Abstract
J Clin Hypertens (Greenwich). 2010;12:203–212. ©2010 Wiley Periodicals, Inc.
This paper evaluates the relationship of blood pressure (BP) levels at Women’s Health Initiative (WHI) baseline, treatment of hypertension, and white matter abnormalities among women in conjugated equine estrogen (CEE) and medroxyprogesterone acetate and CEE‐alone arms. The WHI Memory Study—Magnetic Resonance Imaging (WHIMS‐MRI) trial scanned 1424 participants. BP levels at baseline were significantly positively related to abnormal white matter lesion (WML) volumes. Participants treated for hypertension but who had BP ≥140/90 mm Hg had the greatest amount of WML volumes. Women with untreated BP ≥140/90 mm Hg had intermediate WML volumes. Abnormal WML volumes were related to hypertension in most areas of the brain and were greater in the frontal lobe than in the occipital, parietal, or temporal lobes. Level of BP at baseline was strongly related to amount of WML volumes. The results of the study reinforce the relationship of hypertension and BP control and white matter abnormalities in the brain. The evidence to date supports tight control of BP levels, especially beginning at younger and middle age as a possible and perhaps only way to prevent dementia.
Hypertension is the primary risk factor for vascular disease in the brain and especially for small vessel disease, which subsequently leads to white matter abnormalities, brain infarction, and possibly loss of gray matter neurons. 1 , 2 , 3 , 4 The small blood vessels in the brain and kidneys are more susceptible to even moderately elevated blood pressure (BP). 5 , 6 Hypertensive renal disease is strongly related to white matter abnormalities in the brain. 7 Longitudinal studies have now documented that white matter abnormalities are a probable independent risk factor for dementia, 8 , 9 , 10 , 11 lower extremity functional abnormalities, 12 clinical stroke, 13 , 14 and survivorship among older individuals. 15 Prevalence of elevated BP is very high in the United States and in many other countries, and good control of BP remains poor. 16 , 17 Elevated BP, especially in midlife, has been associated with an increased risk of dementia, including both vascular and Alzheimer disease. 18 , 19 , 20 , 21 , 22 , 23 , 24 The evidence that lowering BP in clinical trials will reduce the risk of dementia or Alzheimer disease is limited. 25 , 26 , 27 , 28 , 29 There is also little evidence that better control of BP slows the progression of white matter lesions (WMLs) in the brain. 25 , 30 BP lowering is associated with substantial reduction in risk of stroke, renal failure, and congestive heart failure. 31 , 32
The Women’s Health Initiative Memory Study—Magnetic Resonance Imaging (WHIMS‐MRI) trial has provided an important opportunity to evaluate the relationship of BP levels, treatment of hypertension in the context of hormone therapy, and WML volume. Does the higher BP among women on hormone therapy increase the volume of WMLs? Does baseline BP at entry to the trial 8 years prior to magnetic resonance imaging (MRI) or changes in BP during the trial predict WML volumes? Is there any relationship between control of BP by antihypertensive drug therapy and WML volumes?
We have reported in the WHIMS‐MRI trial that the mean WML volume was slightly larger for the conjugated equine estrogen (CEE) and CEE with medroxyprogesterone acetate (CEE+MPA) active compared with their placebo. The differences were not statistically significant. 33 There was no difference in the extent of WML volume in the CEE‐alone trial between placebo and active treatment. Increased WML volumes were significantly related to baseline age, smoking, history of cardiovascular disease (CVD), and hypertension as well as scores on the Modified Mini‐Mental State Examination (3MSE) and development of mild cognitive impairment (MCI) or probable dementia during or after the trial but prior to MRI. 33
In this paper, we focused primarily on the relationships that BP levels at baseline and at follow‐up, hypertension, treatment, and control of hypertension have with total and region‐specific brain white matter abnormalities in the WHIMS‐MRI trial.
Methods
WHIMS is an ancillary study to the Women’s Health Initiative (WHI); 38 of 40 WHI clinical centers participated in WHIMS. WHIMS recruited women age 65 or older from among those randomized into the CEE‐alone or CEE+MPA arms of the WHI. To be enrolled in WHIMS, women must have participated in either of the hormone trials of the WHI, free of probable dementia, and willing to undergo annual cognitive assessment. 34 , 35 Of age‐eligible WHI participants, 2947 (92.1%) of the CEE‐alone women and 4532 (92.6%) of the CEE +MPA women consented to enroll in the WHIMS CEE‐alone and CEE+MPA arms, respectively. All WHIMS participants underwent cognitive screening with the modified 3MSE at baseline, and women who scored below a certain cutpoint on the 3MSE (≤80 for ≤8th grade education or 88 for >8th grade education) underwent more comprehensive neuropsychologic evaluation. Details of WHIMS have previously been published. 34 , 35 , 36 At the conclusion of the WHI hormone trials, participants continued to be followed annually for cognitive testing and for adjudication of possible dementia and MCI in the WHIMS Extension Study.
