from /i/ to /u/ occurred only in the UIU and ALT stimulus strategies. The response window for this change was from 1000 to 1250 ms relative to stimulus onset for the UIU and from 750 to1000 ms relative to the trigger for the ALT strategy. The duration of these latency windows was sufficient for use with both subject groups.
Figure 2.
Grand mean waveforms at each electrode site in the UI2 condition are overlaid for adults and children. The waveforms obtained at electrode site Cz are shown in black.
The magnitude of the ACC was expressed as the root mean squared (rms) ACC amplitude within the appropriate response latency window. The rms rather than peak amplitude was used for two reasons. The first was to facilitate comparison of the data from adults and children, who have different morphology. The second was to avoid the subjectivity involved in identifying and labeling components within the ACC for individual participants. The rms amplitude has the advantage of indexing overall response magnitude without making assumptions about morphology1.
Noise
Noise in the averaged waveform was defined as the square root of the mean of the variances at each point in the averaged waveform within the response window divided by the square root of the number of accepted sweeps. This metric is described in more detail in the Appendix. Ideally noise would be measured in a portion of the waveform without an evoked potential response (such as the pre-stimulus interval). In all strategies except UI2 there was no window that could be guaranteed to be response-free.
ACC-to-noise ratio
The ACC-to-noise ratio was determined by dividing the rms ACC amplitude by the noise amplitude.
Efficiency
As indicated earlier, efficiency was defined for present purposes as the ACC-to-Noise ratio divided by testing time (Hyde and Blair, 1981; Picton et al., 1977; 1983; 1984). The latter was the inter-onset interval multiplied by the number of sweeps, in this case 500. Testing time for the UI2 and UIU strategies was 17 minutes. Testing time for the UI1 and ALT strategies was 8 minutes.
Statistical Analyses
Separate repeated measures analyses of variance were completed on the ACC magnitude, noise magnitude, ACC-to-noise ratio, and efficiency data. In each case, age (adults vs. children) was the grouping variable and stimulus strategy was the within-participant variable.
Results
Grand Mean Waveforms
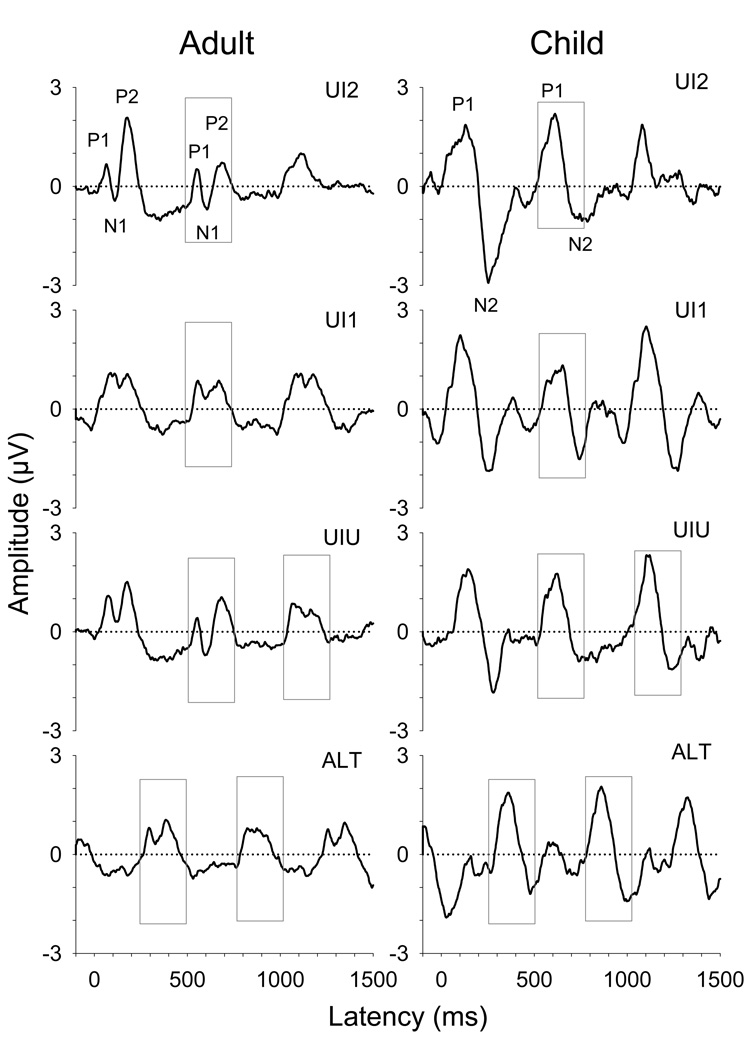
Grand mean waveforms for the adults and children are shown for each stimulus strategy in Figure 3. For UI2, the adults show a P1-N1-P2 complex occurring approximately 50–200 ms after stimulus onset, followed by the ACC at approximately 550–700 ms relative to stimulus onset (shown in the inset box). A small response to stimulus offset is also present at 1000–1200 ms after stimulus onset. In contrast, children show the expected P1-N2 response typically seen in this age group in response to stimulus onset (100–250 ms), followed by the ACC P1-N2 response to the acoustic change (550–750 ms, shown in the inset box), which in turn is followed by a response to stimulus offset. As would be expected, the amplitude of the ACC is larger for the children than for the adults (Gomes et al., 2001; Ponton et al., 2000; Sharma et al., 1997; Wunderlich and Cone-Wesson, 2006).
Figure 3.
Grand mean waveforms for the adults (n = 10) and children (n = 9) are displayed as a function of stimulus strategy. The acoustic change complex is indicated by the gray inset boxes.
The waveform pattern obtained in the UI1 strategy shows less distinct components, particularly for the adults, compared to the UI2 strategy. There is overlap between the response to stimulus offset and stimulus onset and as a result, the third set of deflections, are a combination of offset and onset responses (stimulus onset occurs at 0 ms and again at 1000 ms).
In the UIU strategy, the response to stimulus onset (50–200 ms) is followed by a second response to the acoustic change from /u/ to /i/ (550–700 ms) and a third response to the acoustic change from /i/ to /u/ (1000–1200 ms).
In the ALT strategy, there is a response to the acoustic change from /u/ to /i/, followed by a response to the acoustic change from /i/ to /u/. Temporal overlapping of components is present. The third response is identical to the first because there is no offset response. That is, the third deflection of one epoch is the first deflection in the next epoch.
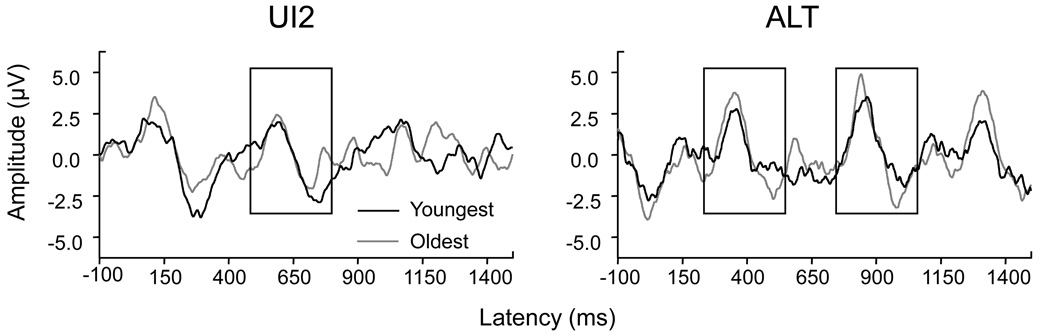
Children
The waveforms for the youngest child and the oldest child in the study are superimposed in Figure 4 in the UI2 and ALT conditions. While subtle maturation of the ACC is apparent, it is also apparent that the ACC shows only small changes in the timing of the response across the 6 to 9 year old age range.
Figure 4.
The waveforms for the youngest and oldest children participating are overlaid for the UI2 and ALT conditions.
