Abstract
Introduction
Long-term care facilities (LTCF) residents have been estimated to have the highest incidence of diarrheal illness among adults living in the developed world. This study describes undiagnosed diarrhea, intestinal inflammation, and Clostridium difficile colonization in a LTC population and explores whether these are associated with functional decline, as defined by weight loss or a change in cognitive or ADL status.
Methods
An observational study of a convenience sampling of residents in a 180-bed LTCF was obtained; evaluation of stool and medical records was done. Stool specimens were evaluated for consistency, gross blood, inflammation (via quantitative fecal lactoferrin, IBD-SCAN), and C difficile (via PCR for gdh). SPSS and STATA were used and significance was set at P <.05.
Results
There were 46 stools collected; 13 of the subjects were male, 28 were older than 65 years, and 35 were prescribed 5 to 15 medications. Twenty-six of the 46 stools collected had elevated quantitative fecal lactoferrin levels. Although only 5 subjects were reported to have diarrhea (4 with elevated lactoferrin), 28 stool specimens were observed to be liquid or semi-solid (19 with elevated lactoferrin), and these liquid/semisolid stools were significantly correlated with lactoferrin positivity (P = .017). In analysis of functional status, there was no statistically significant association between change in ADL (n = 17) or cognitive status (n = 5) and elevated lactoferrin. However, all 3 subjects who had significant weight loss had elevated lactoferrin, although the mean fecal lactoferrin was not statistically different from those without weight loss. Of the 2 samples with C difficile, both were liquid and, when compared with all other liquid stools (n = 22), the mean lactoferrin was statistically higher (134.1 versus 28.8 μg/mL, P = .008). These 2 subjects had neither weight loss nor change in cognitive status, but 1 had a change in ADL status.
Discussion and conclusions
Diarrhea in LTCF residents is underdiagnosed. Diarrhea and the presence of C difficile in the stool are associated with intestinal inflammation, as detected by fecal lactoferrin. With our small numbers, we were not able to identify a specific link; however, we were able to identify a correlation between weight loss and intestinal inflammation, but, with just 2 samples, not C difficile colonization. This relationship highlights the importance of larger studies to further examine the rate of diarrhea in LTCF; the effect of diarrhea and intestinal inflammation on weight loss; and the interaction of C difficile colonization with weight loss, malnutrition, and functional decline.
Keywords: Diarrhea, long-term care, Clostridium difficile, functional decline, fecal lactoferrin
Diarrhea is major cause of illness among residents of long-term care facilities (LTCF) and skilled nursing facilities. In 1993, Bennett and Greenough1 reported that “perhaps the most important risk factor for developing diarrhea for an older person is whether that individual resides in a nursing home. Among adults in the developed world, residents of nursing homes, no doubt, have the highest incidence of diarrheal illness.” A report of deaths from diarrheal illnesses from 1979 to 1987 found that 51% of mortalities occurred in those older than 74 years.2 There are many causes of diarrhea in the LTC setting; however, the most common infectious cause of nonepidemic acute diarrhea in LTC is Clostridium difficile.3,4 There are multiple population factors that contribute to these high rates, including the following:
Transfers between the LTCF and acute care hospitals introduce C difficile and other enteric pathogens into the environment on a continuous basis
Large numbers of medically frail residents with incontinence and cognitive disorders
Close quarters
Social interactions are encouraged
Antibiotic use is frequent
Staff–patient ratios are high
Infection control resources are few5
C difficile is a major cause of nosocomial diarrhea, but the full and distinct impact on LTCF residents is not fully understood. Internationally, there have been multiple recent reports of an increase in the number and severity of C difficile infections,6–8 hospitalizations,9 and C difficile–related mortality.10 Locally, we have found that the number of cases of C difficile has steadily increased since 2002 for hospitalized patients.
Although risk factors for C difficile infection in LTCF are similar to those for patients in acute care settings, immuno-compromised and elderly patients have always been at increased risk for C difficile infection. This perhaps reflects a frail population that can acquire C difficile from their environment and, in the setting of waning immunity and antibiotic use, develop symptomatic infection.5
Studies that have attempted to evaluate the prevalence of C difficile in LTCF have varied greatly in rates, methodology, and risk-factor analyses. Nonetheless, in nonepidemic settings,
Point prevalence surveys detected rates of 1.8% and 7.7%, based on positive culture or toxin assays, in a chronic care facility for older adults.11 Although many of these patients had been hospitalized previously, antibiotic use was not found to be a risk factor.