The WHIMS‐MRI trial was designed to contrast MRI findings among women older than 70 who had been assigned to either active vs placebo therapy in both the CEE‐alone and CEE+MPA arms during the WHIMS ancillary study of the WHI. 34 , 35 It was conducted in 14 of the original 38 centers that participated in the WHIMS. Recruitment began in January 2005 and was completed in April 2006. The description of recruitment has been previously published (Figure). 33 , 37 A standardized protocol was used in all 14 of the clinical centers. No baseline (entry to the WHI) MRI scans are available. The average time from the WHIMS CEE+MPA and CEE‐alone trial randomization to the MRI scans was 8.02 and 7.97 years, respectively, and from termination of the trial to scanning was 3.03 and 1.36 years, respectively. The details of the MRI scanning protocol have been previously published and include the following33, 38, 39
Figure.
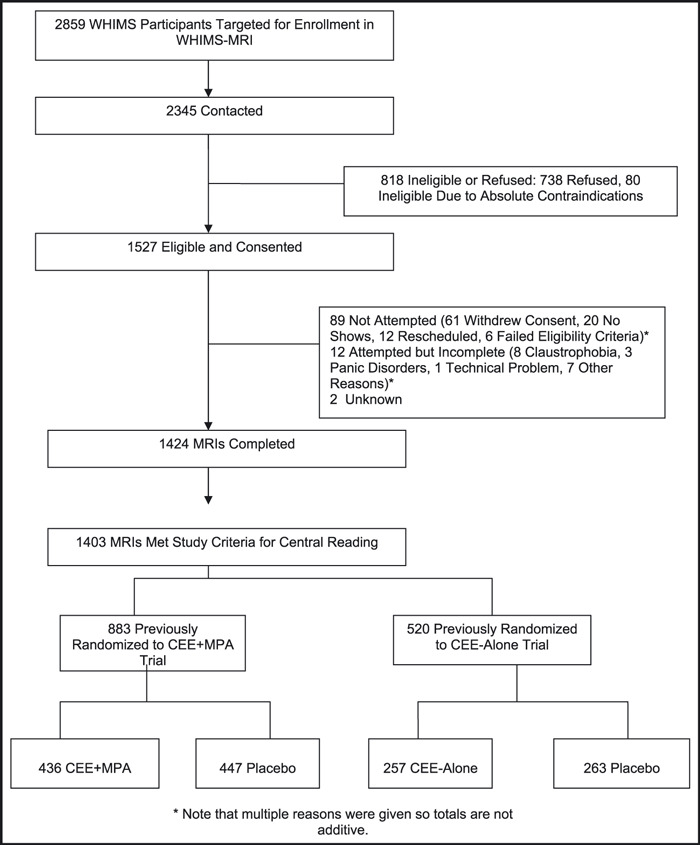
Enrollment and flow of participants through the Women’s Health Initiative Memory Study— Magnetic Resonance Imaging (WHIMS‐MRI). MRIs indicates magnetic resonance images; CEE, conjugated equine estrogen; MPA, medroxyprogesterone acetate.
-
•
Series 1: Three‐plane gradient echo localizer for positioning
-
•
Series 2: Sagittal T1‐weighted spin echo (300/0/8/1/5 TR/TI/TE/slice thickness) mid‐slice image to demonstrate anatomical location of the AC/PC for slice angle and slice position
-
•
Series 3: Oblique axial spin density/T2‐weighted spin echo (3200/0/30,120/3) images from the vertex to skull base parallel to the AC/PC plane
-
•
Series 4: Oblique axial fluid‐attenuated inversion‐recovery (FLAIR) T2‐weighted spin echo (8000/2000/100/3) images matching slice positions in series 2
-
•
Series 5: Oblique axial 3D T1‐weighted gradient echo (flip angle 30; 21/0/8/1.5) images from the vertex to the skull base parallel to the AC/PC plane (field of view: 22 cm; matrix: 256×256 for all scans)
Detailed training was done prior to the beginning of the WHIMS‐MRI trial, and all of the MRI technologists at the centers were certified prior to the beginning of the study.
The primary outcome measures of the WHIMS‐MRI trial are total white matter brain lesion volumes measured in cubic centimeters as detected from a standardized imaging and reading protocol. The lesion volume as defined and identified by this methodology generally corresponds to what has been called, for example, leukoaraiosis, ischemic white matter disease, and small vessel ischemia. The methodology for detecting and quantifying ischemic tissue used in the WHIMS‐MRI was automated quantitative computerized digital imaging analytical techniques that have been previously described. 33 , 38 , 39 These are correlated with human observations and by semiquantitative scoring systems. The methodology for detecting and quantifying ischemic tissue used in this report reflects the evolution in image processing from manual human observer to automatic quantitative computerized digital image analytical techniques that are not only correlated with human observers and the semiquantitative scoring systems, but are very reproducible and offer a greater dynamic range. 40 , 41
The automated segmental technique for WML volume was validated in 44 patients compared with the Cardiovascular Health Study (CHS) grading of WMLs. This CHS grading system was developed by Dr Bryan who was also director of the WHIMS‐MRI trial. 42 , 43 , 44 Brain infarcts based on MRI were not measured.