Analyses
Direction of Acoustic Change
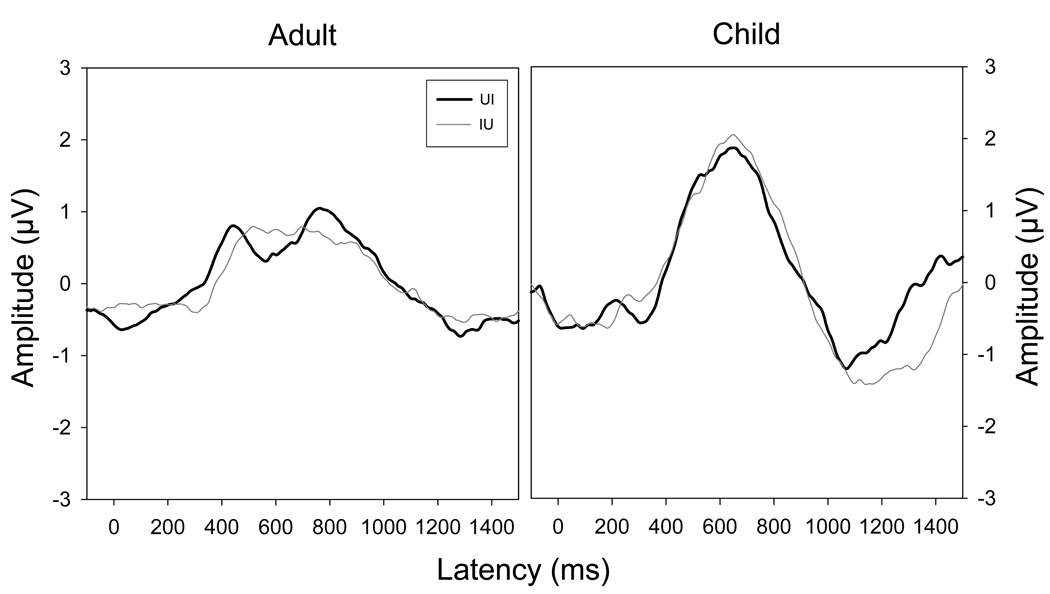
Stimuli in the ALT and UIU strategies contained two directions of acoustic change--/ui/ and /iu/. Figure 5 shows the waveforms obtained for the two directions of change overlaid for the ALT strategy. Despite small differences in peak amplitude, RMS amplitudes appear quite similar for the two acoustic changes. N1 is smaller in the adults for the second direction of acoustic change. The UIU condition showed a similar pattern of results (see Figure 3). Figure 6 shows a plot comparing magnitude of the ACC complex as a function of direction of change for the ALT and UIU strategies. The effects of neither strategy nor direction reached the 0.05 level of significance (Table 1), justifying the decision to collapse the data across the two directions of acoustic change in subsequent sections of this paper, thus reducing noise amplitude by the square root of two.
Figure 5.
Grand mean waveforms for the two directions of acoustic change (/ui/ and /iu/) are overlaid for the two age groups.
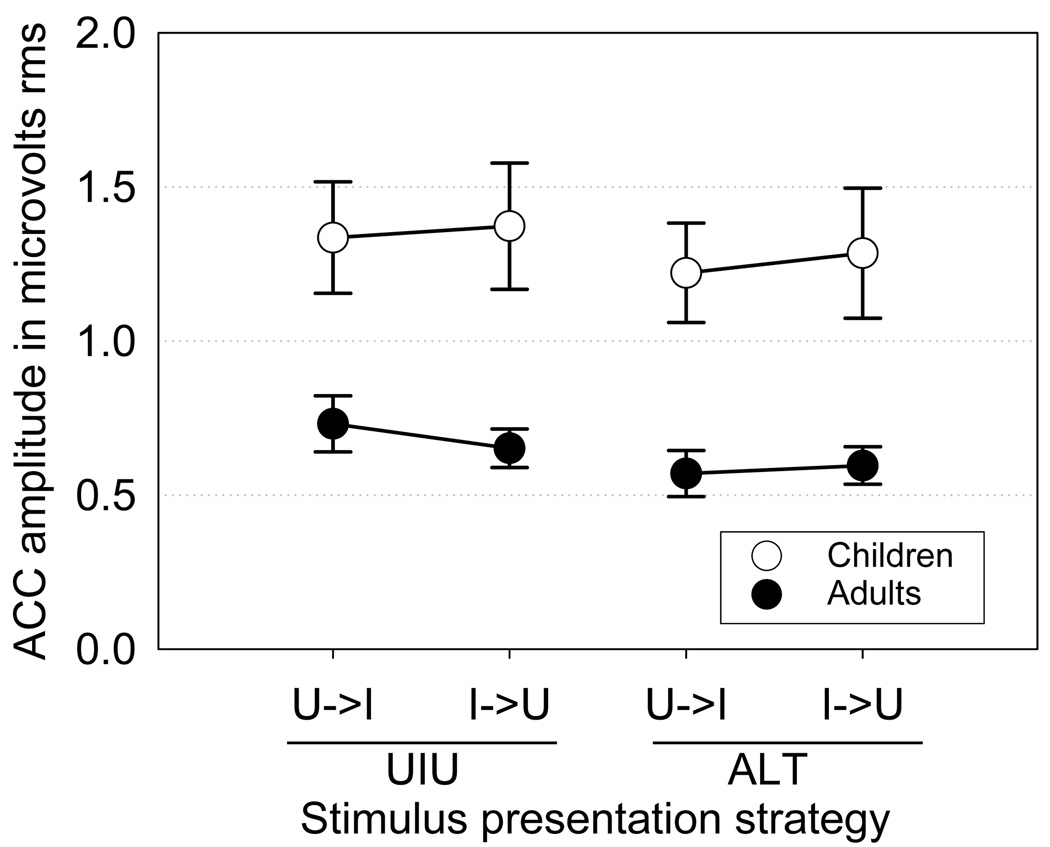
Figure 6.
Magnitude of the ACC complex as a function of change direction for the two stimulus strategies involving both directions of change between /u/ and /i/ (UIU and ALT). The effects of neither strategy nor direction reached the 0.05 level of significance.
Table 1.
A repeated measures analysis of variance in the direction of change data.
