Abstract
Anterograde amnesia is characterised by a profound inability to retain new information. Recent research suggests that at least some of this severe memory impairment may be the product of retroactive interference. What specifically interferes with memory in amnesic patients however remains unknown. Here we reveal a substantial non-specific retroactive interference effect in densely amnesic patients: Whereas 7 out of 10 amnesic patients were able to recall some prose material following an unfilled delay period, only 1 of them was able to recall any material after a delay period in which they were required to listen for piano notes. The data reveal that some amnesic patients have the capacity to retain new material for much longer than usual but that any new post-learning information profoundly interferes with such retention. This non-specific retroactive interference effect deviates from the item-specific interference effect that is typically assessed in clinical practice, and which is frequently observed in patients with executive impairment. We hypothesise that these interference effects are qualitatively different, occurring during distinct memory processes, namely retrieval (item-specific interference) and consolidation (non-specific interference).
Keywords: Amnesia, forgetting, retroactive interference, consolidation
Introduction
Anterograde amnesia patients present with an inability to remember explicitly events and information experienced only moments before. Here we show that at least some of these patients benefit profoundly from the removal of all material in the 10 min following prose learning, whereas even a distracting task very different from the memoranda, tone detection, greatly exacerbates the amnesia.
Over the last 50 years each of the main memory processes - encoding, consolidation, storage and retrieval - has been considered as a potential locus of impairment underlying anterograde amnesia (see Kopelman, 2002 for a review). However, none of the resulting hypotheses have been able to provide a sufficiently solid account of the impaired and spared functions that are observed in the majority of patients with anterograde amnesia. Pioneer work on the patient H.M. led Milner (1966) to propose that amnesic patients were wholly unable to transfer any new information from short term memory (STM) to long term memory (LTM). This general consolidation deficit theory was however soon dismissed on the grounds that it could not account for amnesic patients’ spared procedural long term memory formation (Milner et al., 1968), their ability to identify previously presented fragmented pictures and words following long delays (Warrington and Weiskrantz, 1968) and their improved test recall performance when cues (e.g. the first letter of a word) were provided (Warrington and Weiskrantz, 1970). The latter findings led Warrington and Weiskrantz (1970) to put forward that amnesic patients could consolidate new memories but that they were unable to access these unless retrieval cues were provided which sufficiently differentiated the to-be-retrieved items from competing stored items. Later work by themselves however showed that minimisation of competing responses did not improve retention in amnesic patients (Warrington and Weiskrantz, 1978). It has since become evident that the memory improvement observed in Warrington and Weiskrantz’ work on fragmented pictures and cued recall tests in fact reflected the patients’ intact implicit memory formation capacity (c.f. Graf et al., 1984) rather than the ameliorated retrieval of explicit memories.
It could be argued that amnesic patients are unable to retrieve information, whether cues are present or absent. However, it would be difficult reconcile such theory with the intact retrieval of remote memories that is typically observed in patients with anterograde amnesia (Squire, 1980; Wilson, 1987). This latter finding is more indicative of an impairment in the initial processing of new explicit information. Butters and Cermak (1975) proposed that patients with anterograde amnesia might fail to spontaneously encode new perceptual input in a meaningful (i.e. semantic) and thorough manner, thus resulting in the formation of weaker memory traces. This theory was however based on patients with Korsakoff syndrome and thus was largely unrepresentative of patients with other aetiologies. Moreover, work has since indicated that Korsakoff patients as well as amnesic patients with other aetiologies do spontaneously encode new information in a meaningful way (c.f. Mayes et al., 1993), and that even in Korsakoff patients, ‘deep’ semantic encoding only leads to minimal memory benefits, no larger than those observed in neurologically intact individuals (e.g. Mayes et al., 1978; 1980). A semantic encoding deficit is thus an unlikely cause of anterograde amnesia.
Huppert and Piercy (1978) instead suggested that amnesia in Korsakoff patients was the result of an acquisition deficit. They showed in a recognition test that forgetting rates did not differ between Korsakoff patients and controls if initial memory performance was equated for the two groups by increasing the presentation duration for each stimulus in the patient group. The amnesic patient H.M. however showed faster forgetting than controls even when his initial recognition performance matched that of the controls, suggesting a cognitive difference between patients with medial temporal and diencephalic lesions (Huppert and Piercy, 1979). Other researchers however failed to replicate this finding. Work by Freed et al. (1987) for example indicates that H.M. showed normal forgetting if his initial 10 minute recognition performance was equated to that of controls via extended stimulus exposure. This work implies that, on the whole, anterograde amnesia is likely to result from an impairment in the initial acquisition, i.e. formation of new explicit memories (Squire, 1980; Kopelman, 2002) rather than from an encoding, retrieval or storage deficit, and that residual explicit memory formation capacities might benefit from extended periods of learning (Reed at al., 1997). This said, it should be highlighted that such increases in explicit memory via extended stimulus presentation are restricted to delayed recognition – amnesic patients’ delayed free recall does not appear to increase via such method (Kopelman and Stanhope, 1997; Isaac and Mayes, 1999).
In contrast, recent clinical observations and work have shown that free delayed recall does increase substantially in some amnesic patients via the removal of post-learning stimuli (Cowan et al., 2004; Della Sala et al., 2005). In everyday life the learning of new material is almost invariably followed by further information or activity. The same applies to the clinical assessment of anterograde memory capacity, which is typically tested via the recall of recently presented material following a delay interval filled with cognitive testing. Within clinical practice such interpolated cognitive testing is utilised (a) to create a temporal delay and (b) to block any potential conscious working memory rehearsal that amnesic patients might attempt in order to maintain to-be-retained material. It has however generally been overlooked that such interpolated cognitive testing might in fact also block LTM processing in amnesic patients.
Cowan et al. (2004) recently examined this possibility by testing 6 severely amnesic patients’ delayed word list recall following a 10 min standard delay (filled with cognitive testing) as well as following a 10 min unfilled delay, during which patients rested alone in a dark, quiet testing room. Whereas 2 patients showed no retention following either delay, 4 patients performed substantially better following the unfilled delay (49% retention) than the standard filled delay (14% retention). These 4 patients also performed significantly better following an unfilled delay (79% retention) than a filled delay (7% retention) when the duration of the delay was increased to 1 h and when the to-be-retained material consisted of prose passages.
Given the long delay intervals and the finding that some patients remembered well even after sleeping through at least part of the retention interval, it seems improbable that the observed memory improvement was the mere product of continuous rehearsal within intact STM (Cowan et al., 2004). Instead, it appears that the unfilled delay enabled a LTM process to function in these amnesic patients, and thus that interpolated cognitive testing, and post-learning material in general, in fact has a direct effect on forgetting in such patients.
Here we further examine this novel ‘retroactive interference’ hypothesis of amnesia (Cowan et al., 2004). In particular, we question what specifically interferes with memory in amnesic patients.
The term ‘retroactive interference’ (die ‘rückwirkende Hemmung’) was coined in 1900 by Georg Müller, an experimental psychologist, and Alfons Pilzecker, a medical student and former doctoral student of Müller’s (Lüer, 2007). They defined retroactive interference as memory interference by any post-learning material (see Dewar et al. 2007 and Wixted, 2004).