In a larger study, 70% of chronic-care ward patients and 26% of nursing home residents (overall 33% of the LTCF residents) had C difficile in their stool within 2 weeks of antibiotic treatment, but most did not have symptoms of diarrhea.12
Numerous studies from 1980s and 1990s have reported the prevalence of asymptomatic carriage among LTCF residents from 4% to 20%.3,11,13–20
C difficile infection in the elderly has also been shown to cause a protein-losing enteropathy, which increases the risk for malnutrition in an already vulnerable population.21 We evaluated the strains of C difficile present in local residents of a LTCF and to analyze the strain-specific effects of infection on mortality, nutritional status (diarrhea, weight loss, malnutrition), and quality of life/functional status. This is a pilot study to look at an asymptomatic LTCF population and determine if undiagnosed diarrhea, intestinal inflammation, and colonization can be associated with functional decline; specifically weight loss, change in activities of daily living (ADL), and change in cognitive status.
METHODS
Institutional review board exemption for this study was obtained because the data analyzed by the research group was de-identified. An observational study of a convenience sampling of residents in a 180-bed LTCF was done; 46 stool samples were collected and the medical records of those residents were evaluated. Stools were evaluated for consistency, gross blood, inflammation (via IBD Scan ELISA (TechLab, Inc.) for quantitative fecal lactoferrin), and C difficile (via polymerase chain reaction [PCR] for gdh (glutamate dehydrogenase, a C. difficile housekeeping gene) and tcdB (gene that encodes for C. difficile toxin B)). To be classified as having diarrhea, a subject would either be noted on the Minimum Data Set (MDS) to have diarrhea on “Bowel Elimination Pattern” (item H2) or the subject ’s stool specimen took the shape of the container, having liquid or semi-solid stool. Review of the medical record included the most recent MDS available for each resident and a review of the medical chart was done by the facility staff. MDS item B6 was used to evaluate for recent change in cognitive status. The statement of B6 is as follows:
Resident ’s cognitive status, skills, or abilities have changed as compared to status of 90 days ago (or since last assessment if less than 90 days) had the possible replies as follows:
No change
Improved
Deteriorated.22
Only subjects noted to have deteriorated cognitive status via MDS were categorized as having a change in cognitive status for study purpose. Similarly, item G9 was used to evaluate for change in ADL function, with identical categorizations. SPSS (SPSS, Inc., Chicago, IL) and STATA (StataCorp LP, College Station, TX) were used for analysis and significance was set at P less than .05.
RESULTS
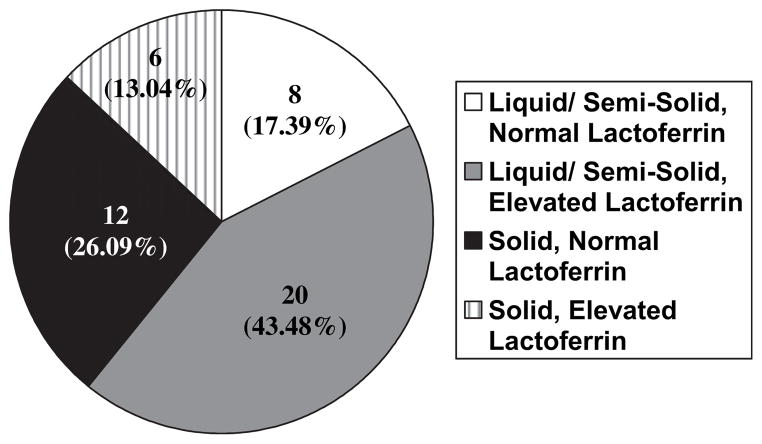
See Table 1 for detailed demographics. Of the 46 residents investigated, only 5 were reported to have diarrhea via MDS. Of those 5 samples, 4 were positive for quantitative fecal lactoferrin. However, on examination of the samples, 28 stools were liquid or semi-solid and 19 of these samples were positive for quantitative fecal lactoferrin (see Figure 1).
Table 1.
Available Demographics (N = 46)
| Gender | 34 Female |
| Age | 28 ≥ 65 years old |
| No. of medications | 35 prescribed 5–15 medications |
| 11 prescribed >15 medications |
Fig. 1.
Stool consistency and quantitative fecal lactoferrin status.