| Direction | ||||||
|---|---|---|---|---|---|---|
| Source Of Variance |
Sum Of Squares |
Degrees Of Freedom |
Estimated Mean Square |
F ratio |
p- level |
|
| Group | 8.40478 | 1 | 8.40477728 | 15.06 | 0.001 | ** |
| within G | 9.49049 | 17 | 0.5582642 | |||
| Stimulus | 0.20851 | 1 | 0.20850683 | 2.27 | 0.150 | |
| S×G | 0.00030 | 1 | 0.0002994 | 0.00 | 0.955 | |
| S×G×wG | 1.56091 | 17 | 0.09181815 | |||
| D | 0.00260 | 1 | 0.00259696 | 0.05 | 0.824 | |
| D×G | 0.02854 | 1 | 0.02853775 | 0.56 | 0.463 | |
| D×G×wG | 0.86078 | 17 | 0.05063411 | |||
| S×D | 0.02034 | 1 | 0.02034425 | 0.73 | 0.405 | |
| S×D×G | 0.00718 | 1 | 0.00717825 | 0.26 | 0.619 | |
| S×DswG | 0.47467 | 17 | 0.02792156 | |||
Amplitude
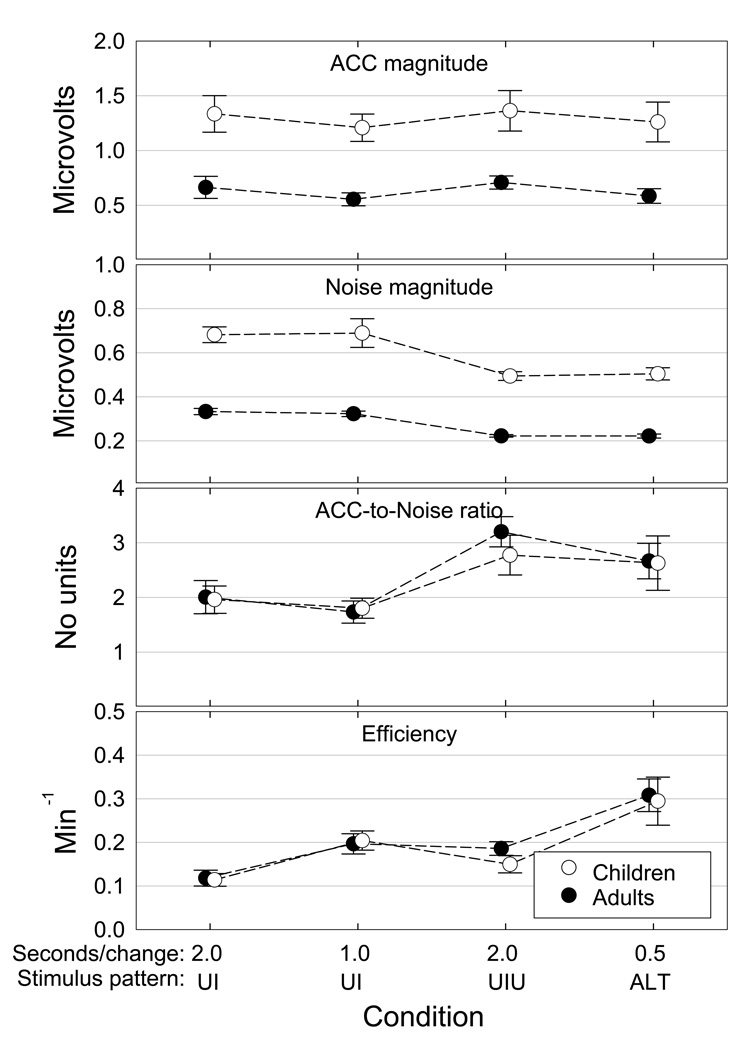
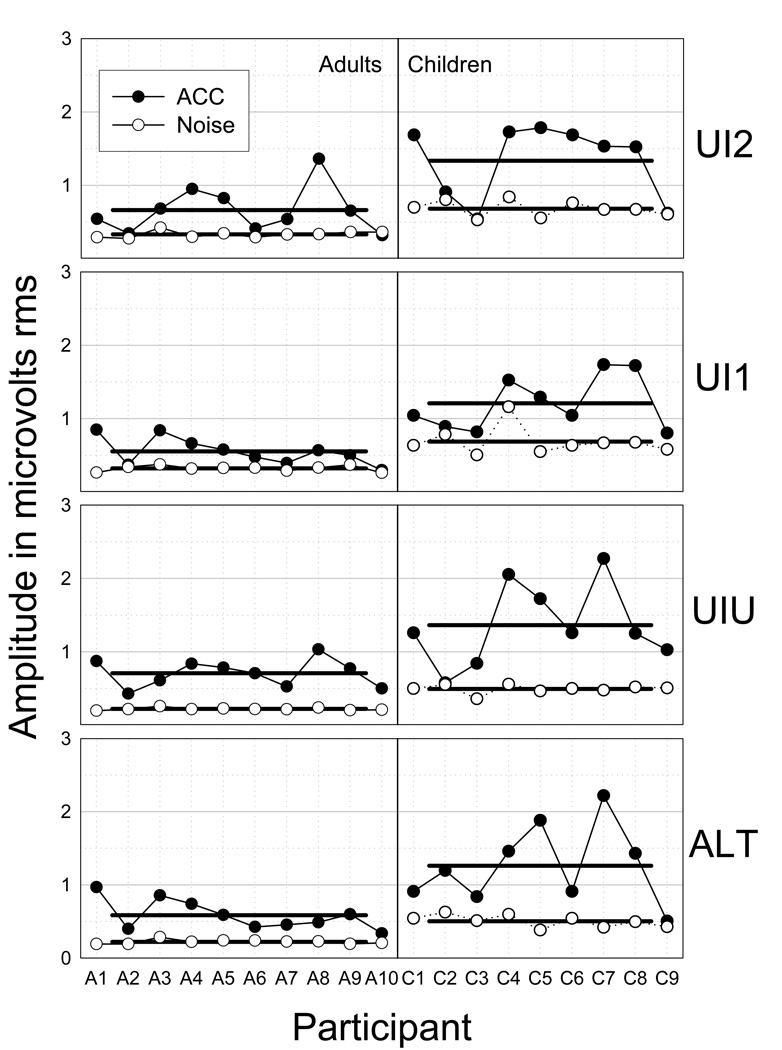
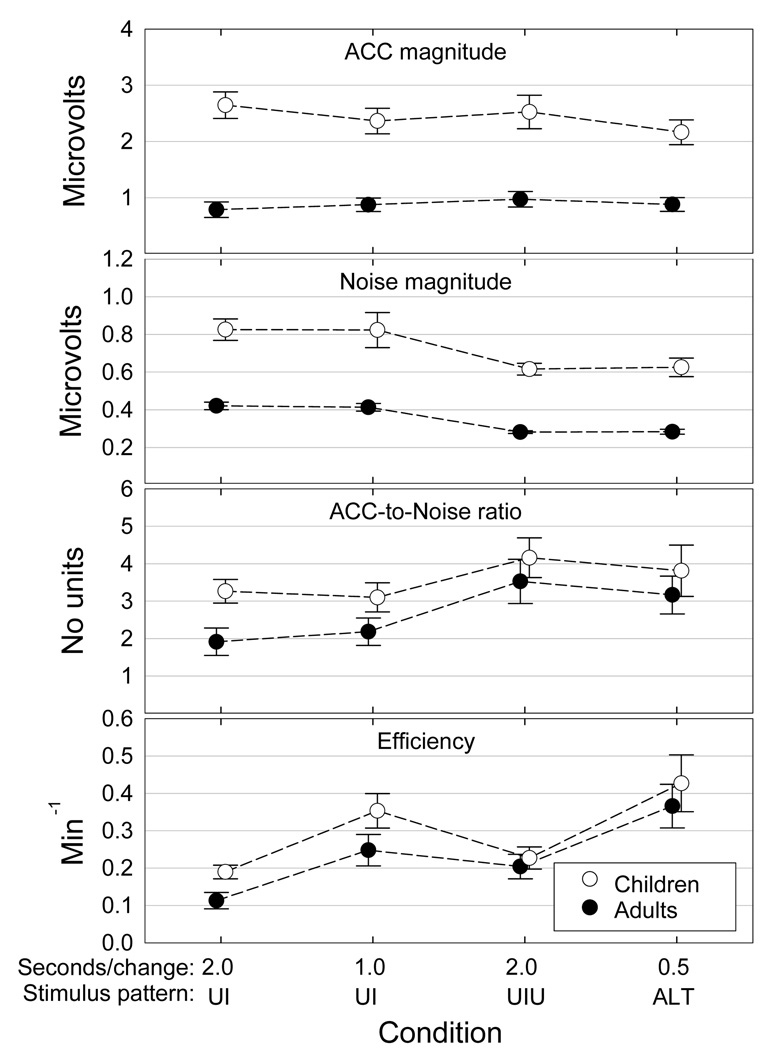
Grand mean ACC magnitude data for each stimulus strategy are shown in Figure 7 (top) along with corresponding standard deviations for the adults and children. Repeated measures ANOVAs were used to compare ACC magnitudes obtained in each stimulus strategy as a function of group. Data from the 10th adult participant were eliminated for this and for subsequent adult-child comparisons so that the two groups would have an equal number of participants. The effect of participant group (adult vs. child) was highly significant [F(1,17) = 19.54; p < 0.001]. As expected, ACC magnitude was significantly larger for children than for adults. There were, however, no interactions between group and stimulus strategy [F(3,51) = 0.01; p = 0.998]. The individual participant’s amplitude and noise data for the four stimulus strategies are shown in Figure 8. Individual differences are apparent in response magnitude, ACC-to-noise ratio, and the effects of the different stimulus strategies.
Figure 7.
ACC magnitude, noise magnitude, ACC-to-noise ratio, and testing efficiency as functions of stimulus strategy and age group. Data points show group grand means + 1 standard deviation.
Figure 8.
Individual amplitude and noise data for four stimulus strategies. Horizontal lines show group grand means. Individual differences are apparent in response magnitude, ACC-to-noise ratio, and the effects of different stimulus strategies.