Today, however, the term is mainly used to refer to interference of previously learned material by more recently learned, highly similar material (c.f. McGeoch and McDonald, 1931; Mensink and Raajmakers, 1988; Anderson, 2003). Such item-specific interference can also be produced by highly similar material that was learned prior to the to-be-retrieved stimuli, and is referred to as proactive interference. Various clinical tools exist to check for an increased susceptibility to such item-specific retroactive or proactive interference (e.g. The Rey Auditory Verbal Learning Test and the California Verbal Learning Test).
Indeed, research has shown that some patients with subtle memory impairment associated with executive dysfunction present with an increased susceptibility to such item-specific interference (e.g. Shimamura et al., 1995; Baldo and Shimamura, 2002). For example, Shimamura et al. (1995) report that in their dysexecutive patients the learning of a list of paired associates such as ‘lion-hunter’ interfered substantially with the subsequent learning of a second list of paired associates, in which the cue word matched that of the first list, e.g. ‘lion-circus’. Their work hints that such increased interference also occurs in dysexecutive patients when to-be-retained information is followed by, rather than preceding, highly similar material (i.e. item-specific retroactive interference).
A heightened susceptibility to item-specific interference has, in the past, also been put forward as a possible cause of the severe memory impairment observed in anterograde amnesia (Warrington and Weiskrantz, 1970; 1974). However, as noted above, this interference hypothesis was later rejected by Warrington and Weiskrantz (1978) themselves on the grounds that minimising potential competing responses did not ameliorate retention in patients with anterograde amnesia, and that proactive interference effects were not larger for the patients than the controls on the first learning trial of new material.
A study by Mayes et al. (1994) provides further evidence against such an item-specific retroactive interference hypothesis of anterograde amnesia. They asked amnesic patients to recall sets of 10 photos of faces following a 12 min delay interval. During the delay interval participants were either presented with further sets of photos of faces (i.e. item-specific interference) or were ‘engaged in conversation and other activities (not involving faces)’ (p.549) (i.e. non-item-specific interference). The patients performed significantly poorer than the controls following both delays. Moreover, the difference in retention between the two conditions was equivalent in the amnesic and control group, leading Mayes et al. (1994) to conclude that there was no evidence that their amnesic patients were ‘more susceptible to the type of sustained retroactive interference’ (p.558) applied in their study.
Importantly however, it is possible that both delay conditions could have, to some extent, interfered with Mayes et al.’s (1994) patients’ memory because of non-specific retroactive interference, in which case the effect would be obscured for lack of the appropriate no-interference control condition.
The aim of the present study was to examine this possibility. In particular, we sought to investigate whether or not highly dissimilar post-learning material would interfere with amnesic patients’ memory. We therefore compared amnesic patients’ prose retention following an unfilled delay with that following a delay in which they were required to listen for piano notes.
Material and Methods
Participants
We tested 10 amnesic patients (7m/3f, mean age = 41.90 years, age range = 20 – 72 years; mean education = 12 years, education range = 8 – 17 years) and 10 age and education matched controls (5m/5f, mean age = 43.90 years, age range = 21 – 74 years; mean education = 14.50 years, education range = 8 – 20 years) (see Table 1). Four patients (P1–P4) and four controls (C1–C4) were British (tested at the University of Edinburgh), and six patients (P5–P10) and six controls (C5–C10) were Italian (tested in the rehabilitation unit in Somma Lombardo). Patients and controls were matched for age and education on a one-to-one basis.
Table 1.
Selected demographic, anatomical and neuropsychological measures for each patient
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 67 | 72 | 49 | 30 | 43 | 25 | 32 | 20 | 34 | 47 | |
| Education (years) | 10 | 17 | 12 | 11 | 8 | 15 | 16 | 11 | 12 | 8 | |
| Sex | m | M | m | f | f | m | m | m | m | f | |
| Aetiology | s | Le | le | an | an | h | h | h | h | an | |
| Known lesion sites | LO, LT (Lhc), LTh |
LRT(LRhc) | LRT(LRhc) | None detected |
LRF | RTh | LRF, LT, RP |
LT, RF | RF | Diffuse atrophy |
|
| Years(y)/months(m) since damage | 1y | 2y 2m | <1m | Since birth | 8m | 1y 8m | 6y 1m | 2y 2m | 6y 4m | 1y 2m | |
| Rivermead screening | 0* | 0* | 0* | 2* | 2* | 2* | 2* | 2* | 3* | 3* | [1, 2] |
| Rivermead classification | Very severe |
Very severe |
Very severe |
Very severe |
Very severe |
Very severe |
Very severe |
Very severe |
Severe | Severe | [1, 2] |
| Word list learning – Total immediate |
34* | 53* | 58* | 48* | 44 | 31* | 46 | 45 | 52 | 48 | P1–P4: [3]^; P5–P10: [4]^ |
| Word list learning – Delayed Recall |
1* | 1* | 0* | 0* | 0* | 1.05* | 0* | 0* | 0* | 0* | P1–P4: [3]^; P5–P10: [4]^ |
| Rey Figure Copy | 25.4* | 34 | 29.58 | 35 | 36 | 36 | 36 | 36 | / | 36 | [5]^ |
| Rey Figure Delayed | 10.53 | 0* | 0* | 0* | 3.75* | 1.75* | 0* | 2.5* | / | 0* | [5]^ |
| Digit span | 6 | 5 | 6 | 6 | 4 | 4 | 5.25 | 5.25 | 5.5 | 4 | P1-4: [6]#; P5–P10: [7]^ |
| Corsi span | 6 | 4 | 6 | / | 4 | 3.25 | 3.25 | 3.25 | 2.5* | 4 | P1-4: [6]#; P5–P10: [7]^ |
| Frenchay Aphasia Screening Test | 28 | 30 | 30 | / | [8]# | ||||||
| Boston Naming Test | 42 | 49 | 51 | 45 | 49 | 53 | [9]^ | ||||
| Verbal reasoning | 5* | 30 | 12 | 15 | 46.25 | 52.5 | 49.25 | 52.6 | 42.25 | 44.5 | P1-4: [6]#, P5–10: [4]^ |
| Non-verbal reasoning | 31 | 35 | 32 | / | 23 | 23 | 24 | 29 | 26 | 27 | [10]# |
| Trail Making (B-A) | 82 | 275* | 46 | 70 | 644* | 157 | 99 | 42 | / | 86 | [11]^ |
Aetiology: an = anoxia, h = head injury, le = limbic encephalitis, s = stroke.
Lesion site: L = left, R = right, F = frontal, O = occipital, P = parietal, T = temporal, hc = hippocampus, Th = thalamus, according to CT or MRI.
Score below/above cut-off;
data corrected for age and education,
raw data
/ P4 was reluctant to undergo further testing, P9 could not copy the Rey Figure or do the trail making test due to a motor impairment
1.Wilson et al., 1985; 2 Brazzelli et al., 1993; 3. Buschke, 1991; 4. Spinnler and Tognoni, 1987; 5. Caffarra et al., 2002; 6. Wechsler, 1997; 7. Novelli et al., 1986; 8. Enderby et al.,1987; 9. Kaplan et al., 1983; 10. Basso et al., 1987; 11. Giovagnoli et al., 1996.