At baseline and annual clinic visits, women sat quietly for 5 minutes before BP was measured with a mercury manometer. Two measures, taken 30 seconds apart, were recorded. The average of the 2 measurements was used in these analyses. Appropriate cuff bladder size was determined at each visit based on arm circumference. 45
Hypertension was defined as baseline a systolic BP (SBP) ≥140 mm Hg or diastolic BP (DBP) ≥90 mm Hg or being on antihypertensive drug therapy. 45
Statistical Analysis
Three outcomes were examined, including WML volume in cubic centimeters, specific basal ganglia WML volume, and WML volumes by specific region of the brain. There were 10 regions of interest included in the number of anatomic locations with WMLs: frontal, occipital, parietal, temporal white matter, frontal matter, occipital matter, parietal gray matter, temporal gray matter, limbic gray matter, and corpus callosum. Presence of WML tissue in any of the 10 regions of interest generated a count of 1, with all regions summed for a maximum of 10 for the outcome number of white matter abnormalities. A very small percentage of the total lesion volume probably represents cortical pathology, ie, grey matter, similar to white matter pathology. The small lesions found in the grey matter resemble WMLs and are grouped as such in the results. Due to extreme skewness of the data, log‐transformations were performed on white matter and basal ganglia lesion volumes. The mean lesion volume in frontal occipital, parietal, temporal lobes; right and left and limbic lobes; and corpus callosum was also reported. Analysis of covariance was performed to examine relationships between baseline hypertension status, WHI treatment assignment, a combination of treatment, and BP control. Hypertension was defined as baseline use of antihypertensive medication, SBP ≥140 mm Hg, or DBP ≥90 mm Hg. We also examined the relation of alternate definitions of hypertension, including ≥1 post‐baseline BP ≥140/90 mm Hg or use of antihypertensive medication, with white matter abnormalities. Fourteen participants were excluded from all analyses because of history of clinical stroke during the trial but prior to MRI. Change in BP (last visit – baseline visit) was analyzed to determine whether a treatment effect existed, using t tests. Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC). All tables were adjusted for age, race, treatment assignment, cranial volume, time between termination of study and MRI, and clinic.
Results
There were 2345 women who were contacted for participation in the WHIMS‐MRI trial; 72% of these women consented to undergo screening for the WHIMS‐MRI trial. Scanning was completed in 1424 (61%) participants, and 1403 were included in this analysis. Of the 1403 participants, 883 had been randomized in the CEE+MPA study (436 active and 447 placebo) and 520 in the CEE‐alone study (257 active and 263 placebo). Detailed characteristics of the women who were included in the WHIMS‐MRI trial have been published, 33 , 46 as well as comparison between women who did or did not have an MRI (Figure). 37 Factors that are independently associated with having an MRI were: younger age (odds ratio [OR], 1.7), 70–75 vs 82–88; education, college vs < high school (OR, 0.51); no history of CHD (OR, 1.58); no diabetes (OR, 1.5); and never smokers (OR, 1.9). Therefore, the participants tended to be healthier than the population from which they were selected. The percent accepting the MRI (71%) and actually completing the MRI (61%) is similar to findings in the CHS (88% and 72%), the Atherosclerosis Risk In Communities Study (ARIC) (73% and 67%), the Rotterdam Study (90% and 57%), the Framingham Heart Study (80% and 71%), and the Honolulu‐Asia Aging Study (71% and 68%). Similarly, the determinants of accepting the MRI are very similar across these studies. 37
Key variables at the time of enrollment into WHI are listed in Table I, comparing hypertensive with nonhypertensive patients. The mean age was 78.5 years at time of MRI. We have previously reported that age and smoking were significantly related to WML volumes. 33 The low prevalence of current smoking and diabetes precluded further detailed evaluation of these two variables. There were no measures of kidney function. Electrocardiographic estimate of left ventricular hypertrophy (LVH) by Minnesota code criteria 3.1–3.3 was only 93 (6.9%): 38 (0.3%) for those not on antihypertensive drug therapy and 55 (5.6%) for those on antihypertensive drug therapy. Prevalence of LVH by voltage criteria was also very low. Echocardiography was not performed.
Table I.
WHI Baseline Demographic, Socioeconomic Status, and Lifestyle Characteristics of WHIMS‐MRI Trial Women by Hypertension Status
| Variable | No Hypertension (N=735) | Hypertension (N=668) |
|---|---|---|
| Age, y, No. (%) | ||
| 65–69 | 402 (55) | 313 (47) |
| 70–74 | 245 (33) | 247 (37) |
| 75+ | 88 (12) | 108 (16) |
| Ethnicity, No. (%) | ||
| American/Indian | 2 (0) | 2 (0) |
| Asian/Pacific Islander | 12 (2) | 11 (2) |
| Black/African American | 21 (3) | 43 (6) |
| Hispanic/Latina | 14 (2) | 7 (1) |
| White, non‐Hispanic | 679 (93) | 597 (90) |
| Other | 6 (0) | 5 (1) |
| Smoking status, No. (%) | ||
| Never | 412 (57) | 394 (59) |
| Former | 279 (38) | 247 (37) |
| Current | 35 (5) | 24 (4) |
| Diabetes | 23 (3) | 52 (8) |
Abbreviation: WHIMS‐MRI, Women’s Health Initiative Memory Study—Magnetic Resonance Imaging.