Noise
Grand mean noise magnitude measures are shown in Figure 7 (second panel) along with corresponding standard deviations. Group [F(1,17) = 95.36; p < 0.001] and stimulus strategy [F(3,51) = 37.94; p < 0.001] were highly significant and there was some evidence that the effect of stimulus strategy differed for the two groups [F(3,51) = 2.99; p = 0.039]. Noise was significantly higher for the children compared to the adults. In addition, post-hoc analyses indicated that there was no difference in noise magnitude between the UI2 and UI1 strategies or between the UIU and ALT strategies. There was, however, a significant difference between the single change and double change waveforms for both adults and children. This effect is attributable to doubling the number of samples in the average.
Amplitude-to-noise Ratio
Grand mean amplitude-to-noise ratios obtained in response to each stimulus strategy are shown in Figure 7 (third panel) along with corresponding standard deviations. The individual participants’ ACC-to-noise ratio data are shown in Table 2. Nine out of the ten adults and six out nine children showed highest ACC-to-noise ratios in the ALT condition. There is no evidence of a difference between adults and children [F(1,17) = 0.01; p = 0.919]. There was a highly significant effect of stimulus strategy [F(3,51) = 14.79; p < 0.001], but there was no evidence that the effect of stimulus strategy differed for the two groups [F(3,51) = 0.05; p = 0.985]. Post-hoc analyses showed no significant difference between UI2 and UI1. All other differences reached at least the 0.05 level of significance. These findings suggest that while children had larger amplitudes than adults, the effect was offset by higher noise levels.
Table 2.
ACC-to-noise ratio as a function of stimulus strategy and participant.
| ACC/N | UI2 | UI1 | UIU | ALT |
|---|---|---|---|---|
| Adult 1 | 1.84 | 3.21 | 0.58 | 5.08 |
| Adult 2 | 1.25 | 1.09 | 1.15 | 2.08 |
| Adult 3 | 1.61 | 2.22 | 0.72 | 3.00 |
| Adult 4 | 3.18 | 2.07 | 1.53 | 3.35 |
| Adult 5 | 2.38 | 1.75 | 1.36 | 2.45 |
| Adult 6 | 1.41 | 1.44 | 0.97 | 1.79 |
| Adult 7 | 1.63 | 1.36 | 1.20 | 2.02 |
| Adult 8 | 4.07 | 1.70 | 2.39 | 2.16 |
| Adult 9 | 1.79 | 1.34 | 1.33 | 3.09 |
| Adult 10 | 0.88 | 1.13 | 0.78 | 1.63 |
| mean | 2.00 | 1.73 | 1.20 | 2.66 |
| s.d. | 0.96 | 0.64 | 0.52 | 1.03 |
| Child 1 | 2.41 | 1.64 | 1.47 | 1.68 |
| Child 2 | 1.13 | 1.14 | 1.00 | 1.91 |
| Child 3 | 1.02 | 1.62 | 0.63 | 1.65 |
| Child 4 | 2.05 | 1.31 | 1.57 | 2.45 |
| Child 5 | 3.20 | 2.35 | 1.36 | 4.92 |
| Child 6 | 2.22 | 1.64 | 1.35 | 1.68 |
| Child 7 | 2.29 | 2.59 | 0.88 | 5.30 |
| Child 8 | 2.27 | 2.54 | 0.89 | 2.88 |
| Child 9 | 1.02 | 1.38 | 0.74 | 1.18 |
| mean | 1.96 | 1.80 | 1.10 | 2.63 |
| s.d. | 0.75 | 0.55 | 0.34 | 1.49 |
Efficiency
Grand mean efficiency data for each stimulus strategy are shown in Figure 7 (bottom). On average, efficiency was highest for the ALT strategy for both participant groups. The individual participants’ efficiency data are shown in Table 3. Nine of the ten adults showed highest efficiency for the ALT strategy, while for the remaining adult the ALT and UIU strategies were equally efficient. Six of the nine children showed highest efficiency for the ALT strategy, while two additional children showed equally high efficiency for the ALT and UI1 strategies. There is no evidence for a difference in efficiency between adults and children [F(1,17) = 0.03; p = 0.856]. There was a highly significant effect of stimulus strategy [F(3,51) = 28.81; p < 0.001], but no evidence that the effect of stimulus strategy differed for the two groups of participants [F(3,51) = 0.21; p = 0.889]. Post-hoc analyses showed no significant difference between the UI2 and UIU strategies. All other differences reached at least the 0.05 level of significance.
Table 3.
Efficiency data as a function of stimulus strategy and participant.
| Efficiency | UI2 | UI1 | UIU | ALT |
|---|---|---|---|---|
| Adult | 0.11 | 0.38 | 0.27 | 0.61 |
| Adult | 0.07 | 0.13 | 0.12 | 0.25 |
| Adult | 0.10 | 0.27 | 0.14 | 0.36 |
| Adult | 0.19 | 0.25 | 0.23 | 0.40 |
| Adult | 0.14 | 0.21 | 0.21 | 0.29 |
| Adult | 0.08 | 0.17 | 0.19 | 0.21 |
| Adult | 0.10 | 0.16 | 0.15 | 0.24 |
| Adult | 0.24 | 0.20 | 0.26 | 0.26 |
| Adult | 0.11 | 0.16 | 0.22 | 0.37 |
| Adult | 0.05 | 0.14 | 0.14 | 0.20 |
| mean | 0.12 | 0.21 | 0.19 | 0.32 |
| s.d. | 0.05 | 0.07 | 0.05 | 0.12 |
| Child | 0.14 | 0.20 | 0.15 | 0.20 |
| Child | 0.07 | 0.14 | 0.06 | 0.23 |
| Child | 0.06 | 0.19 | 0.14 | 0.20 |
| Child | 0.12 | 0.16 | 0.22 | 0.29 |
| Child | 0.19 | 0.28 | 0.22 | 0.59 |
| Child | 0.13 | 0.20 | 0.15 | 0.20 |
| Child | 0.14 | 0.31 | 0.29 | 0.64 |
| Child | 0.14 | 0.31 | 0.14 | 0.35 |
| Child | 0.06 | 0.17 | 0.12 | 0.14 |
| mean | 0.12 | 0.22 | 0.17 | 0.32 |
| s.d. | 0.04 | 0.07 | 0.07 | 0.18 |
Reference Electrode
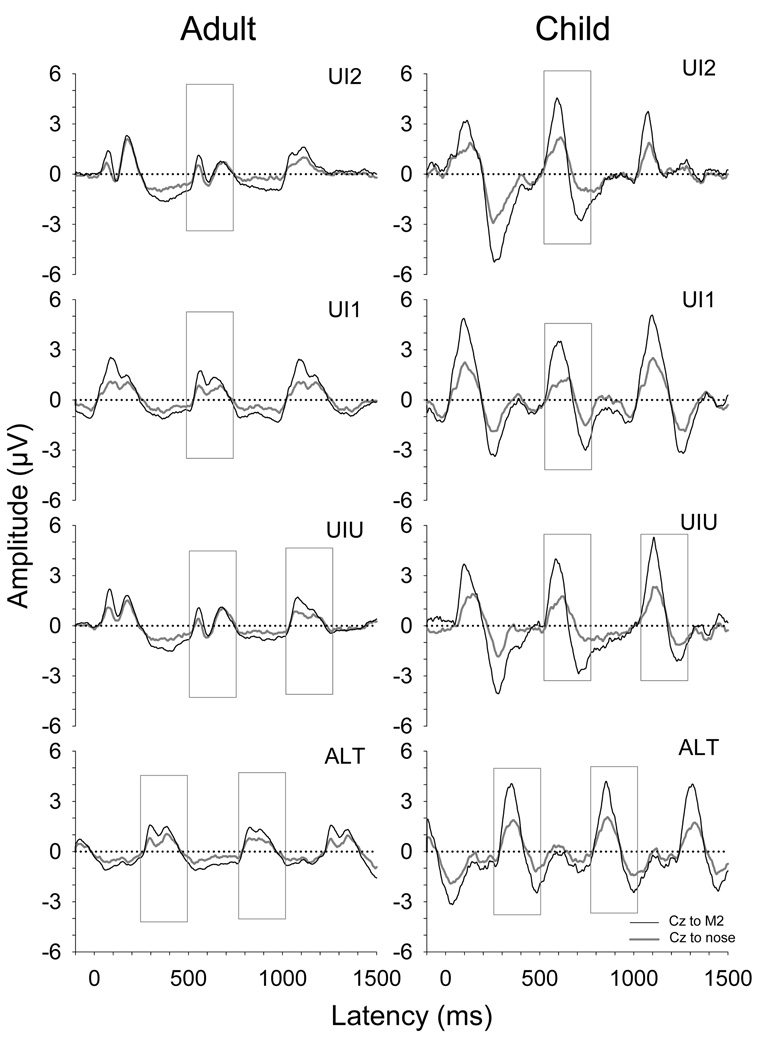
The effects of re-referencing the waveforms obtained in this study to a mastoid electrode (M2) are shown in Figure 9. Amplitudes are typically increased near the vertex when referenced to an electrode below the Sylvian fissure such as M2 (Vaughan and Ritter, 1970). The original grand mean waveforms (from Figure 3) are shown along with the re-referenced waveforms. The grand mean ACC magnitude, noise, ACC-to-noise ratio, and efficiency data for each stimulus strategy after re-referencing are shown in Figure 10 along with corresponding standard deviations for the adults and children. Of interest is whether re-referencing the data to M2 results in increases in these values relative to the original data, and whether reference electrode interacts with age group or stimulus strategy. Only statistically significant results are reported.