All patients except P3 were outpatients. None of the patients had any known pre-morbid psychiatric or neurological histories. Four of the patients had closed-head injuries (P6, P7, P8 and P9), two had been affected by anoxia following cardiac arrest (P5 and P10), one by probable birth anoxia (P4), one by a stroke (P1), one by limbic encephalitis (P2) and the other by probable limbic encephalitis (P3). CT or MRI scans indicated probable lesion sites (see Table 1).
The selection criteria were the same as those used by Cowan et al. (2004) and included the following: (1) Complaints by family members of an abrupt onset of memory loss as the main symptom; (2) classification as amnesic according to the Rivermead Behavioural Memory Test (Wilson et al., 1985, Brazzelli et al., 1993); (3) performance below cut-off for normality in verbal delayed recall (Buschke, 1991; Spinnler and Tognoni, 1987) and non-verbal delayed recall (Caffarra et al., 2002); (4) normal performance in verbal and non-verbal short term memory tasks (Wechsler, 1997; Novelli et al., 1986); (5) score within the normal range on an aphasia test including comprehension (Kaplan et al., 1983; Enderby et al., 1987); (6) scores within the normal range in verbal reasoning (Spinnler and Tognoni, 1987; Wechsler, 1997), (7) scores within the normal range in non-verbal reasoning (Basso et al., 1987) (see Table 1).
The study was approved by the local Ethical Committee, and informed consent was obtained from each participant according to the Declaration of Helsinki.
Procedure
The experimental testing included four trials, in each of which all participants were verbally presented with a prose passage by the experimenter. Participants were instructed to attend to the story and try to remember as much of it as possible for subsequent immediate recall. The four prose passages were taken from the Rivermead Behavioural Memory Test (Wilson et al., 1985; Brazzelli et al., 1993) and contained 21 ‘ideas’ each. Prose presentation was followed by free immediate verbal recall in each of the 4 trials. The end of immediate recall marked the start of a 10-min delay interval, which was always followed by delayed recall.
The critical manipulation occurred during the delay interval, which was either unfilled (Trials 1 and 3) or filled (Trials 2 and 4).
Unfilled Delay Condition
Following immediate recall participants were asked to rest in the room for a short duration while the experimenter left the room to set up the next part of the study. The experimenter subsequently left the room and dimmed the lights (having previously informed the participants that she would do so), returning 10 min later.
Filled Delay Condition – Tone Detection
Following immediate recall the participant engaged in a tone detection task. The stimuli consisted of a 10-min sound track of brown noise (random noise akin to the sound of a distant waterfall), within which a piano note was randomly embedded on 50 occasions. While the note remained the same throughout the track, its loudness varied (i.e. its decibel level was reduced by either 13 dB, 22 dB, 24 dB or 36 dB). The track was played back digitally on a laptop via E-prime (Psychology Software Tools, Inc.) and presented to the participants via headphones. Participants were given a PC mouse and required to press the left mouse button whenever they heard the piano note. Number of mouse presses in each trial was recorded by E-prime.
In order to minimise any extra interference, full instructions for the tone detection task as well as a 1 min practise trial were given prior to commencement of Trials 2 and 4: Participants were informed that they would hear a waterfall sound, within which a piano note was embedded on numerous occasions. They were told that the note would be easy to hear on some occasions, and a little harder to hear on other occasions, and thus that they should attend well in order to be able to hear as many of the notes as possible. A brief reminder, not longer than the instructions given prior to the unfilled delay, was provided before the 10 min tone detection delay.
In Trials 1 and 2 participants were not informed that they would be asked to recall the prose passage again after the delay. After the delay in Trial 1 the experimenter stated that there had been a problem with the tape recorder during prose recall before the delay, and asked the participant to try again to recall as much as possible. This was done in order to minimise any suspicion regarding delayed recall in Trial 2, in which the experimenter simply asked the participant to recall again as much as possible following the delay.
Only 2/6 of Cowan et al.’s (2004) patients indicated that they had attempted subvocal rehearsal of the to-be-retained information when asked following testing. These patients may have been more able to remember delayed recall in previous trials or at least suspected that it would occur in future trials. In the present study we sought to minimise any variance in proportion retention that could result from the use of rehearsal in some but not other participants due to varying degrees of insight into the study. Thus, in Trials 3 and 4, all participants were informed, after immediate recall, that delayed recall would follow.
It was further reasoned that such procedure would allow for the examination of delayed recall following an unfilled delay as well as following tone detection under both ‘incidental’ conditions (when explicit rehearsal would be assumed to be unlikely) as well as ‘intentional’ conditions (when explicit rehearsal might occur).
Order and counterbalancing
The variation in lesion loci and sizes among the patients within the present sample could result in behavioural differences at test. In order to ensure that any such behavioural differences were the product of lesion variations rather than variations in trial order, the order of trials with and without interference was kept constant across participants as noted above. The order of prose passages was, however, counterbalanced across participants (A – B – C – D and B – A – D – C).
Prose Scoring
Only story ideas that were recalled verbatim or close synonyms were scored as correct. Scoring took place during testing and was subsequently checked against a tape recording after testing had been completed. The recordings were also scored by a second rater who was blind to the experimental conditions and aims. The resulting interrater-reliability was very high (1.00 for immediate and delayed recall in both the unfilled and the tone detection condition in the patient group).
Statistical analyses
Memory
As in Cowan et al. (2004) and Della Sala et al. (2005) a proportion retention score was computed for each participant for each of the 4 trials by dividing the number of correct story ideas recalled at delayed recall by the number of correct story ideas recalled at immediate recall in the same trial. Such procedure controls for potential individual and group differences as well as any inter-trial variation at immediate recall. Other studies have utilised extended and/or repeated stimulus presentation in the patient group to equate patient and control immediate recall levels. We chose not to do so on the grounds that it could prove difficult to disentangle the relative beneficial effects of minimal interference and such techniques, and that the groups would be unmatched in the number of experimental manipulations (i.e. only the patients would be exposed to both extended/repeated stimulus presentation and the unfilled delay).
Any story ideas recalled at delayed recall but not immediate recall were included in the delayed recall total, though this occurred rarely (if the delayed recall score exceeded the immediate recall score – as was the case in 3 controls – a maximum proportion retention score of 1 was used). A mixed factor ANOVA on proportion retention with within subjects factors delay condition (unfilled vs. tone detection) and rehearsal condition (incidental vs. intentional) and between subjects factor group (patients vs. controls) was run to examine the effects of the delay condition and rehearsal condition in the patient and control samples. Further examination of the memory data was undertaken via additional ANOVAs.
Tone detection performance
A tone-detection mean score (i.e. number of mouse presses) was computed for each participant from the two tone detection trials. A one-way ANOVA was run to examine whether or not the groups differed in their tone detection performance. Moreover, Pearson correlations were utilized to check for potential trade-off effects between memory and tone detection performance.
Executive function and memory performance
In order to examine a potential relationship between performance levels in the memory test and executive function, Pearson correlations were run between the patients’ trail making score as well as their experimental memory scores. The alpha level was set to 0.05 for all analyses.