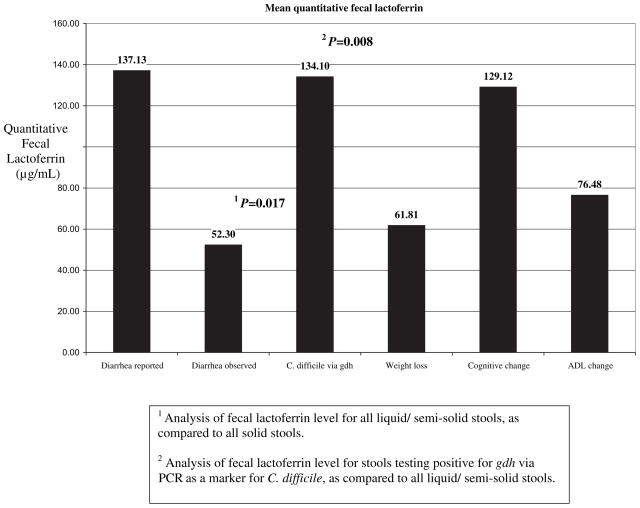
Liquid/semisolid stools were significantly correlated with positive quantitative fecal lactoferrin (P = .017). Of the 46 stools collected, 26 had elevated quantitative fecal lactoferrin levels. See Figure 2 for details of mean values of quantitative fecal lactoferrin and Table 2 for detailed lactoferrin levels by subset of our population. There was no statistically significant association between change in ADL (n = 17) or cognitive status (n = 5) and quantitative fecal lactoferrin levels. However, each of the 3 subjects who had significant weight loss had elevated quantitative fecal lactoferrin levels, although mean lactoferrin level was not statistically different from those without weight loss. Of the 2 samples with C difficile, both were liquid and, when compared with all other liquid stools (n = 22), the mean lactoferrin was statistically higher (134.1 versus 28.8 μg/mL, P = .008). Each of the 2 samples that were positive for gdh were negative for toxin B (via tcdB). See Table 3 for details.
Fig. 2.
Mean levels of quantitative fecal lactoferrin.
Table 2.
Quantitative Fecal Lactoferrin for Each Subset Population
| Diarrhea Reported | Diarrhea Observed | Clostridium difficile via PCR for gdh | Weight Loss | Cognitive Change | ADL Change | |
|---|---|---|---|---|---|---|
| No. of samples total | 5 | 28 | 2 | 3 | 5 | 17 |
| No. of samples with elevated fecal lactoferrin | 3 | 19 | 2 | 3 | 2 | 10 |
| Minimum level fecal lactoferrin (μg/mL) | 137.13 | 10.2 | 14.29 | 13.78 | 99.37 | 11.41 |
| Mean level fecal lactoferrin (μg/mL) | 14.00 | 52.3 | 134.10 | 61.87 | 129.12 | 76.48 |
| Maximum level fecal lactoferrin (μg/mL) | 253.00 | 254.00 | 253.95 | 152.76 | 158.86 | 253.95 |
ADL, activity of daily living; PCR, polymerase chain reaction.
Table 3.
Description of Subjects with Weight Loss and C difficile in Stool
| Subjects with C difficile (n = 2) |
|---|
| Age < 65 years old and 85 years old |
| Male and female |
| 9 and 8 medications prescribed |
| Housed on different wards |
| Both had liquid stools |
| 1 had change in ADL status |
| Neither had weight loss or cognitive change |
| Statistically higher fecal lactoferrin than all other subjects (134.1 versus 28.8 μg/mL; P =.008) |
ADL, activity of daily living.
DISCUSSION
One of the limitations of this study is the level of detail available from the MDS data. All data were recorded for clinical purposes and not for this research study. Changes in cognitive status and ADL function were based on a subjective assessment by LTCF staff and not further characterized in the MDS. Residents who had functional or cognitive changes, but had not been assessed via the MDS during the study period, would be missed. Additionally, incomplete or inaccurate reporting of changes in cognitive status and ADL function on the MDS also limits this study. Given the underrepresentation of diarrhea on the MDS, it may correlate that changes in ADL and/or cognitive status were also under-represented. The ability to generalize this study is further limited by the fact that this was a convenience sampling of residents of a single facility during a nonepidemic time.