Figure 9.
Grand mean waveforms for the adults and children are displayed as a function of stimulus strategy and reference electrode.
Figure 10.
ACC magnitude, noise magnitude, ACC-to-noise magnitude, and testing efficiency as functions of stimulus strategy and age group are shown after re-referencing the waveforms to electrode M2.
The effect of reference electrode site on ACC magnitude was highly significant [F(1,17) = 154.50; p < 0.001]. As expected, ACC magnitude was higher in the re-referenced condition. In addition, there was a significant interaction between reference electrode and group [F(1,17) = 63.10; p < 0.001]. Post-hoc analysis indicated that ACC magnitude increased more for children than for adults with re-referencing. Similarly, noise also showed a significant effect of reference electrode [F(1,17) = 101.30; p < 0.001] with higher noise in the re-referenced condition. This increase was significantly greater for children than for adults [F(1,17) = 7.40; p < 0.001]. ACC-to-noise ratio showed significant increases with re-referencing [F(1,17) = 25.48; p < 0.001]. This effect interacted with age group [F(1,17) = 9.39; p < 0.001] such that increases were greater for children than adults. Therefore, while noise was greater for children in the re-referenced condition, this effect was offset by their higher ACC magnitudes. There was a highly significant effect of reference electrode on test efficiency [F(1,17) = 0.172; p <0.001] along with some evidence of an interaction between reference electrode and group [F(1,17)= 8.25; p = 0.011]. Post-hoc analyses indicate that the increase in efficiency in the re-referenced condition is greater for children. There was also a significant interaction between reference electrode and stimulus strategy [F(3,51) = 6.87; p = 0.001]. Post-hoc analyses indicate complex patterns. Of note, efficiency is higher for the re-referenced ALT condition compared to all other conditions (re-referenced or not) and the original UI2 condition shows smaller efficiency than all other conditions. In addition, the re-referenced UI2 condition shows smaller efficiency than all other re-referenced conditions.
Discussion
Neural refractoriness is always of concern when measuring cortical auditory evoked potentials, especially when testing children (Ceponiene et al., 2002; Gilley et al., 2005; Gomes et al., 2001). There was no evidence of a detrimental trade-off between shortened testing time and response amplitude in this study. That is, the benefits of increased speed of testing resulting from elimination of the silent period between stimuli along with the increased number of acoustic changes obtained in a given time period were not offset by a serious reduction in response amplitude. This is a positive finding for the potential clinical application of continuously alternating stimuli.
Averaging efficiency has been examined previously for onset responses (Hyde and Blair, 1981: Picton et al., 1977; 1983; 1984) and an inter-onset interval of 1–2 seconds was recommended as most efficient. In this study, the ALT strategy, which involved a 1 second interval between repetitions of the same direction of acoustic change, was most efficient for the ACC. Picton et al. (1983) suggested that a simple technique for increasing the efficiency of the auditory brainstem response is to record two responses at the same time. This study accomplished the same goal for the ACC, by recording responses to two directions of acoustic change concurrently in the continuous strategy.
The adult-child comparisons in this study facilitate the interpretation of the efficiency data obtained in this study. Continuous alternating stimulus presentation was more efficient than interrupted stimulus presentation. All adults followed this pattern and all but one of the children did as well. On average, noise in the averaged waveforms was higher for the children compared to the adults. This did not result in poorer efficiency (on average) for the children, largely because of the high amplitude of their ACC responses. These data emphasize the importance of techniques designed to maximize response amplitude while minimizing noise, as well as the potential value of continuously alternated stimuli for the evaluation of young children.
Efficiency gain, the ratio of efficiency obtained for two stimulus strategies, helps to explain why the ALT condition was beneficial in this study. The mean efficiency gain by shortening test time (UI1 vs. UI2) was 1.73 (with 95% confidence limits of +/-0.04). This is somewhat less than the expected value of 2 (from halving test time). The discrepancy may indicate a small refractory effect. The efficiency gain resulting from doubling the number of changes (UIU vs. UI2) was 1.45 (+ 0.03), which is very close to the predicted value of 1.41 (i.e. the √2). The efficiency gain resulting from both halving the onset interval and doubling the number of changes (ALT vs. UI2) was 2.60 (+ 0.07). This is only a little less than the predicted value of 2.82 (i.e. 2*√2).
Maximizing response amplitude by optimizing the electrode recording montage is another way of potentially increasing efficiency. In this study, the re-referencing the data to electrode site M2 had a greater effect for children than for adults, with higher ACC magnitude, noise, ACC-to-noise ratio and efficiency for an M2 than for a noise reference. A full electrode array may not be feasible for clinical application and it will be important to use an efficient recording montage to optimize the probability of recording a response with a high ACC-to-noise ratio. Efficiency gain in the re-referenced condition from shortening test time (UI1 vs. UI2) was 2.0 (+/-0.07), from doubling the number of changes (UIU vs. UI2) was 1.44 (+/-0.05) and from doing both (ALT vs. UI2) was 2.65 (+/-0.10). Therefore, efficiency gain from shortening test time was higher in the re-referenced condition and was equal to the predicted value of 2. Efficiency gain for the other comparisons was comparable to that obtained with the original reference electrode.
The individual data are important in relation to clinical application. Similar to the Fsp approach commonly used in auditory brainstem response testing (see Appendix), it may be possible to set a criterion for a significant ACC-to-noise magnitude ratio. Similar to Fsp, this criterion could conceivably be used to determine if 500 sweeps is sufficient for a given individual or if the ACC-to-noise ratio needs to be monitored to set a stopping point for averaging.
There was relatively large variability in efficiency across participants in this study, particularly for the ALT strategy. It is likely that this variability was caused, in part, by overlapping components. Maturation and refractoriness issues likely played dominant roles behind the larger variance found in the ALT strategy in children compared to adults. A few of the older children in this study were beginning to show a more adult-like response, with an emerging N1 component. Therefore, knowledge regarding maturation of cortical auditory evoked potentials in general, and the ACC specifically in children will be critical for detailed response interpretation in clinical populations and additionally in order to set an appropriate response interval for measuring the ACC.
Clinical decisions regarding whether to use a continuous alternating or an interrupted stimulus should be based upon the question one is looking to answer. If one is interested in determining whether the ACC is present or absent (that is, whether an individual patient has the potential to discriminate the acoustic change of interest), then a continuous alternating stimulus is optimal because of its efficiency. This would be the most cost-effective approach in terms of obtaining maximal information regarding speech discrimination capacity in minimal time. However, if one is interested in examining specific components within the ACC response in order to better evaluate how brain processing of sound is altered in different clinical populations, then an interrupted stimulus, with a longer onset-interval would be more appropriate.
The ACC is a tool in development. The clinical population for which the ACC technique holds greatest promise is infants and young children, particularly those with hearing loss, for whom we do not have good behavioral tests of suprathreshold resolution. The high amplitude and efficiency of the continuous alternating strategy in the 6 to 9 year olds tested in the present study bodes well for its application to infants and young children. Further research will be needed prior to clinical application of the ACC, to test efficiency in these young children and in those with hearing loss, and also to determine whether the stimulus presentation parameters can be further optimized.