Results
Memory performance
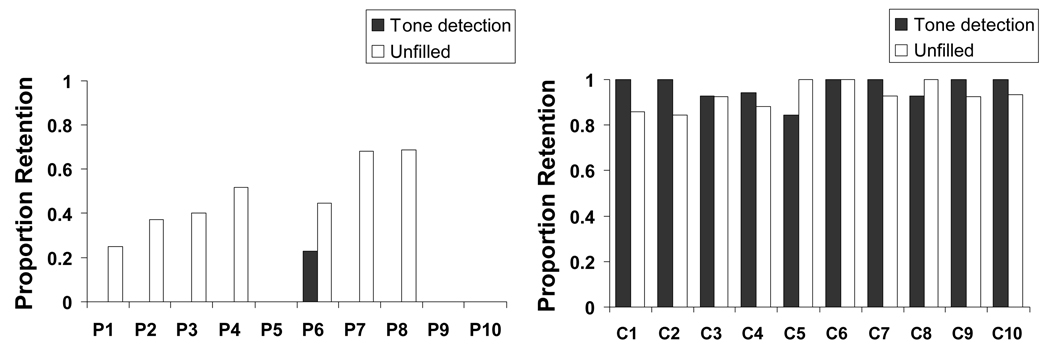
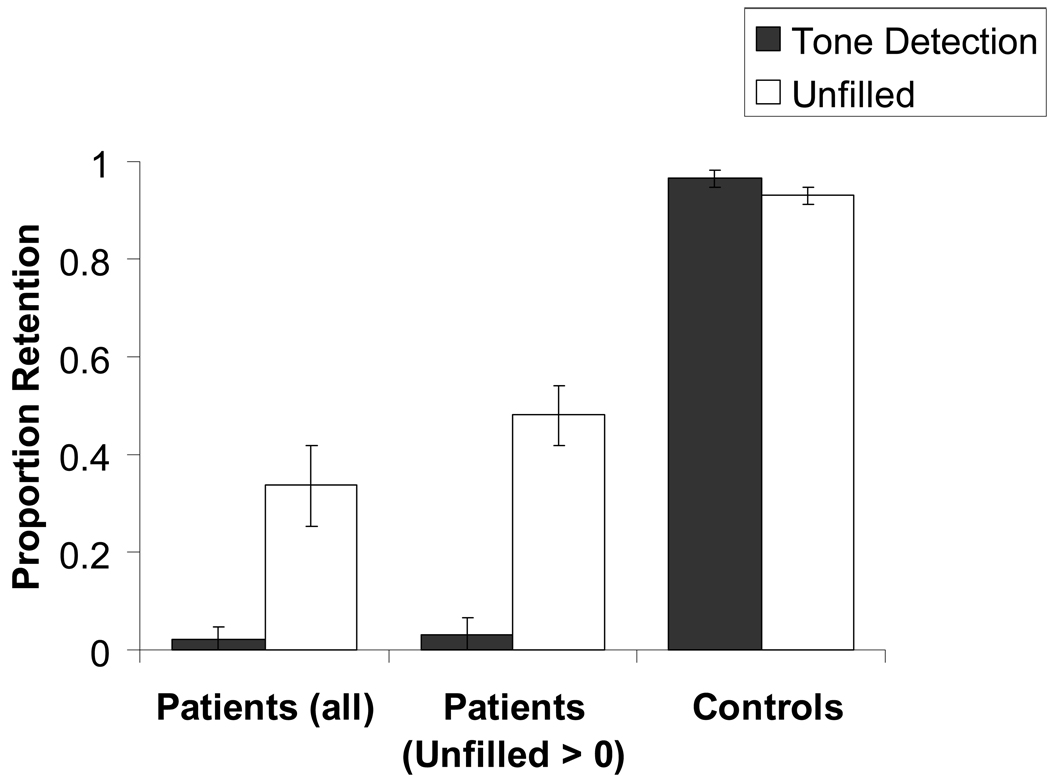
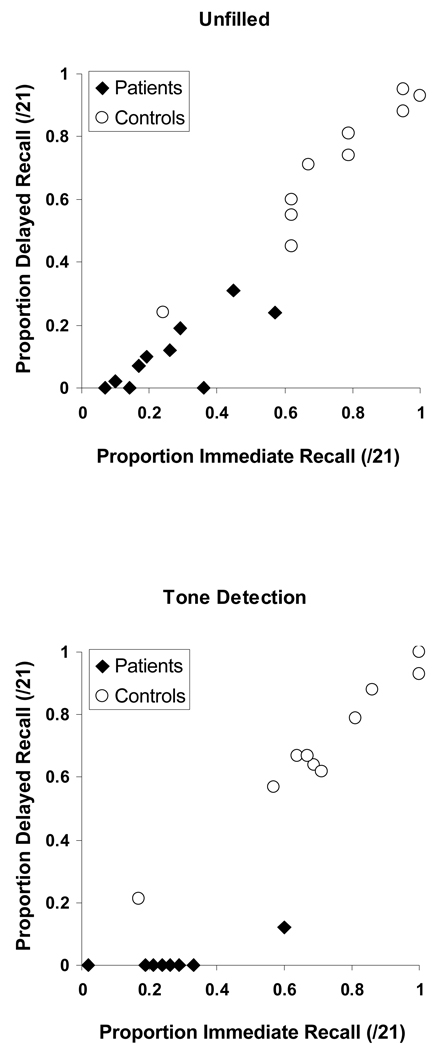
Initial analysis of the memory data revealed a main effect of delay condition, F(1, 18) = 9.722, p < 0.01, a main effect of group, F(1, 18) = 277.23, p < 0.001 and, most importantly, an interaction of the delay condition with the group, F(1, 18) = 15.245, p < 0.01. Given that the type of rehearsal condition did not significantly affect performance in either group (Patients − F(1, 9) = 0.001, p = 0.979; Controls − F(1, 9) = 2.126, p = 0.179) in either delay condition (Unfilled − F(1, 18) = 0.006, p = 0.941; Tone detection − F(1, 18) = 2.721, p = 0.116), the data from the incidental and intentional conditions was collapsed to compute a mean unfilled delay proportion retention score and mean tone detection proportion retention score for each participant. As is elucidated in Figure 1, 7 out of the 10 patients were able to recall some material following the unfilled delay (P1, P2, P3, P4, P6, P7, P8). However, only 1 patient (P6) could retain any material following the tone detection delay. This improvement from the tone detection condition to the unfilled condition was significant, both when only those patients performing > 0 in the unfilled delay were included, F(1, 6) = 39.028, p < 0.002, and when all 10 patients were included, F(1, 9) = 13.89, p < 0.004 (see Figure 2). No significant condition difference was revealed in the controls, who performed at ceiling in both conditions. The improvement from the tone detection condition to the unfilled condition in the patients, and the absence of such improvement in the controls were also demonstrated when proportion delayed recall (number of correctly recalled prose ideas / 21), was considered (F(1, 9) = 8.725, p < 0.05; F(1, 9) = 0.061, p > 0.8, for the patients and controls respectively) (see Figure 3). Figure 3 further shows that the 2 groups differed substantially in their proportion immediate recall performance. However, immediate recall performance did not differ between the unfilled condition and the tone detection condition in either group. Moreover and importantly, the interaction of the delay condition with the group remained significant even when these group differences in immediate recall performance were accounted for F(1, 16) = 10.966, p < 0.01.
Figure 1.
Individual mean proportion retention of the amnesic patients (P1–P10) and controls (C1–C10) in the unfilled and tone detection delay condition. Proportion Retention = (Delayed Recall/Immediate Recall).
Figure 2.