This study finds that diarrhea is underdiagnosed in our LTCF. Of the patients who were found to have diarrhea, 26% had elevated quantitative fecal lactoferrin, as a marker of intestinal inflammation. This could skew our results that patients with multiple stools may have been more likely to have a stool sample submitted. However, because of the limited scope of this project, we were unable to obtain a stool specimen from every resident in the facility. Such studies will be important to define the frequency, causes, and impact of diarrhea and intestinal inflammation in the elderly.
CONCLUSIONS
Diarrhea in LTCF residents is underdiagnosed. Diarrhea is a major cause of morbidity and mortality for children and the elderly. In 1992, Gangarosa et al23 evaluated the case-fatality rate for diarrhea. They found that, “while children aged less than 5 years and adults aged 60 years or more each comprised one fourth of hospitalizations involving gastroenteritis, the older group represented 85% of diarrheal deaths. Age was the most important risk factor for death subsequent to a hospitalization involving gastroenteritis (odds ratio = 52.6, 95% confidence interval 37.0–76.9 for age greater than or equal to 70 years vs. less than 5 years).” In the May 2009 issue of this Journal, in an article entitled “Diarrhea in Long-Term Care: A Messy Problem,” Drs. Morley and Steinberg24 describe that “diarrhea is a major and often under-recognized problem in long-term care. It is a major cause of morbidity (especially weight loss, dehydration, and delirium) and mortality, as well as being costly.” Our study reemphasizes that in our facility, diarrhea was a substantially underdiagnosed problem.
In this study, diarrhea, even without C difficile in the stool, was associated with intestinal inflammation, as detected by fecal lactoferrin. This suggests that the diarrhea seen in this study was not medication or diet related, but reflected an underlying inflammatory pathology. Despite the small sample size, we are able to identify a correlation between weight loss and intestinal inflammation; although with our small numbers, not C difficile colonization. This relationship highlights the importance of examining further the rate of diarrhea in LTCF; the effect of diarrhea and intestinal inflammation on weight loss; and the interaction of C difficile colonization with weight loss, malnutrition, and functional decline.
Acknowledgments
A special thank you to Jonathan Evans, MD, Suzann Williams-Rosenthal, NP, and Suparat Phisaiphanth for their assistance with stool and data collection.
Footnotes
These data were presented as an abstract at the American Medical Directors Association Convention in Charlotte, NC, in March, 2009 and published as follows: Archbald-Pannone L, Sevilleja J, Evans J, Guerrant R. Asymptomatic C. difficile colonization and weight loss among LTCF residents. J Am Med Dir Assoc 2009;10(3):B5–B5.
Richard Guerrant, MD, licensed fecal lactoferrin testing to Techlab, Inc., Blacksburg, VA. He is cofounder of A1Glutamine, LLC and on the Probiotics Scientific Advisory Board for Danone-Yakult. All other authors have stated there are no disclosures to be made that are pertinent to this article.
References
- 1.Bennett RG, Greenough WB., 3rd Approach to acute diarrhea in the elderly. Gastroenterol Clin North Am. 1993;22:517–533. [PubMed] [Google Scholar]
- 2.Lew JF, Glass RI, Gangarosa RE, et al. Diarrheal deaths in the United States, 1979 through 1987. A special problem for the elderly. JAMA. 1991;265:3280–3281. 3281, 3283, 3284. [PubMed] [Google Scholar]
- 3.Simor AE, Bradley SF, Strausbaugh LJ, et al. Clostridium difficile in long-term–care facilities for the elderly. Infect Control Hosp Epidemiol. 2002;23:696–703. doi: 10.1086/501997. [DOI] [PubMed] [Google Scholar]
- 4.Makris AT, Gelone S. Clostridium difficile in the long-term care setting. J Am Med Dir Assoc. 2007;8:290–299. doi: 10.1016/j.jamda.2007.01.098. [DOI] [PubMed] [Google Scholar]