In conclusion, continuous alternating stimulus presentation is more efficient than interrupted stimulus presentation in eliciting the acoustic change complex. The benefits resulting from eliminating silent periods and doubling the number of acoustic changes presented in a given time period, are not offset by a serious reduction in rms response amplitude. This conclusion, however, applies only to adults and to children aged six years or more.
Acknowledgements
Work supported by NIH-NIDCD grant #DC05386 (BAM). The authors would like to thank Brian Cairns, Kelly Shea-Miller, Vikram Dayalu, and Mary Boyle for their contributions to this work. The potential benefit of switching to a continuous alternating presentation was first suggested by Paul Abbas.
Appendix: Estimation of rms noise amplitude
The metric used in this study to assess noise amplitude was the square root of the mean variance within sample points, divided by the square root of the number of sweeps contributing to the averaged waveform. That is:
| (A1) |
Where: N = noise amplitude, p = point number, n = the number of sampled points within the response window, Vp = the variance at sample p, and s = the number of sweeps contributing to the averaged waveform.
The result is, essentially, the standard error of all voltage values, after the main effect of sample point (a combination of signal and noise) has been removed.
To illustrate, consider the matrix of Table A1 in which there are 20 sweeps covering 10 sample points. A schematic N1/P2 complex is shown in the first row of data. The next 20 rows show the voltages at each sample point in each sweep. These rows are filled with random numbers to which have been added the N1/P2 complex from the first row. The bottom two rows show the means and variances at each sample point. At the right are, 1) the standard deviation of the means at the 10 sample points, giving a metric for signal plus noise amplitude (S+N), and 2) the metric for noise amplitude (N) derived from equation A1. Table A2 shows an analysis of variance in these data. In this analysis, the main factors are Point at 10 levels and Sweep at 20 levels. The error term is the interaction between Point and Sweep. The bottom row of this table shows the variance derived from the sum of squares attributable to a combination of the main effect of sample and the interaction term. The square root of this term, divided by the square root of the number of sweeps, is identical to the noise estimate derived from equation A1. In other words, this estimate does not eliminate a significant effect of sample – for example a baseline drift. The use of sample-by-sample baseline correction, however, minimizes this effect. An alternative, but equivalent method of minimizing the sample effect is to compute an average waveform in which alternate sweeps were inverted, otherwise known as the +/- technique (Glaser and Ruchkin, 1976; Picton, Linden, Hamel, and Maru, 1983; Schimmel, 1967; Schimmel, Rapin and Cohen, 1974; Wong and Bickford, 1980). An appropriate noise metric would then be the standard deviation of the resulting point means. An advantage of both techniques is that one does not need a waveform region that is free of stimulus-synchronized potentials. Clearly, this condition did not apply to some of the stimulus conditions used in the present study. The most popular noise metric used in the ERP literature is the Fsp (Don and Elberling, 1996; Don, Elberling, and Waring, 1984; Elberling and Don, 1984; 2007). The metric in this case is the standard deviation of samples at a single point. It is used to assess statistical significance of the mean potential at that point. The technique is applicable when response amplitude is specified in terms of peak voltages. In the present study, however, amplitude was assessed in terms of the standard deviation of mean voltages within an expected response window, so as to avoid making assumption about individual waveform morphology. It was appropriate, therefore to base the noise estimate on data from all sample points within the same window. In a sense, this is the Fsp metric averaged across the response window.
Table A1.
Illustrative voltage data. Each sample point entry consists of a random noise value plus the hypothetical N1/P2 complex shown in the top row.
| N1/P2 complex in µv |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | −0.5 | −0.7 | −0.5 | 0 | 1 | 1.4 | 1.4 | 1 | 0 | |
| Sample point | ||||||||||
| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 1 | −1.10 | −1.42 | −1.17 | 1.32 | 0.48 | 1.83 | −0.20 | 0.74 | −0.29 | −0.06 |
| 2 | 0.29 | −1.85 | 0.39 | 1.17 | −1.56 | 2.38 | 0.04 | 1.47 | −0.25 | 0.09 |
| 3 | 0.81 | −0.63 | −0.84 | 0.01 | 0.03 | 2.08 | 2.40 | 2.38 | 1.60 | 1.45 |
| 4 | 0.28 | 0.30 | −1.15 | 0.09 | −0.10 | −0.06 | 2.78 | −0.38 | 0.25 | 1.25 |
| 5 | −1.27 | 0.45 | 0.84 | −2.30 | 0.19 | −0.76 | 2.82 | 0.58 | −0.25 | 0.23 |
| 6 | 0.15 | 1.03 | −1.63 | −1.51 | 0.82 | 2.69 | 1.92 | −0.40 | 1.66 | 1.89 |
| 7 | −1.53 | −0.83 | 0.69 | 0.64 | −0.75 | 0.38 | 0.24 | 3.05 | 0.81 | 0.87 |
| 8 | −0.88 | −0.25 | −0.27 | 0.54 | 0.24 | −0.34 | 2.52 | 0.44 | 2.92 | −1.59 |
| 9 | 0.09 | −0.79 | −2.16 | −0.22 | −0.27 | 0.11 | 1.13 | 2.82 | 1.48 | 1.09 |
| 10 | −0.08 | 0.65 | −1.38 | −0.38 | 1.07 | 1.25 | 1.50 | −0.43 | 2.36 | 1.15 |
| 11 | −0.54 | −0.77 | −1.54 | −0.04 | −0.95 | 0.39 | 3.31 | 3.36 | 0.22 | 1.07 |
| 12 | 0.23 | −2.23 | 0.80 | −0.73 | 1.40 | −0.35 | 1.97 | 1.61 | −0.08 | 0.62 |
| 13 | 1.73 | −1.24 | −2.34 | −1.37 | 1.99 | 1.75 | 3.20 | −0.53 | 1.94 | 0.89 |
| 14 | 1.47 | −1.12 | −2.43 | −1.68 | −0.10 | 0.81 | 1.48 | 0.52 | −0.90 | 1.59 |
| 15 | 0.47 | 1.26 | 0.64 | 0.87 | −0.11 | −0.40 | 3.11 | 1.82 | −0.59 | 0.30 |
| 16 | 0.32 | −1.87 | 0.57 | 0.93 | 0.16 | 1.33 | 1.59 | 3.11 | 1.80 | −0.22 |
| 17 | 0.90 | 0.43 | −0.14 | −0.22 | 0.52 | 0.71 | 1.78 | 1.52 | −0.50 | −0.74 |
| 18 | −0.24 | −1.99 | −1.91 | 0.20 | 0.00 | 0.32 | 2.40 | 1.42 | 1.61 | −1.53 |
| 19 | 0.34 | −0.67 | 0.00 | −0.02 | −1.11 | 0.95 | 2.49 | 2.35 | −0.85 | 0.57 |
| 20 | 1.03 | 0.24 | 1.18 | −0.45 | 1.55 | −0.79 | −0.53 | 0.59 | 1.03 | −0.85 |
| Mean | 0.12 | −0.57 | −0.59 | −0.16 | 0.18 | 0.71 | 1.80 | 1.30 | 0.70 | 0.40 |
| Variance | 0.76 | 1.09 | 1.43 | 0.96 | 0.81 | 1.10 | 1.33 | 1.60 | 1.35 | 0.99 |
| Standard deviation of column means = Amplitude (S+N) = |
0.768 |
|||||||||
| Standard error based on average column variance = Estimated noise (N) = |
0.239 | |||||||||
Table A2.