Group mean proportion retention as a function of delay condition, including all patients and including only those patients who retained > 0 following the unfilled delay. Proportion Retention = (Delayed Recall/Immediate Recall). Error bars = SEM.
Figure 3.
Individual mean proportion delayed recall as a function of individual mean proportion immediate recall, in patients and control participants (graph parameter). Both recall measures shown here are absolute proportions of the 21 story ideas presented. Top panel, tone detection condition; bottom panel, unfilled condition.
Seven patients (P2, P5–P10) and controls (C4–C10) received a third unfilled delay trial after the study as described above. This trial replicated the first unfilled delay trial of the present study but was followed by a 5 min conversation (entirely unrelated to the prose passage or the study) between the experimenter and the patient, and a subsequent second surprise delayed recall. It was found that three out of the four patients who had been able to recall some prose material following the unfilled delay continued to be able to recall some of this material after the subsequent conversation (patient mean proportion retention from immediate recall = 0.42, SD = 0.083, individual patient proportion retention = 0.50, 0.33 and 0.42; the group mean and SD for the 7 controls was 0.96 and 0.048, respectively).
Tone detection performance
The mean number of tones detected (out of 50) did not differ significantly between the two groups (MPatients = 47.89, SD = 3.66; MControls = 43.95, SD = 5.38). Moreover, no significant correlations were obtained between tone detection mean score and proportion retention following tone detection for the two groups overall, nor for the two separate groups.
Executive function and memory performance
No significant correlations emerged between the patients’ trail making scores and their retention in the tone detection condition, unfilled condition, or their degree of benefit from the unfilled condition (retention in the unfilled condition – retention in the tone detection condition).
Discussion
Seven out of ten severely amnesic patients, who performed at floor on standard tests of delayed recall, were able to recall new verbal material following a delay interval when this was spent quietly and alone in a darkened, empty room. In contrast, when the delay interval was spent listening for piano notes, only one patient (P6) was able to recall any new verbal material at subsequent delayed recall. Indeed, following tone detection some patients could not even remember that a story had been presented to them. Patient P7, for example, showed a mean proportion retention score of 0.68 following the unfilled delay interval. However, when asked to recall the story presented to him prior to tone detection he responded ‘What story?’ and stated that he had no recollection of there having been a story.
These findings not only support Cowan et al.’s (2004) retroactive interference hypothesis of amnesia, but demonstrate for the first time that the retroactive interference effect observed in amnesic patients is non-specific. Indeed, in line with Müller and Pilzecker’s (1900) original definition of retroactive interference our data show that post-learning stimuli need not be similar to to-be-retained information or even semantically meaningful for a large memory interference effect to occur in amnesic patients.
Of course an improvement in retention following the absence of further stimuli need not automatically imply that the presence of further stimuli hinders such retention. Indeed, it could be argued that the amnesic patients’ severe forgetting is entirely unrelated to retroactive interference and that the unfilled delay simply allowed patients to use an intact compensatory mechanism, which is susceptible to interference in both neurologically intact people as well as amnesic patients. The most obvious potential ‘compensatory mechanism’ is conscious maintenance within working memory, which remains intact in amnesic patients. In both neurologically intact individuals as well as amnesic patients information can be maintained within working memory via conscious rehearsal, but decays rapidly (~ 30 seconds) as soon as such rehearsal is interrupted (Scoville and Milner, 1957; Milner, 1968; Baddeley and Hitch, 1979; Odgen, 1996). It could be argued that the present patients had no LTM capacity and that the unfilled delay simply allowed them to consciously maintain new information within working memory, thus effectively protecting it from working memory decay.
This account of our findings seems unlikely for various reasons. Four out of the seven patients who showed enhanced retention following the unfilled delay reported during post experimental feedback that they had not attempted to rehearse the material in any of the delay intervals. Such subjective evidence was further supported by the objective Trial 1 and Trial 3 retention data. Given that all participants were forewarned about delayed recall in Trial 3, the likelihood of rehearsal was predicted to be higher in this trial than the ‘incidental’ Trial 1. Therefore, if rehearsal had taken place, retention should have been better in Trial 3 than Trial 1. However, such pattern was only observed in one of the four patients who reported no rehearsal and showed enhanced retention.
Further evidence against this working memory account of our data comes from the additional data gathered from seven of the ten patients. As described above, these seven patients received a third unfilled delay trial that was followed by a 5 min conversation between the experimenter and the patient, and a subsequent second surprise delayed recall. Three out of the four patients who had shown some prose retention following the unfilled delay, continued to be able to recall some of this material after the subsequent conversation. Given that explicit working memory would have been near-to-impossible during the conversation, the extended enhanced retention observed in these three patients is unlikely to have been the mere product of explicit working memory rehearsal (though it is of course possible that any such rehearsal could have aided a LTM process).
The delay condition effect elucidated in the present amnesic patients thus appears to reflect a genuine non-specific LTM retroactive interference effect. This novel finding is of interest given existing accounts of item-specific LTM interference for the subtle memory impairment observed in some dysexecutive patients.
It seems unlikely that the interference susceptibility in the present case is merely an augmented form of that observed in patients with executive dysfunction. The tone detection stimuli bore no resemblance to the to-be-retained material. It would thus be difficult to account for the observed interference effect in terms of a confusion of similar stimuli. Moreover, only two of the tested patients (P2 and P5) presented with executive impairment (trail making B-A) and did not perform differently than the other patients in the present memory study (one of them showed increased retention in the unfilled condition (P2), the other showed no retention in the unfilled condition (P5)). Furthermore, in comparison to studies on memory and executive function in dysexecutive patients (c.f. Simard et al., 2003; Diamond et al., 1997) no correlation was found between executive function and memory performance in the present patients. If the interference susceptibility observed in the present patients were indeed associated with executive function one would have expected there to have been a relationship between executive performance (trail making B-A) and (a) retention following tone detection and (b) the degree of improvement from the tone detection to the unfilled delay. It thus appears likely that the difference in interference susceptibility between the present patients with anterograde amnesia and those with executive dysfunction is one of kind as opposed to degree. In order to interpret such differences it is useful to consider the cognitive loci of these interference effects.
Item-specific interference is assumed to occur during LTM retrieval, when to-be- retained stimuli become confused with other stored stimuli due to a high resemblance in these stimuli or their retrieval cues (Skaggs, 1933; McGeoch and Nolen, 1933; Mensink and Raajmakers, 1988; Anderson and Bjork, 1994). Patients with executive dysfunction are hypothesised to be especially susceptible to such confusion at retrieval (Baldo and Shimamura, 2002).
Given that psychologists have tended to focus on item-specific interference (Wixted, 2004; Dewar et al., 2007) less is known about the possible cognitive locus of non-specific retroactive interference. Müller and Pilzecker (1900) defined such retroactive interference as interference of the consolidation (i.e. strengthening) of to be retained material by further material. Their work as well as that by their fellow pioneer in retroactive interference, Skaggs (1925) showed that interpolated material, even if dissimilar to the to-be-retained material, had a more detrimental effect when placed immediately following learning of to-be-retained material than when placed following an unfilled delay. Müller and Pilzecker (1900) explained such temporal gradient of retroactive interference in terms of weak new memory traces, which gradually strengthen, i.e. consolidate, thus becoming less susceptible to interference over time. Such hypothesis is supported by more recent animal neuroscience work on protein synthesis inhibitors. Protein synthesis inhibitors, usually antibiotics or toxins, interfere with the neural processes associated with memory formation in animals (Dudai, 2004; Agranoff et al., 1966). Retention of recently learned material is low if a protein synthesis inhibitor is introduced immediately following learning, but improves steadily with augmenting delay in the introduction of the protein synthesis inhibitor, thus indicating a decrease in interference susceptibility over time.