- 5.Rao A, Bradley S. Clostridium difficile in older adults and residents of long-term care facilities. Annals of Long-Term Care. 2003;11:42–47. [Google Scholar]
- 6.Stubbs S, Rupnik M, Gibert M, et al. Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. FEMS Microbiol Lett. 2000;186:307–312. doi: 10.1111/j.1574-6968.2000.tb09122.x. [DOI] [PubMed] [Google Scholar]
- 7.Terhes G, Urban E, Soki J, et al. Community-acquired Clostridium difficile diarrhea caused by binary toxin, toxin A, and toxin B gene-positive isolates in Hungary. J Clin Microbiol. 2004;42:4316–4318. doi: 10.1128/JCM.42.9.4316-4318.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 9.Zilberberg MD. Clostridium difficile–related hospitalizations among US adults. Emerg Infect Dis. 2006;2009(15):122–124. doi: 10.3201/eid1501.080793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile–related mortality rates, United States, 1999–2004. Emerg Infect Dis. 2007;13:1417–1419. doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cefai C, Elliot TS, Woodhouse KW. Gastrointestinal carriage rate of Clostridium difficile in elderly, chronic care hospital patients. J Hosp Infect. 1988;11:335–339. doi: 10.1016/0195-6701(88)90086-2. [DOI] [PubMed] [Google Scholar]
- 12.Thomas D, Bennett R, Laughon B, et al. Postantibiotic colonization with Clostridium difficile in nursing home patients. J Am Geriatr Soc. 1990;38:415–420. doi: 10.1111/j.1532-5415.1990.tb03539.x. [DOI] [PubMed] [Google Scholar]
- 13.Simor AE, Yake SL, Tsimidis K. Infection due to Clostridium difficile among elderly residents of a long-term-care facility. Clin Infect Dis. 1993;17:672–678. doi: 10.1093/clinids/17.4.672. [DOI] [PubMed] [Google Scholar]
- 14.Monsieur I, Mets T, Lauwers S, et al. Clostridium difficile infection in a geriatric ward. Arch Gerontol Geriatr. 1991;13:255–261. doi: 10.1016/0167-4943(91)90047-t. [DOI] [PubMed] [Google Scholar]
- 15.Walker KJ, Gilliland SS, Vance-Bryan K, et al. Clostridium difficile colonization in residents of long-term care facilities: Prevalence and risk factors. J Am Geriatr Soc. 1993;41:940–946. doi: 10.1111/j.1532-5415.1993.tb06759.x. [DOI] [PubMed] [Google Scholar]
- 16.Fulton JD, Fallon RJ. Is Clostridium difficile endemic in chronic-care facilities? Lancet. 1987;2:393–394. doi: 10.1016/s0140-6736(87)92409-3. [DOI] [PubMed] [Google Scholar]
- 17.Campbell RR, Beere D, Wilcock GK, Brown EM. Clostridium difficile in acute and long-stay elderly patients. Age Ageing. 1988;17:333–336. doi: 10.1093/ageing/17.5.333. [DOI] [PubMed] [Google Scholar]
- 18.Corrado OJ, Mascie-Taylor BH, Hall MJ, Bolton RP. Prevalence of Clostridium difficile on a mixed-function ward for the elderly. J Hosp Infect. 1990;21:287–292. doi: 10.1016/0163-4453(90)94005-k. [DOI] [PubMed] [Google Scholar]
- 19.Bentley DW. Clostridium difficile-associated disease in long-term care facilities. Infect Control Hosp Epidemiol. 1990;11:434–438. doi: 10.1086/646204. [DOI] [PubMed] [Google Scholar]
- 20.Larson E, Bobo L, Bennett R, et al. Lack of care giver hand contamination with endemic bacterial pathogens in a nursing home. Am J Infect Control. 1992;20:11–15. doi: 10.1016/s0196-6553(05)80118-x. [DOI] [PubMed] [Google Scholar]
- 21.Rybolt AH, Bennett RG, Laughon B, et al. Protein-losing enteropathy associated with Clostridium difficile infection. Lancet. 1989;1:1353–1355. doi: 10.1016/s0140-6736(89)92803-1. [DOI] [PubMed] [Google Scholar]
- 22.Minimum Data Set (MDS)- Version 2.0 For Nursing Home Resident Assessment and Care Screening. CMS; Sep, 2000. [Google Scholar]
- 23.Gangarosa R, Glass R, Lew J, Boring J. Hospitalizations involving gastroenteritis in the United States, 1985: The special burden of the disease among the elderly. Am J Epidemiol. 1992;135:281–290. doi: 10.1093/oxfordjournals.aje.a116282. [DOI] [PubMed] [Google Scholar]
- 24.Morley JE, Steinberg KE. Diarrhea in long-term care: A messy problem. J Am Med Dir Assoc. 2009;10:213–217. doi: 10.1016/j.jamda.2009.01.007. [DOI] [PubMed] [Google Scholar]