At baseline, 435 (31%) of the participants had an SBP ≥140 mm Hg and 270 of these 435 (62%) were not on antihypertensive drug therapy (Table II). Only 215 (57%) of the 380 women taking antihypertensive medication had SBP control <140 mm Hg.
Table II.
White Matter Lesion Volumes in Cubic Centimeters (SE) by Baseline BP Levelsa
| SBP, mm Hg | No. | Baseline | No. | Last BP |
|---|---|---|---|---|
| Not on antihypertensive drug therapy | ||||
| <100 | 102 | 4.07 (0.44) | 108 | 3.25 (0.35) |
| 100–119 | 187 | 4.11 (0.32) | 136 | 4.13 (0.37) |
| 120–139 | 450 | 3.89 (0.20) | 331 | 3.86 (0.22) |
| 140+ | 270 | 5.20 (0.33) | 155 | 4.82 (0.40) |
| P=.0044 | P=.03 | |||
| On antihypertensive drug therapy | ||||
| <100 | 11 | 5.56 (1.75) | 50 | 4.52 (0.71) |
| 100–119 | 33 | 2.73 (0.56) | 91 | 4.25 (0.50) |
| 120–139 | 171 | 5.01 (0.40) | 295 | 5.10 (0.32) |
| 140+ | 165 | 6.09 (0.48) | 223 | 5.76 (0.41) |
| P=.002 | P=.12 | |||
Abbreviations: BP, blood pressure; SBP, systolic blood pressure; SE, standard error. aNot on Antihypertensive drug therapy (n=1009) or on antihypertensive drug therapy (n=380) at baseline and at last on‐treatment visit.
SBP levels at baseline (entry to the WHI) for patients both on and not on antihypertensive drug therapy were significantly positively related to WML volumes (Table II). There was little difference in WML volumes by BP level <140 mm Hg SBP for women not on antihypertensive drug therapy. There was no difference within either the CEE+MPA or CEE‐alone arms on the volume of WML volumes by whether the women were randomized to the active or placebo group (not shown). Abnormal WML volumes were substantially greater for the CEE‐alone arm vs the CEE+MPA arm for the nonhypertensive women only (not shown). Women on antihypertensive therapy tended to have greater WML volumes than those not on antihypertensive therapy (P=.03). Note that there were only 11 women on antihypertensive therapy with SBPs <110 mm Hg (Table II).
We repeated this analysis based on the last on‐trial BP data and antihypertensive medication status (Table II). There were now 659 (47%) of the 1389 women on antihypertensive drug therapy. Approximately two thirds of the 659 women on antihypertensive drug therapy had an SBP <140 mm Hg. A higher percentage compared with baseline (223 of 378, 72.3%) of women with BP ≥140 mm Hg were on antihypertensive drug therapy. WML volumes were still significantly related to BP levels for the women not on antihypertensive drug therapy, but not among women on antihypertensive therapy.
Hypertension as defined in this study was significantly related to total WML volumes, basal ganglion lesion volumes, and number of regions containing WML volumes after adjustment for age, race, total cranial volume, time between termination of study and MRI, and clinic.
Participants who were treated for hypertension but had uncontrolled BP, defined as BP ≥140/90 mm Hg (n=172) (Table III), had the greatest amount of total WML volumes, basal ganglion lesion volumes, and number of regions containing WMLs, while women who had BP <140/90 mm Hg (with or without treatment) had the least. There were no significant differences in number of regions containing WMLs, volume of WMLs, or basal ganglion lesion volumes between untreated normotensive women and treated women, as long as the baseline BP was controlled <140/90 mm Hg. Thus, control of hypertension appeared to attenuate the adverse effects of hypertension on lesion volume and number of regions containing WML volumes. Further adjustment for smoking, CVD, and education did not impact these results. We repeated this analysis (Table III) using last on‐trial BP. Women on antihypertensive drug therapy with controlled elevated BP had the highest prevalence of white matter and basal ganglia lesion volumes, but there was no difference by BP level and treatment for number of regions containing WMLs (Table III).
Table III.