Analysis of variance of the data shown in Table A1. Note: 1) the Standard Error based on the main effect of sample Point is identical to signal-plus-noise estimate from Table A1, 2) the Standard error based on the combined effects of Sample and noise (the interaction term) is identical to the noise estimate derived from the column variances of Table A1 (i.e., equation A1).
| Source of Variance |
Sum of Squares |
Degrees of Freedom |
Vari- ance |
F ratio |
Stand- ard error |
|---|---|---|---|---|---|
| Point | 106.15 | 9 | 11.79 | 9.91 | 0.768 |
| Sample | 13.37 | 19 | 0.70 | 0.265 | |
| P*S | 203.45 | 171 | 1.19 | 0.244 | |
| Total | 322.96 | 199 | 0.70 | 0.839 | |
| P*S+S | 216.81 | 190 | 1.14 | 0.239 |
|
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In fact, we computed the standard deviation of the means within the response window so as to remove any contribution from a non-zero grand mean. Potential causes of a non-zero grand mean include asymmetry of the positive and negative components of the ACC, prolonged negativity following an onset response, initiation of the response window after the ACC has begun, and termination of the response window before the ACC has ended.
References Cited
- American National Standards Institute. ANSI S3.6, Specification for Audiometers. 1996 [Google Scholar]
- Arlinger S, Elberling C, Bak C, Kofoed B, Lebech J, Saermark K. Cortical magnetic fields evoked by frequency glides of a continuous tone. Electroencephalogr Clin Neurophysiol. 1982;54:642–653. doi: 10.1016/0013-4694(82)90118-3. [DOI] [PubMed] [Google Scholar]
- Arlinger SD, Jerlvall LB, Ahren T, Holmgren EC. Slow evoked cortical responses to linear frequency ramps of a continuous pure tone. Acta Physiol. Scand. 1976;98:412–424. doi: 10.1111/j.1748-1716.1976.tb10330.x. [DOI] [PubMed] [Google Scholar]
- Billings CJ, Tremblay KL, Souza PE, Binns MA. Effects of hearing aid amplification and stimulus intensity on cortical auditory evoked potentials. Audiol Neurootol. 2007;12:234–246. doi: 10.1159/000101331. [DOI] [PubMed] [Google Scholar]
- Boothroyd A. Measuring auditory speech-perception capacity in young children, Chapter 9. In: Seewald RC, Bamford JM, editors. A Sound Foundation through Early Amplification: Proceedings of the 3rd International Conference; Phonak AG; 2005. pp. 129–140. [Google Scholar]
- Boothroyd A. Assessment of speech perception capacity in profoundly deaf children. Am J Otol. 1991;12 (Suppl.):67–72. [PubMed] [Google Scholar]
- Brown CJ, Etler C, He S, O’Brien S, Erenberg S, Kim JR, Dhuldhoya AN, Abbas PJ. The electrically evoked auditory change complex: Preliminary results from Nucleus cochlear implant users. Ear Hear. 2008;29:704–717. doi: 10.1097/AUD.0b013e31817a98af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceponiene R, Rinne T, Naatanen R. Maturation of cortical sound processing as indexed by event-related potentials. Clin Neurophysiol. 2002;113:870–882. doi: 10.1016/s1388-2457(02)00078-0. [DOI] [PubMed] [Google Scholar]
- Clynes M. Dynamics of vertex evoked potentials: The R-M brain functions. In: Donchin E, Lindsley DB, editors. Average evoked potentials: Methods, results and evaluations. Washington, D.C.: US Government Printing Office; 1969. pp. 363–374. NASA SP-191. [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: Age, sleep and modality. Clin Neurophysiol. 2004;115:732–744. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic A, Michalewski HJ, Zeng FG, Pratt H, Starr A. Frequency changes in a continuous tone: Auditory cortical potentials. Clin Neurophysiol. 2008;119:2111–2124. doi: 10.1016/j.clinph.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don M, Elberling C. Use of quantitative measures of auditory brain-stem response peak amplitude and residual background noise in the decision to stop averaging. J. Acous Soc Am. 1996;99:494–499. doi: 10.1121/1.414560. [DOI] [PubMed] [Google Scholar]
- Don M, Elberling C, Waring M. Objective detection of averaged auditory brainstem responses. Scandinavian Audiology. 1984;13:219–228. doi: 10.3109/01050398409042130. [DOI] [PubMed] [Google Scholar]
- Elberling C, Don M. Quality estimation of averaged auditory brainstem responses. Scand Audiol. 1984;13:187–197. doi: 10.3109/01050398409043059. [DOI] [PubMed] [Google Scholar]
- Elberling C, Don M. Detecting and assessing synchronous neural activity in the temporal domain (SNR, response detection. In: Burkard RF, Don M, Eggermont JJ, editors. Auditory Evoked Potentials: Basic Priniciples and Clinical Application. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007. pp. 102–123. [Google Scholar]
- Friesen LM, Tremblay KL. Acoustic change complexes recorded in adult cochlear implant listeners. Ear Hear. 2006;27:678–685. doi: 10.1097/01.aud.0000240620.63453.c3. [DOI] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman M, Martin K. Developmental changes in refractoriness of the cortical auditory evoked potential. Clin Neurophysiol. 2005;116:648–657. doi: 10.1016/j.clinph.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Ruchkin DS. Principles of Neurobiological Signal Analysis. New York: Academic Press; 1976. [Google Scholar]
- Gomes H, Dunn M, Ritter W, Kurtzberg D, Brattson A, Kreuzer JA, Vaughan HG. Spatiotemporal maturation of the central and lateral N1 components to tones. Brain Res Dev Brain Res. 2001;129:147–155. doi: 10.1016/s0165-3806(01)00196-1. [DOI] [PubMed] [Google Scholar]
- Gomes H, Sussman E, Ritter W, Kurtzberg D, Cowan N, Vaughan HG. Electrophysiological evidence of developmental changes in the duration of auditory sensory memory. Dev Psychol. 1999;35:294–302. doi: 10.1037//0012-1649.35.1.294. [DOI] [PubMed] [Google Scholar]
- Hari R. Activation of the human auditory cortex by speech sounds. Acta Otolaryngol. 1991;491 Suppl:132–138. doi: 10.3109/00016489109136790. [DOI] [PubMed] [Google Scholar]
- Harris FC, Mills JH, Dubno JR. Electrophysiologic correlates of intensity discrimination in cortical evoked potentials of younger and older adults. Hear Res. 2007;228:58–68. doi: 10.1016/j.heares.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Picton TW. On and off components in the auditory evoked potential. Percep Psychophys. 1978;24:391–398. doi: 10.3758/bf03199736. [DOI] [PubMed] [Google Scholar]
- Hyde M. The N1 response and its applications. Audiol Neurootol. 1997;2:281–307. doi: 10.1159/000259253. [DOI] [PubMed] [Google Scholar]
- Hyde ML, Blair RL. The auditory brainstem response in neuro-otology: Perspectives and problems. J Otolaryngol. 1981;10:117–125. [PubMed] [Google Scholar]
- Imaizumi S, Mori K, Kiritani S, Yumoto M. Neural representation of concurrent sounds: A magnetoencephalographic study. In: Hashimoto I, Okada YC, Ogawa S, editors. Visualization of information processing in the human brain: Recent advances in MEG and functional MRI. Amsterdam: Elsevier Science; 1996. pp. 191–197. EEG. [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr. Clin. Neurophysiol. 1958;10:370–375. [PubMed] [Google Scholar]
- Jerger J, Jerger S. Evoked responses to intensity and frequency change. Arch Otolaryngol. 1970;91:433–436. doi: 10.1001/archotol.1970.00770040627007. [DOI] [PubMed] [Google Scholar]
- Kaukoranta E, Hari R, Lounasmaa OV. Responses of the human auditory cortex to vowel onset after fricative consonants. Exp Brain Res. 1987;69:19–23. doi: 10.1007/BF00247025. [DOI] [PubMed] [Google Scholar]
- Kohn M, Lifshitz K, Litchfield D. Average evoked potentials and frequency modulation. Electroencephalogr Clin Neurophysiol. 1978;45:236–243. doi: 10.1016/0013-4694(78)90007-x. [DOI] [PubMed] [Google Scholar]
- Kohn M, Lifshitz K, Litchfield D. Average evoked potentials and amplitude modulation. Electroencephalogr Clinl Neurophysiol. 1980;50:134–140. doi: 10.1016/0013-4694(80)90330-2. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee T, Carrell T, Sharma A, Micco A, Nicol T. Speech-evoked cortical potentials in children. J Am Acad Audiol. 1993;4:238–48. [PubMed] [Google Scholar]