Might dissimilar interpolated stimuli, such as the ones applied in the present study interfere with memory consolidation in patients with anterograde amnesia? Recent work by us suggests that such might well be the case (Dewar et al., 2009). 12 patients with amnestic MCI were presented with a list of 15 words, which had to be recalled immediately afterwards as well as after a delay of 9 minutes. A picture naming task was interpolated either during the first third, the middle third or the last third of this 9 minute interval. The patients’ retention was at floor when interference occurred during the first third of the delay. However, retention significantly increased with augmenting delay in the onset of interference, indicating that aMCI patients have the capacity to consolidate some new information but that such process is severely disrupted by immediately following post-learning material (Dewar et al., 2009).
In the present study’s additional trial, 3 out of 4 patients who had shown some retention following the 10 min unfilled delay continued to be able to recall some prose material following a further 5 min delay that was filled with conversation. This finding tentatively suggests that these patients too have the capacity to consolidate, provided that the time immediately following new learning is devoid of further material. While further work is necessary it appears highly plausible that the non-specific interference effect obtained here occurred because tone detection impeded consolidation. If this is the case, the implication is that any post-learning material is likely to disrupt memory consolidation profoundly in some patients with anterograde amnesia, provided that at least some consolidation capacity remains intact.
This spared consolidation capacity might be insufficient for the processing and strengthening of much new incoming information, i.e. to-be-retained material and subsequent material. However, it may suffice for the processing and strengthening of small amounts of new information (Dewar et al., 2009). The removal of all new material, including dissimilar stimuli, may make for an ideal learning condition.
The need for removal of even highly dissimilar material in amnesic patients fits such a consolidation hypothesis of minimal interference well. Functional imaging has shown that the medial temporal lobes, i.e. the areas most closely associated with early memory processing and consolidation (Alvarez and Squire, 1994), are always and automatically active when one attends to any new event (Martin, 1999). It seems reasonable that all such events should be removed for a weak and capacity limited consolidation system to be able to function.
In line with this weak consolidation system hypothesis 5 of the 7 patients who benefited from minimal interference had temporal lobe lesions (P1, P2, P3, P7, P8), which included the hippocampus in 3 of the patients (P1, P2, P3). However, the other 2 patients who benefited from minimal interference did not have any known temporal lesions. Further work based on selected patients with precisely identified focal lesions of one or two types (c.f. Allen et al., 2006; Barense et al., 2007) is thus necessary to allow for the pinning down of the lesion sites, which are associated with the postulated consolidation interference and those which might be associated with other types of memory impairment. Moreover, volumetric structural data might provide clues as to the individual differences in the degree of post-learning interference.
It is unclear why little or no post-learning interference occurred in the controls in this study. Indeed, some controls even performed marginally better following tone detection than following the unfilled delay. It is possible that tone detection required only few consolidation resources, and thus that it did not sufficiently tax the controls’ intact consolidation system. The psychometric tests used by Cowan et al., (2004) on the other hand might have been sufficiently demanding to tax the controls’ consolidation systems to some extent, thus leading to a mild interference effect. Future work will examine whether or not the degree of cognitive load does indeed play a role in the magnitude of non-specific retroactive interference.
A caveat of the present study is the group difference in immediate recall levels. On the whole the present amnesic patients’ immediate prose recall was lower than that of the controls (as also found in the prose recall study by Cowan et al., 2004), indicating that, even prior to the onset of the delay period, the patients’ memory traces were already quantitatively different from those of the controls. It is unlikely however that the delayed recall effects demonstrated in the present study were mere artefacts related to weak initial memory traces in the patients.
Figure 3 shows that the proportion immediate recall – proportion delayed recall ratio was somewhat similar for the 2 groups in the unfilled condition, i.e. a common line could fit most of the participants in both groups. This is not at all true in the tone detection condition. Even those patients whose immediate recall levels were similar to those of some of the controls performed substantially worse at delayed recall than did these ‘matched’ controls in the tone detection condition. Indeed, tone detection continued to have a very detrimental effect upon retention in the patients but not in the controls, even when group differences in immediate recall were accounted for. Such findings should not have emerged if the degree of memory interference were solely governed by immediate recall level.
It should be noted that significant group differences at delayed recall were also observed in Cowan et al.’s (2004) word list experiment, in which immediate recall performance was comparable for patients and controls. The same was true in a subsequent prose recall study on MCI patients (Della Sala et al., 2005).
Other work on amnesic patients with impaired immediate recall has however led to conflicting findings. This shows that the artificial matching of patients’ and controls’ immediate memory levels via extended and/or repeated stimulus presentation in the patient group can lead to normal delayed memory levels in patients (c.f. Freed et al., 1987; Kopelman and Stanhope, 1997, Isaac and Mayes, 1999). It is important to highlight however that such findings are based on yes/no recognition and forced choice paradigms rather than on free recall paradigms. Indeed, Isaac and Mayes (1999) showed that their mild to moderately amnesic patients’ immediate memory levels could be matched to that of controls when the patients received several learning trials. However, whereas the patients’ delayed prose recognition was normal, their delayed free prose recall was well below that of the controls, indicating that impaired delayed free recall cannot be solely attributed to lower immediate memory levels.
These findings also hint that, in at least some amnesic patients, the here proposed weakened consolidation system limits recall but not recognition performance. Immediate-post learning information might not completely block the consolidation of new material in amnesic patients. New memory traces might survive albeit in a very weak state, in which they can only be retrieved via specific reminders or cues (c.f. Squire, 2006). Moreover, some boosting of the memory traces prior to the filled delay, i.e. via extended and/or repeated stimulus presentation (c.f. Freed et al., 1987; Kopelman and Stanhope, 1997, Isaac and Mayes, 1999) might be necessary for recognition to be successful in amnesic patients. In contrast, successful free recall appears to be dependent upon a relatively long period of uninterrupted consolidation in amnesic patients.
It remains to be examined whether the immediate prose recall deficit that is observed in many amnesic patients might be related to consolidation interference. Given that the earliest stages of consolidation (synaptic consolidation) are thought to occur within seconds of learning (Dudai, 2004) it is conceivable that new material as complex as a story might exceed the patients’ spared consolidation capacity during presentation, thus resulting in interference and poorer immediate memory levels. It also remains to be established to what extent the immediate recall process itself might affect memory retention. Cowan et al.’s (2004) work showed that amnesic patients’ word list retention was not differentially affected by the presence vs. absence of an immediate recall phase. However, future work is required to examine whether this finding also holds for the retention of prose.
What is clear and novel from the present study is the finding that essentially all post-learning material, including dissimilar stimuli, must be removed following new learning in order for amnesic patients to show enhanced retention.
Some of the present patients and their carers were amazed by such seemingly hidden memory capacity. Indeed one patient and his wife stated that the findings were ‘very encouraging’ and that they would try to use this ‘technique’ at home.