Mean (SE) Lesion Volumes by Baseline and at Last On‐Trial Hypertension/BP Control, After Adjustment for Age, Race, Treatment Assignment, Total Cranial Volume, Time Between Termination of Study and MRI, and Clinic
| No Treatment and BP Control <140/90 mm Hg (n=728) | No Treatment and BP Uncontrolled ≥140/90 mm Hg (n=281) | Treatment and BP Control <140/90 mm Hg (n=208) | Treatment and BP Uncontrolled ≥140/90 mm Hg (n=172) | Overall P Value Comparing 4 Groups | |
|---|---|---|---|---|---|
| Lesion volumes by baseline BP | |||||
| White matter lesion volume | 3.98 (0.16) | 5.17 (0.32) | 4.29 (0.32) | 6.46 (0.49) | <.0001 |
| Basal ganglia lesion volume | 0.59 (0.03) | 0.79 (0.05) | 0.61 (0.05) | 0.90 (0.07) | <.0001 |
| Number of regions containingwhite matter lesion volumes | 3.97 (0.30) | 4.17 (0.33) | 4.18 (0.34) | 4.53 (0.36) | .0345 |
| No Treatment and BP Control <140/90 mm Hg (n=570) | No Treatment and BP Uncontrolled ≥140/90 mm Hg (n=160) | Treatment and BP Control <140/90 mm Hg (n=434) | Treatment and BP Uncontrolled ≥140/90 mm Hg (n=225) | Overall P Value Comparing 4 Groups | |
| Lesion volumes by last on trial BP | |||||
| Abnormal white matter lesion volume | 4.85 (0.18) | 5.65 (0.40) | 5.92 (0.25) | 6.55 (0.39) | <.0001 |
| Abnormal basal ganglia lesion volume | 1.55 (0.03) | 1.75 (0.06) | 1.74 (0.04) | 1.79 (0.05) | <.0001 |
| Number of regions containing white matter lesion volumes | 4.81 (0.25) | 4.94 (0.29) | 5.03 (0.27) | 5.15 (0.29) | .2044 |
Abbreviations: BP, blood pressure; MRI, magnetic resonance imaging; SE, standard error.
Baseline pulse pressure was significantly related to white matter (P=.002) and basal ganglia (P=.000) volumes and number of regions containing WML volumes (P=.03). However, none of these relationships were significant when hypertension was included in the model. There was a significant interaction of pulse pressure and hypertension only for WML volumes (P=.02).
WML volumes were related to hypertension in most areas of the brain (Table IV). Lesion volumes among hypertensives and nonhypertensives were substantially greater in the frontal lobe than the occipital, parietal, or temporal lobes of the brain.
Table IV.
Mean (SE) Lesion Volumes by Hypertension Status After Adjustment for Age, Race, Total Cranial Volume, Time Between Termination of Study, and MRI
| Analysis | Hypertension (n=661) | No Hypertension (n=728) | P Value Hypertension vs No Hypertension |
|---|---|---|---|
| Left frontal lobe | 1.21 (0.05) | 0.90 (0.04) | <.0001 |
| Right frontal lobe | 1.31 (0.05) | 1.02 (0.04) | <.0001 |
| Left occipital lobe | 0.20 (0.01) | 0.19 (0.01) | .5140 |
| Right occipital lobe | 0.20 (0.01) | 0.19 (0.01) | .5837 |
| Left parietal lobe | 0.72 (0.04) | 0.55 (0.03) | .0002 |
| Right parietal lobe | 0.66 (0.03) | 0.51 (0.03) | .0011 |
| Left temporal lobe | 0.69 (0.03) | 0.54 (0.03) | <.0001 |
| Right temporal lobe | 0.62 (0.03) | 0.50 (0.02) | .0005 |
| Limbic lobe | 0.05 (0.00) | 0.03 (0.00) | .0052 |
| Corpus callosum | 0.17 (0.01) | 0.17 (0.01) | .6667 |
Abbreviations: MRI, magnetic resonance imaging; SE, standard error.
There was about a 1.5‐mm difference in SBP in women assigned to active therapy compared with those given placebo in the CEE+MPA arm and about a 1‐mm difference in the CEE‐alone active vs placebo groups during the course of the trials. 47 , 48 There were no statistically significant differences in change in SBP between hormone therapy users (active) and nonusers (placebo) in this relatively small sample size from the WHIMS‐MRI trial.
There were only 75 diabetics and no significant differences in white matter volumes by diabetes status.
Discussion
The results of the WHIMS‐MRI trial indicate that increased WML volumes were significantly related to the elevated BP at entry to the trial. These results of the WHIMS‐MRI trial are consistent with previous observations in WHI of the relationship of BP levels to risk of CVD even among women with “prehypertension.” 45
The WHI clinical trial previously reported that there was an increased risk of stroke in both women taking CEE or CEE with MPA. 47 , 48 The incidence of stroke was substantially higher in the women in the CEE‐alone compared with the CEE+MPA arms of the trial for the women both taking the active hormone therapy and placebo.