- Kujala T, Tervaniemi M, Schroger E. The mismatch negativity in cognitive and clinical neuroscience: Theoretical and methodological considerations. Biol Psychol. 2007;74:1–19. doi: 10.1016/j.biopsycho.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lavikainen J, Huotilainen M, Ilmoniemi RJ, Simola JT, Naatanen R. Pitch change of a continuous tone activates two distinct processes in human auditory cortex: A study with whole-head magnetometer. Electroencephalogr Clin Neurophysiol. 1995;96:93–36. doi: 10.1016/0013-4694(94)00283-q. [DOI] [PubMed] [Google Scholar]
- Lenhardt ML. Effects of frequency modulation on auditory averaged evoked responses. Audiology. 1971;10:18–22. doi: 10.3109/00206097109072536. [DOI] [PubMed] [Google Scholar]
- Martin BA. Can the acoustic change complex be recorded in an individual with a cochlear implant? Separating neural responses from cochlear implant artifact. Journal of the American Academy of Audiology. 2007;18:126–140. doi: 10.3766/jaaa.18.2.5. [DOI] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A. Cortical, auditory, event-related potentials in response to periodic and aperiodic stimuli with the same spectral envelope. Ear Hear. 1999;20:33–44. doi: 10.1097/00003446-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A. Cortical, auditory, evoked potentials in response to changes of spectrum and amplitude. J Acoust Soc Am. 2000;107:2155–2161. doi: 10.1121/1.428556. [DOI] [PubMed] [Google Scholar]
- Martin BA, Tremblay KL, Stapells DR. Principles and applications of cortical auditory evoked potentials. In: Burkard RF, Don M, Eggermont JJ, editors. Auditory Evoked Potentials: Basic Principles and Clinical Application. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2007. [Google Scholar]
- Morr ML, Shafer VL, Kreuzer JA, Kurtzberg D. Maturation of mismatch negativity in typically developing infants and preschool children. Ear Hear. 2002;23:118–136. doi: 10.1097/00003446-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Naatanen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Beh Brain Sci. 1990;13:201–288. [Google Scholar]
- Naatanen R. Attention and Brain Function. New Jersey: Lawrence Erlbaum; 1992. [Google Scholar]
- Naatanen R. Mismatch negativity: Clinical research and possible applications. Int J Psychophysiol. 2003;48:179–188. doi: 10.1016/s0167-8760(03)00053-9. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Gaillard AWK, Mantysalo S. Early selective attention effect on evoked potential reinterpreted. Acta Psychol. 1978;42:313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Picton TW. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Ostroff JM, Martin BA, Boothroyd A. Cortical evoked response to acoustic change within a syllable. Ear Hear. 1998;19:290–297. doi: 10.1097/00003446-199808000-00004. [DOI] [PubMed] [Google Scholar]
- Picton TW, Alain C, Otten L, Ritter W, Achim A. Mismatch negativity: Different water in the same river. Audiology and Neurootology. 2000;5:111–139. doi: 10.1159/000013875. [DOI] [PubMed] [Google Scholar]
- Picton TW, Alain C, Woods DL, John MS, Scherg M, Valdes-Sosa P, Bosch-Bayard J, Trujillo NJ. Intracerebral sources of human auditory-evoked potentials. Audiology and Neurootology. 1999;4:64–79. doi: 10.1159/000013823. [DOI] [PubMed] [Google Scholar]
- Picton TW, Hink RF, Perez-Abalo M, Linden RD, Wiens AS. Evoked potentials: How now? Journal of Electrophysiological Technology. 1984;10:177–221. [Google Scholar]
- Picton TW, Linden RD, Hamel G, Maru J. Aspects of averaging. Seminars in Hearing. 1983;4:327–339. [Google Scholar]
- Picton TW, Woods DL, Baribeau-Braun J, Healey TMG. Evoked potential audiometry. J Otolaryngol. 1977;6:90–119. [PubMed] [Google Scholar]
- Ponton CW, Don M, Eggermont JJ, Kwong B. Integrated mismatch negativity (MMNi): A noise-free representation of evoked responses allowing single-point distribution-free statistical tests. Electroencephalogr Clin Neurophysiol. 1997;104:143–150. doi: 10.1016/s0168-5597(97)96104-9. [DOI] [PubMed] [Google Scholar]
- Ponton C, Eggermont JJ, Khosla D, Kwong B, Don M. Maturation of human central auditory system activity: separating auditory evoked potentials by dipole source modeling. Clin Neurophysiol. 2002;113:407–20. doi: 10.1016/s1388-2457(01)00733-7. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Clin Neurophysiol. 2000;111:220–36. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Ross B, Tremblay KL, Picton TW. Physiological detection of interaural phase differences. Journal of the Acoustical Society of America. 2007;121:1017–1027. doi: 10.1121/1.2404915. [DOI] [PubMed] [Google Scholar]
- Ruhm HB. Rate of frequency change and the acoustically evoked response. J Aud Res. 1970;10:29–34. [Google Scholar]
- Ruhm HB, Jansen JW. Rate of stimulus change and the evoked response: 1: Signal and rise-time. J Aud Res. 1969;3:211–216. [Google Scholar]
- Schimmel H. The + reference: Accuracy of estimated mean components in average response studies. Science. 1967;157:92–94. doi: 10.1126/science.157.3784.92. [DOI] [PubMed] [Google Scholar]
- Schimmel H, Rapin I, Cohen MM. Improving evoked response audiometry with special reference to the use of machine scoring. Audiology. 1974;13:33–65. doi: 10.3109/00206097409089335. [DOI] [PubMed] [Google Scholar]
- Semlitch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kraus N, McGee TJ, Nicol TG. Developmental changes in P1 and N1 central auditory responses elicited by consonant-vowel syllables. Electroencephalogr Clin Neurophysiol. 1997;104:540–545. doi: 10.1016/s0168-5597(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Spoor A, Timmer F, Odenthal DW. The auditory evoked responses to intensity modulated and frequency modulated tones and tone bursts. Int Audiol. 1969;8:410–415. [Google Scholar]
- Stapells DR. Cortical event-related potentials to auditory stimuli. In: Katz J, Burkard RF, Medwetsky L, editors. Handbook of Clinical Audiology. 5th edition. Philadelphia, PA: Lippincott, Williams, and Wilkins; 2002. pp. 378–406. [Google Scholar]
- Tremblay KL, Billings CJ, Friesen LM, Souza PE. Neural representation of amplified speech sounds. Ear Hear. 2006;27:93–103. doi: 10.1097/01.aud.0000202288.21315.bd. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Friesen L, Martin BA, Wright R. Test-retest reliability of cortical evoked potentials using naturally produced speech sounds. Ear Hear. 2003;24:225–232. doi: 10.1097/01.AUD.0000069229.84883.03. [DOI] [PubMed] [Google Scholar]
- Tyler RS. Speech perception by children. In: Tyler RS, editor. Cochlear implants: Audiological Foundations. San Diego: Singular Publishing Group; 1993. pp. 191–256. [Google Scholar]
- Vaughan HG, Ritter W. The sources of auditory evoked responses recorded from the human scalp. Electroenceph Clin Neurophysiol. 1970;28:360–367. doi: 10.1016/0013-4694(70)90228-2. [DOI] [PubMed] [Google Scholar]
- Wong PKH, Bickford RG. Brain stem auditory evoked potentials: The use of noise estimate. Electroenceph Clin Neurophysiol. 1980;50:25–34. doi: 10.1016/0013-4694(80)90320-x. [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK. Maturation of CAEP in infants and children: A review. Hear Res. 2006;212:212–223. doi: 10.1016/j.heares.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK, Shepherd R. Maturation of the cortical auditory evoked potential in infants and young children. Hear Res. 2006;212:185–202. doi: 10.1016/j.heares.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Yingling CD, Nethercut GE. Evoked response to frequency shifted tones: Tonotopic and contextual determinants. Int J Neurosci. 1983;22:107–118. doi: 10.3109/00207459308987389. [DOI] [PubMed] [Google Scholar]
Reference Note
- 1.Ostroff JM. Parametric study of the acoustic change complex elicited by second formant change in synthetic vowel stimuli. City University of New York: Unpublished doctoral dissertation; 1999. [Google Scholar]