Of course it would be impossible to implement such a period of minimal interference after all new learning in everyday life. Nonetheless the technique could be applied to teach some amnesic patients selective information, important to them.
Might minimal interference also aid more complex autobiographical (i.e. personal episodic) memory in amnesic patients? Some anecdotal observations in the present study hint that such might be the case. Following the tone detection interval patient P2 for example had no recollection of the story material, the presence of a story or the experimenter. Following the unfilled delay on the other hand he greeted the experimenter with enthusiasm and was able to recall some story material as well as some contextual information about the time of story presentation. Moreover, patient P6’s parents reported that on collecting their son from the hospital he told them many more details about the assessment than they would have expected. Furthermore, a year after the assessment patient P7 freely recalled that there had been an ‘English doctor’. This memory was clearly not a mere intelligent guess. The patient was Italian and tested at his local Italian hospital where ‘English doctors’ are not often found. However on the day of testing the team of experimenters did indeed include a visiting UK psychologist. Whether or not such instances of enhanced memory were the result of minimal interference or some entirely unrelated factor can of course not be deduced from the present data. However, given the reported remarkable improvement in prose memory in some patients with amnesia such possibility does not appear unfeasible, and indeed future work is planned to test this hypothesis empirically.
In conclusion, the present study reveals that even highly dissimilar post-learning material interferes substantially with recently learned material in patients with severe anterograde amnesia. Given the usual presence of such post-learning material in everyday life, we hypothesize that at least some of the severe forgetting in amnesic patients is the product of non-specific retroactive interference. This kind of non-specific retroactive interference effect deviates from the item-specific interference that is frequently reported and clinically assessed in dyexecutive patients. It is postulated that these two interference effects differ in kind as opposed to degree, occurring during different cognitive processes, with item-specific interference affecting retrieval and non-specific retroactive interference (i.e. interference from any material, similar or non-similar) affecting consolidation. Given that at least some patients with severe amnesia are able to learn new material when all post-learning material is removed, it appears important to not only assess a patient’s susceptibility to item-specific interference, but to also check for a potential susceptibility to non-specific interference.
Acknowledgements
We wish to thank Prof. Adam Zeman, Dr Jon Stone, Dr Colin Mumford (Western General Hospital, Edinburgh, UK) and Dr Francesco Zaro (Rehabilitation Unit in Somma Lombardo, Italy) for allowing us to test their patients. We also thank Dr Sharon Abrahams for providing us with neuropsychological test data for patient P4, and Dr Benjaman Schoegler for his help with the generation of the tone detection stimuli. We are extremely grateful to all patients and controls who volunteered to take part in this study, as well as to 3 anonymous reviewers who provided very helpful comments on an earlier draft of this manuscript.
References
- Allen JS, Tranel D, Bruss J, Damasio H. Correlations between regional brain volumes and memory performance in anoxia. Journal of Clinical and Experimental Neuropsychology. 2006;28:457–476. doi: 10.1080/13803390590949287. [DOI] [PubMed] [Google Scholar]
- Alvarez P, Squire LR. Memory consolidation and the medial temporal lobe: A simple network model. Proceedings of the National Academy of Sciences, USA. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agranoff BW, Davis RE, Brink JJ. Chemical studies on memory fixation in goldfish. Brain Research. 1966;1:303–309. doi: 10.1016/0006-8993(66)90095-3. [DOI] [PubMed] [Google Scholar]
- Anderson MC. Rethinking interference theory: Executive control and the mechanisms of forgetting. Journal of Memory and Language. 2003;49:415–445. [Google Scholar]
- Anderson MC, Bjork RA. Mechanisms of inhibition in long-term memory: A new taxonomy. In: Dagenbach D D, Carrn T, editors. Inhibitory processes in attention, memory and language. Academic Press; 1994. pp. 265–326. [Google Scholar]
- Baddeley A, Hitch GJ. Working memory. In: Bower GA, editor. The Psychology of Learning and Motivation. Academic Press; 1974. pp. 47–89. [Google Scholar]
- Baldo JV, Shimamura AP. Frontal lobes and memory. In: Baddeley A, Kopelman MD, Wilson BA, editors. The Handbook of Memory Disorders. 2nd ed. Chichester: John Wiley; 2002. [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45:2963–2974. doi: 10.1016/j.neuropsychologia.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Basso A, Capitani E, Laiacona M. Raven's Coloured Progressive Matrices: normative values on 305 adult normal controls. Functional Neurology. 1987;2:189–194. [PubMed] [Google Scholar]
- Brazzelli M, Della Sala S, Laiacona M. Supplemento al Manuale del TMCR. Florence: Organizzazioni Speciali; 1993. Taratura della versione Italiana del Rivermead Behavioural Memory Test: un test di valutazione ecologica della memoria. [Google Scholar]
- Buschke H. Buschke Selective Reminding Test (BSRT) In: Spreen O, StraussE E, editors. A compendium of neuropsychological tests: Administration, norms, and commentary. NY: Oxford University Press; 1991. pp. 125–138. [Google Scholar]
- Butters N, Cermak LS. Alcoholic Korsakoff's syndrome: an information-processing approach to amnesia. New York: Academic Press; 1980. [Google Scholar]
- Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A. Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurological Sciences. 2002;22:443–447. doi: 10.1007/s100720200003. [DOI] [PubMed] [Google Scholar]
- Cowan N, Beschin N, Della Sala S. Verbal recall in amnesiacs under conditions of diminished retroactive interference. Brain. 2004;27:825–834. doi: 10.1093/brain/awh107. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Cowan N, Beschin N, Perini M. Just lying there, remembering: Improving Recall of Prose in Amnesic Patients with Mild Cognitive Impairment by Minimizing Interference. Memory. 2005;13:435–440. doi: 10.1080/09658210344000387. [DOI] [PubMed] [Google Scholar]
- Dewar MT, Cowan N, Della Sala S. Forgetting due to retroactive interference: A fusion of Müller and Pilzecker's (1900) early insights into forgetting and recent research on anterograde amnesia. Cortex. 2007;43:616–634. doi: 10.1016/s0010-9452(08)70492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar M, Fernandez Garcia Y, Cowan N, Della Sala S. Delaying interference enhances memory consolidation in amnesic patients. Neuropsychology. 2009;23:627–634. doi: 10.1037/a0015568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond BJ, DeLuca J, Kelley SM. Memory and executive functions in amnesic and non-amnesic patients with aneurysms of the anterior communicating artery. Brain. 1997;120:1015–1025. doi: 10.1093/brain/120.6.1015. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidation, or, how stable is the engram? Annual Review of Psychology. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Enderby P, Wood V, Wade D. Frenchay Aphasia Screening Test (FAST) Windsor: UK: NFER-Nelson; 1987. [DOI] [PubMed] [Google Scholar]