We reported that there were no significant differences between ischemic volumes and 3MSE scores at WHI baseline. Women who scored highest on the 3MSE measured closest to MRI scores had smaller mean ischemic lesion volumes. 33 Also, women who developed post‐trial MCI or dementia had larger ischemic lesion volumes. 33
The primary determinants of total WML volume were BP levels and the degree of control of BP at baseline. Women in the CEE‐alone arm in both the active and placebo groups had significantly higher WML volumes consistent with their higher levels of BP prior to randomization, as previously reported in the WHI, and greater risk of stroke. 48
The volunteer participants in the WHI clinical trial were probably more likely to be in good health, have access to the health care system, and to be adherent to various medical therapies. In spite of this, BP control at baseline was very poor, the majority of women with SBP ≥140 mm Hg were not on antihypertensive drug therapy and only just over half of those on antihypertensive therapy had SBP controlled to <140 mm Hg. There was a substantial association between the BP levels and lack of control of BP on drug therapy and the extent of white matter abnormalities. The level of the BP prior to randomization is a primary determinant of the WML volumes possibly leading to increasing risks of dementia and cognitive decline in this study. 34 , 35 The association of BP levels with white matter abnormalities years before the MRI is consistent with a long incubation period for the development of the white matter abnormalities and the observation, as previously noted, that midlife BP levels are associated with an increased risk of dementia at older ages but BP levels measured at older ages have a much weaker relationship with dementia that has its onset within a few years of the BP measurements. The association of WML volume also with BP measured at the end of the trial is a function of the high tracking of BP over time. A greater percentage of participants were on antihypertensive therapy at the last visit in the study than at entry to the trial. However, BP remained poorly controlled, as 34% of patients on BP therapy at the end of the trial still had a BP >140 mm Hg in spite of repeat BP measurements and information to the participants. Furthermore, participants with poorly controlled BP, as noted in II, III, have a higher prevalence of abnormal WML volumes. The higher prevalence of WMLs in the frontal as compared with the occipital lobes is consistent with the literature and is possibly related to differences in perfusion, especially of the frontal and posterior ventral horn. 49 , 50 , 51 This higher prevalence of white matter abnormalities in the frontal lobe is consistent with the reported characteristics of vascular‐related dementia and abnormal cognitive tests and the reported relationship of BP levels to cognitive abnormalities. 52 , 53 , 54 , 55
Study Limitations
We did not include a measure of brain infarcts on MRI. The risk factors for silent brain infarcts are very similar to white matter abnormalities, including hypertension, diabetes, and cigarette smoking. In a recent report from the Framingham Study, approximately half of the infarcts were located in the basal ganglia and 35% subcortical. 56 There is also a high correlation of white matter abnormalities and brain infarcts. 57 , 58 The separation of small infarcts from white matter abnormalities may be difficult. 59
The smaller MRI sample size as compared with the entire randomized hormone therapy trial precluded any analysis of changes in BP levels during the trial by active vs placebo treatment assignments in the hormone trial, and we therefore could not answer the question as to whether the small increase in BP among women on hormone therapy contributed to their WMLs.
Conclusions
The results of this study reinforce the importance of hypertension in etiology of WML volumes in the brain. We do not know whether treatment of elevated BP will prevent the development of white matter abnormalities, how low the BP needs to be lowered, and whether a class of antihypertensive drugs is better than other drugs. We have only suggestive evidence that the progression of WMLs can be slowed by BP‐lowering therapy.
Further clinical trials are needed to better determine whether white matter abnormalities can be prevented or progression slowed by antihypertensive therapy, the specific drug therapy, and BP levels. Whether prevention of vascular disease in the brain will reduce risk of dementia is an important unanswered question, perhaps the most important for current hypertension treatment. 60 The prevention of WMLs and then progression should therefore be a high priority in future hypertension treatment and prevention studies. Therefore, it is clear that even moderately elevated BP is associated with silent vascular disease in the brain that contributes to risk of dementia. A prudent clinical approach at present would encourage maintaining as low a BP as possible, especially beginning in young and middle ages, in order to possibly prevent dementia as well as stroke. There are no other potentially effective preventive therapies.
Disclosures: The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health and the US Department of Health and Human Services through contracts N01WH22110, 24152, 32100‐2, 32105‐6, 32108‐9, 32111‐13, 32115, 32118‐32119, 32122, 42107‐26, 42129‐32, and 44221. The Women’s Health Initiative Memory Study was funded in part by Wyeth Pharmaceuticals, Inc, St Davids, PA. ClinicalTrials.gov identifier: NCT00000611.
References
- 1. Liao D, Cooper L, Cai J, et al. The prevalence and severity of white matter lesions and hypertension, its treatment, and its control. The ARIC study. Stroke. 1996;27:2262–2270. [DOI] [PubMed] [Google Scholar]
- 2. Kuller LH, Lopez OL, Jagust WJ, et al. Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology. 2005;64:1548–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Longstreth WT, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. [DOI] [PubMed] [Google Scholar]
- 4. Skoog I. A review on blood pressure and ischaemic white matter lesions. Dement Geriatr Cogn Disord. 1998;9(suppl 1):13–19. [DOI] [PubMed] [Google Scholar]
- 5. O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney. Hypertension. 2005;46:200–204. [DOI] [PubMed] [Google Scholar]
- 6. Ikram MA, Vernooij MW, Hofman A, et al. Kidney function is related to cerebral small vessel disease. Stroke. 2008;39:55–61. [DOI] [PubMed] [Google Scholar]
- 7. Seliger SL, Longstreth WT Jr, Katz R, et al. Cystatin C and subclinical brain infarction. J Am Soc Nephrol. 2005;16:3721–3727. [DOI] [PubMed] [Google Scholar]
- 8. Kuller LH. Commentary on “Vascular cognitive impairment: today and tomorrow”. Alzheimers Dementia. 2006;2:195–197. [DOI] [PubMed] [Google Scholar]
- 9. Vermeer SE, Prins ND, Den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 10. White L, Petrovitch H, Hardman J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu‐Asia Aging Study participants. Ann NY Acad Sci. 2002;977:9–23. [DOI] [PubMed] [Google Scholar]
- 11. Schmidt R, Ropele S, Enzinger C, et al. White matter lesion progression, brain atrophy, and cognitive decline: The Austrian Stroke Prevention Study. Ann Neurol. 2005;58:610–616. [DOI] [PubMed] [Google Scholar]
- 12. Rosano C, Brach J, Longstreth WT Jr, et al. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high‐functioning older adults. Neuroepidemiology. 2005;26:52–60. [DOI] [PubMed] [Google Scholar]
- 13. Kuller LH, Longstreth WT Jr, Arnold AM, et al. White matter hyperintensity on cranial magnetic resonance imaging. A predictor of stroke. Stroke. 2004;35:1821–1825. [DOI] [PubMed] [Google Scholar]
- 14. Vermeer SE, Hollander M, Van Dijk EJ, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population. The Rotterdam Scan Study. Stroke. 2003;34:1126–1129. [DOI] [PubMed] [Google Scholar]
- 15. Kuller LH, Arnold AM, Longstreth WT Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the Cardiovascular Health Study. Neurobiol Aging. 2007;28:1307–1315. [DOI] [PubMed] [Google Scholar]
- 16. Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the United States 1999–2000. A rising tide. Hypertension. 2004;44:398–404. [DOI] [PubMed] [Google Scholar]
- 17. Goldstein LB, Adams R, Alberts MJ, et al. A guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerosis Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873–e923. [DOI] [PubMed] [Google Scholar]
- 18. Gorelick PB, William M. Feinberg Lecture: cognitive vitality and the role of stroke and cardiovascular disease risk factors. Stroke. 2005;36:875–879. [DOI] [PubMed] [Google Scholar]
- 19. Farmer ME, White LR, Abbott RD, et al. Blood pressure and cognitive performance. The Framingham Study. Am J Epidemiol. 1987;126:1103–1114. [DOI] [PubMed] [Google Scholar]
- 20. Hebert LE, Scherr PA, Bennett DA, et al. Blood pressure and late‐life cognitive function change: a biracial longitudinal population study. Neurology. 2004;62:2021–2024. [DOI] [PubMed] [Google Scholar]
- 21. Havlik RJ, Foley DJ, Sayer B, et al. Variability in midlife systolic blood pressure is related to late‐life brain white matter lesions: the Honolulu‐Asia Aging Study. Stroke. 2002;33:26–30. [DOI] [PubMed] [Google Scholar]
- 22. Kivipelto M, Helkala EL, Hänninen T, et al. Midlife vascular risk factors and late‐life mild cognitive impairment: a population‐based study. Neurology. 2001;56:1683–1689. [DOI] [PubMed] [Google Scholar]
- 23. Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu‐Asia aging Study. Neurobiol Aging. 2000;21:57–62. [DOI] [PubMed] [Google Scholar]
- 24. Kilander L, Nyman H, Boberg M, et al. Hypertension is related to cognitive impairment: a 20‐year follow‐up of 999 men. Hypertension. 1998;31:780–786. [DOI] [PubMed] [Google Scholar]
- 25. Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;122:1644–1650. [DOI] [PubMed] [Google Scholar]
- 26. Kuller LH. Dementia epidemiology research: it is time to modify the focus of research. J Gerontol A Biol Sci Med Sci. 2006;61A:1314–1318. [DOI] [PubMed] [Google Scholar]
- 27. Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double‐blind placebo‐controlled Systolic Hypertension in Europe (Syst‐Eur) trial. Lancet. 1998;352:1347–1351. [DOI] [PubMed] [Google Scholar]
- 28. Applegate WB, Pressel S, Wittes J, et al. Impact of the treatment of isolated systolic hypertension on behavioral variables. Results form the Systolic Hypertension in the Elderly Program. Arch Intern Med. 1994;154:2154–60. [PubMed] [Google Scholar]
- 29. Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET‐COG): a double‐blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. [DOI] [PubMed] [Google Scholar]
- 30. Schmidt R, Petrovic K, Ropele S, et al. Progression of leukoaraiosis and cognition. Stroke. 2007;38:2619–2625. [DOI] [PubMed] [Google Scholar]