- Freed DM, Corkin S, Cohen NJ. Forgetting in H.M.: A second look. Neuropsychologia. 1987;25:461–471. doi: 10.1016/0028-3932(87)90071-6. [DOI] [PubMed] [Google Scholar]
- Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E. Trail making test: normative values from 287 normal adult controls. The Italian Journal of Neurological Sciences. 1996;17:305–309. doi: 10.1007/BF01997792. [DOI] [PubMed] [Google Scholar]
- Graf P, Squire LR, Mandler G. The information that amnesic patients do not forget. Journal of Experimental Psychology: Learning, Memory and Cognition. 1984;10:164–178. doi: 10.1037//0278-7393.10.1.164. [DOI] [PubMed] [Google Scholar]
- Huppert FA, Piercy M. Dissociation between learning and remembering in organic amnesia. Nature. 1978;275:317–318. doi: 10.1038/275317a0. [DOI] [PubMed] [Google Scholar]
- Huppert FA, Piercy M. Normal and abnormal forgetting in organic amnesia: effect of locus of lesion. Cortex. 1979;15:385–390. doi: 10.1016/s0010-9452(79)80065-9. [DOI] [PubMed] [Google Scholar]
- Isaac CL, Mayes AR. Rate of forgetting in amnesia: I. Recall and recognition of prose. Journal of Experimental Psychology: Learning, Memory and Cognition. 1999;25:942–962. doi: 10.1037//0278-7393.25.4.942. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kopelman MD. Disorders of memory. Brain. 2002;125:2152–2190. doi: 10.1093/brain/awf229. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Stanhope N. Rates of forgetting in organic amnesia following temporal lobe, diencephalic, or frontal lobe lesions. Neuropsychology. 1997;11:343–356. doi: 10.1037//0894-4105.11.3.343. [DOI] [PubMed] [Google Scholar]
- Lüer G. Georg Elias Müller (1850–1934): a Founder of Experimental Memory Research in Psychology. Cortex. 2007;43:579–582. doi: 10.1016/s0010-9452(08)70488-x. [DOI] [PubMed] [Google Scholar]
- Martin A. Automatic activation of the medial temporal lobe during encoding: lateralized influences of meaning and novelty. Hippocampus. 1999;9:62–70. doi: 10.1002/(SICI)1098-1063(1999)9:1<62::AID-HIPO7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Downes JJ, Shoqeirat M, Hall C, Sagart HJ. Encoding ability is preserved in amnesia: Evidence from a direct test of encoding. Neuropsychologia. 1993;31:745–759. doi: 10.1016/0028-3932(93)90126-k. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Downes JJ, Symons V, Shoqeirat M. Do amnesics forget faces pathologically fast? Cortex. 1994;30:543–563. doi: 10.1016/s0010-9452(13)80235-3. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Meudell PR, Neary D. Must amnesia be caused by either encoding or retrieval disorders? In: Gruneberg MM, Morris PE, Sykes RN, editors. Practical aspects of memory. London: Academic Press; 1978. [Google Scholar]
- Mayes AR, Meudell P, Neary D. Do amnesics adopt inefficient encoding strategies with faces and random shapes? Neuropsychologia. 1980;18:527–541. doi: 10.1016/0028-3932(80)90154-2. [DOI] [PubMed] [Google Scholar]
- McGeoch JA, McDonald WT. Meaningful relation and retroactive inhibition. American Journal of Psychology. 1931;43:579–588. [Google Scholar]
- McGeoch JA, Nolen ME. Studies in retroactive inhibition IV. Temporal point of interpolation and degree of retroactive inhibition. Journal of Comparative Psychology. 1933;15:407–417. [Google Scholar]
- Mensink GJ, Raaijmakers JGW. A model for interference and forgetting. Psychological Review. 1988;95:434–455. [Google Scholar]
- Milner B. Amnesia following operations on the medial temporal lobes. In: Whitty CWM, Zangwill OL, editors. Amnesia. London: Butterworths; 1966. pp. 109–133. [Google Scholar]
- Milner B, Corkin S, Teuber HL. Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of H. M. Neuropsychologia. 1968;6:215–234. [Google Scholar]
- Müller GE, Pilzecker A. Experimentelle Beiträge zur Lehre vom Gedächtniss. Zeitschrift für Psychologie. Ergänzungsband. 1900;1:1–300. [Google Scholar]
- Novelli G, Papagno C, Capitani E, Laiacona M, Cappa SF, Vallar G. Tre test clinici di memoria verbale a lungo termine. Taratura su soggetti normali. Archivio di psicologia, neurologia e psichiatria. 1986;47:278–296. [Google Scholar]
- Odgen J. Marooned in the moment. In: Odgen J, editor. Fractured minds. A case-study approach to clinical neuropsychology. New York: Oxford University Press; 1996. pp. 41–58. [Google Scholar]
- Reed JM, Hamann SB, Stefanacci L, Squire LR. When amnesic patients perform well on recognition memory tests. Behavioral Neuroscience. 1997;111:1163–1170. doi: 10.1037//0735-7044.111.6.1163. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP, Jurica PJ, Mangles JA, Gershberg FB, Knight RT. Susceptibility to memory interference effects following frontal lobe damage: Findings from tests of paired-associate learning. Journal of Cognitive Neuroscience. 1995;7:144–152. doi: 10.1162/jocn.1995.7.2.144. [DOI] [PubMed] [Google Scholar]
- Simard S, Rouleau I, Brosseau J, Laframboise M, Bojanowsky M. Impact of executive dysfunctions on episodic memory abilities in patients with ruptured aneurysm of the anterior communicating artery. Brain and Cognition. 2003;53:354–358. doi: 10.1016/s0278-2626(03)00142-8. [DOI] [PubMed] [Google Scholar]
- Skaggs EB. Further studies in retroactive inhibition. Psychological Monographs. 1925;Whole No. 161:1–60. [Google Scholar]
- Skaggs EB. A discussion on the temporal point of interpolation and degree of retroactive inhibition. The Journal of Comparative Psychology. 1933;16:411–414. [Google Scholar]
- Spinnler H, Tognoni G, editors. Italian Journal of Neurological Sciences. Vol. 6. 1987. Standardizzazione e taratura italiana di test neuropsicologici; pp. 1–120. [PubMed] [Google Scholar]
- Squire LR. Specifying the defect in human amnesia: storage, retrieval and semantics. Neuropsycholgia. 1980;18:368–372. doi: 10.1016/0028-3932(80)90134-7. [DOI] [PubMed] [Google Scholar]
- Squire LR. Lost forever or temporarily misplaced? The long debate about the nature of memory impairment. Learning & Memory. 2006;13:522–529. doi: 10.1101/lm.310306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, Weiskrantz L. New method of testing long-term retention with special reference to amnesic patients. Nature. 1968;217:972–974. doi: 10.1038/217972a0. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Weiskrantz L. Amnesic Syndrome: Consolidation or retrieval? Nature. 1970;288:628–630. doi: 10.1038/228628a0. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Weiskrantz L. The effect of prior learning on subsequent retention in amnesic patients. Neuropsychologia. 1974;12:419–428. doi: 10.1016/0028-3932(74)90072-4. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Weiskrantz L. Further analysis of the prior learning effect in amnesic patients. Neuropsychologia. 1978;16:169–177. doi: 10.1016/0028-3932(78)90104-5. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III. San Antonio: Psychological Corporation; 1997. [Google Scholar]
- Wilson BA. Rehabilitation of memory. New York: The Guilford Press; 1987. [Google Scholar]
- Wilson B, Cockburn J, Baddeley AD. The Rivermead Behavioural Memory Test. Reading: Thames Valley Test Group; 1985. [Google Scholar]
- Wixted JT. The Psychology and neuroscience of forgetting. Annual Review of Psychology. 2004;55:235–269. doi: 10.1146/annurev.psych.55.090902.141555. [DOI] [PubMed] [Google Scholar]