- 31. The National Heart, Lung, and Blood Institute Working Group in Future Directions in Hypertension Treatment Trials . Major clinical trials of hypertension. What should be done next? Hypertension. 2005;46:1–6. 15911739 [Google Scholar]
- 32. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The JNC 7 Report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 33. Coker LH, Hogan PE, Bryan NR, et al. Effects of postmenopausal hormone therapy on volumetric sub‐clinical cerebrovascular disease: The Women’s Health Initiative Memory Study‐Magnetic Resonance Imaging Study (WHIMS‐MRI). Neurology. 2009;72:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women. The Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. [DOI] [PubMed] [Google Scholar]
- 35. Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. [DOI] [PubMed] [Google Scholar]
- 36. Shumaker SA, Reboussin BA, Espeland MA, et al. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19:604–621. [DOI] [PubMed] [Google Scholar]
- 37. Jaramillo SA, Felton D, Andrews LA, et al. Enrollment in a brain magnetic resonance study: results from the Women’s Health Initiative Memory Study Magnetic Resonance Imaging Study (WHIMS‐MRI). Acad Radiol. 2007;14:603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goldszal AF, Davatzikos C, Pham DL, et al. An image‐processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998;22:827–837. [DOI] [PubMed] [Google Scholar]
- 39. Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEE Trans Med Imaging. 2002;21:1421–1439. [DOI] [PubMed] [Google Scholar]
- 40. Anbeek P, Vincken KL, Van Osch MJ, et al. Automatic segmentation of different‐sized white matter lesions by voxel probability estimation. Med Image Anal. 2004;8:205–215. [DOI] [PubMed] [Google Scholar]
- 41. Yoshita M, Fletcher E, Harvey D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bryan RN, Manolio TA, Schertz LD, et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain: The Cardiovascular Health Study. Am J Neuroradiol. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 43. Lao Z, Shen D, Jawad A, et al. Automatic segmentation of white matter lesions in 3D brain MR images using multivariate patter classification. ISBI. 2006:307–310. [Google Scholar]
- 44. Moonis G, Liu D, Lao Z, et al. Comparison of an automated segmentation method to established visual criteria of measures of cerebral atrophy and white matter lesion load. (Abstract) Presented at 44th Annual American Society of Neuroradiology, San Diego, CA, April 29–May 5, 2006.
- 45. Hsia J, Margolis KL, Eaton CB, et al. Prehypertension and cardiovascular disease risk in the Women’s Health Initiative. Circulation. 2007;115:855–860. [DOI] [PubMed] [Google Scholar]
- 46. Resnick S, Davatzikos C, Espeland MA. Effects of postmenopausal hormone therapy on region‐specific lesion volumes: The Women’s Health Initiative Memory Study Magnetic Resonance Imaging Study (WHIMS‐MRI). Neurology. 2009;72:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wassertheil‐Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women. The Women’s Health Initiative. JAMA. 2003;289:2673–2684. [DOI] [PubMed] [Google Scholar]
- 48. Hendrix SL, Wassertheil‐Smoller S, Johnson KC, et al. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113:2424–2434. [DOI] [PubMed] [Google Scholar]
- 49. Holland CM, Smith EE, Csapo I, et al. Spatial distribution of white‐matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen Y‐F, Wang H, Chu Y, et al. Regional quantification of white matter hyperintensity in normal aging, mild cognitive impairment and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22:177–184. [DOI] [PubMed] [Google Scholar]
- 51. Gootjes L, Teipel SJ, Zebuhr Y, et al. Regional distribution of white matter hyperintensities in vascular dementia, Alzheimer’s disease and healthy aging. Dement Geriatr Cogn Disord. 2004;18:180–188. [DOI] [PubMed] [Google Scholar]
- 52. Ingles JL, Boulton DC, Fisk JD, et al. Preclinical vascular cognitive impairment and Alzheimer disease. Neuropsychological test performance 5 years before diagnosis. Stroke. 2007;38:1148–1153. [DOI] [PubMed] [Google Scholar]
- 53. Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117:1169–1180. [DOI] [PubMed] [Google Scholar]
- 54. Sierra C, De La Sierra A, Salamero M, et al. Silent cerebral white matter lesions and cognitive function in middle‐aged essential hypertensive patients. Am J Hypertens. 2004;17:529–534. [DOI] [PubMed] [Google Scholar]
- 55. Boone KB, Miller BL, Lesser MI, et al. Neuropsychological correlates of white matter lesions in healthy elderly subjects. Arch Neurol. 1992;49:549–554. [DOI] [PubMed] [Google Scholar]
- 56. Das RR, Seshadri S, Beiser AS, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham Offspring Study. Stroke. 2008;39:2929–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Inzitari DI, Diaz F, Fox A, et al. Vascular risk factors and leuko‐araiosis. Arch Neurol. 1987;44:42–47. [DOI] [PubMed] [Google Scholar]
- 58. Gouw AA, Van Der Flier WM, Pantoni L, et al. On the etiology of incident brain lacunes: longitudinal observations from the LADIS study. Stroke. 2008;39:3083–3085. [DOI] [PubMed] [Google Scholar]
- 59. Wardlaw JM. What is a lacune? Stroke. 2008;39:2921–2922. [DOI] [PubMed] [Google Scholar]
- 60. By the Executive Committee of Vas‐Cog on behalf of the General Assembly . Declaration of San Antonio, Texas. Neuroepidemiology. 2007;28:191–192. [Google Scholar]


