Abstract
Objective
Myasthenia gravis (MG) and its animal model, experimental autoimmune myasthenia gravis (EAMG), are antibody-mediated autoimmune diseases, in which autoantibodies bind to and cause loss of muscle nicotinic acetylcholine receptors (AChRs) at the neuromuscular junction. To develop a specific immunotherapy of MG, we treated rats with ongoing EAMG by intraperitoneal injection of bacterially-expressed human muscle AChR constructs.
Methods
Rats with ongoing EAMG received intraperitoneal treatment with the constructs weekly for 5 weeks beginning after the acute phase. Autoantibody concentration, subclassification, and specificity were analyzed to address underlying therapeutic mechanism.
Results
EAMG was specifically suppressed by diverting autoantibody production away from pathologically relevant specificities directed at epitopes on the extracellular surface of muscle AChRs toward pathologically irrelevant epitopes on the cytoplasmic domain. A mixture of subunit cytoplasmic domains was more effective than a mixture containing both extracellular and cytoplasmic domains or than only the extracellular domain of α1 subunits.
Interpretation
Therapy using only cytoplasmic domains, which lack pathologically relevant epitopes, avoids the potential liability of boosting the pathological response. Use of a mixture of bacterially-expressed human muscle AChR cytoplasmic domains for antigen-specific immunosuppression of myasthenia gravis has the potential to be specific, robust, and safe.
Introduction
Myathenia gravis (MG) is mediated by autoantibodies to skeletal muscle nicotinic acetylcholine receptors (AChRs).1–3 Experimental autoimmune MG (EAMG) can be induced by immunization with AChRs from fish electric organs.2, 4
AChRs are formed by 5 homologous subunits organized around the central cation channel in the order α1γα1δβ1 in fetal muscle.5 In the adult, γ is replaced byε. Each subunit has an N-terminal extracellular domain of about 210 amino acids followed by four transmembrane domains (M1–M4). Between M3 and M4 is a large cytoplasmic domain of 112 to 151 amino acids. After M4 is a short extracellular tail. The main immunogenic region (MIR) at the extracellular tip ofα1 subunits is targeted by more than half of MG autoantibodies.6–9 Immunization with native AChR produces antibodies predominantly to the extracellular surface, while immunization with denatured AChR produces antibodies predominantly to cytoplasmic domains.10
Neuromuscular transmission in MG and EAMG is impaired primarily by loss of AChRs and disruption of postsynaptic membrane morphology.1, 2 Acute and passively transferred EAMG result from antibody bound to the muscle postsynaptic membrane triggering a complement-dependent attack by macrophages.2 Chronic EAMG involves only autoantibodies and complement in the attack on the postsynaptic membrane.2
MG is a chronic affliction.11, 12 Symptomatic therapy uses inhibitors of ACh esterase. Thymectomy, plasmapheresis, intravenous immunoglobulin, and non-specific immunosuppressive drugs are often used. There is no specific immunosuppressive therapy.
Immunization with denatured Torpedo AChR can prevent and suppress EAMG.13, 14 Both systemic, oral, and nasal administration of antigens can induce tolerance.15–17 Mucosal therapy with either native AChR, denatured subunit fragments, or short synthetic subunit sequences inhibits the onset of EAMG.18–24 Suppression of ongoing EAMG is more difficult.
Ongoing EAMG can be reduced by oral administration of bacterially-expressed human or rat AChR α1 extracellular domains.25 This is thought to result from suppression of the autoimmune response by regulatory T lymphocytes. Antigen therapy risks inducing autoimmunity rather than suppressing it.17, 26
We discovered that intraperitoneal (i.p.) treatment with a mixture of bacterially-expressed human α1, β1, γ, δ, and ε subunit extracellular and cytoplasmic domains potently suppresses ongoing EAMG.27 Constructs containing only cytoplasmic domains were even more effective. The therapeutic mechanism is shifting specificities of autoantibodies from pathologically relevant epitopes on the extracellular surface to pathologically irrelevant epitopes on the cytoplasmic surface, rather than suppression of the autoimmune response. This new approach may provide a robust and safe strategy for antigen-specific immunotherapy of MG.
Materials and Methods
Induction of EAMG
Eight-week-old female Lewis rats (Charles River, Wilmington, MA) were used. All studies were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. Rats were immunized once in the base of the tail by the subcutaneous (s.c.) injection of purified Torpedo AChR emulsified in TiterMax adjuvant (CytRx, Norcross, GA).28 Weakness was graded as described previously.29
Antigen preparation
Transmembrane domains were deleted from human AChR subunit constructs because these contain few T-cell epitopes30 and interfered with bacterial expression.21 Sequences forming each construct are shown in Table 1. Construction and purification are described in Supplementary Methods.
Table 1.
Human AChR Subunit Sequences Incorporated in Therapeutic Constructs
| Subunit Constructs | Extracellular Domain | Large Cytoplasmic Domain | C-Terminal Domain |
|---|---|---|---|
| α1 | α1 1-209 | α1 297-408 | α1 428-437 + 6 his |
| β1 | β1 1-220 | β1 309-445 | β1 466-478 + 6 his |
| γ | γ 2-217 | γ 307-457 | γ 472-494 |
| δ | δ 2-224 | δ 313-450 | δ 469-496 |
| ε | ε 1-219 | ε 309-437 | ε 466-473 |
| Cytoplasmic Domain Constructs | Large Cytoplasmic Domain | ||
| β1α1γ | β1 310-446 | α1 297-408 | γ307-451 |
| εα1δ | ε309-408 | α1 297-408 | δ313-451 |
Antibody assay
Antibodies to AChRs were measured by immunoprecipitation of 125I-α bungarotoxin(125I-αBgt) labeled AChRs, and expressed as nmol of toxin binding sites/L serum (nM).
ELISA
Antibodies to the bacterially-expressed subunit constructs were assayed by ELISA. Microtiter plates (Corning, Lowell, MA) were coated overnight at room temperature with 100μl of constructs (20μg/ml in 0.1 M sodium carbonate buffer, pH 9.6). After blocking with 3% BSA and washing, serially diluted EAMG rat sera were added for 2 h at 37°C. Bound antibodies were detected using biotinylated goat anti-rat IgG and horseradish peroxidase (HRP) labeled streptavidin (Kirkegaard & Perry Laboratory, Gaithersburg, MD). HRP activity was measured with QuantaBlu Fluorogenic Peroxidase Substrate Kit (Pierce, Rockford, IL). Titer was defined as the dilution that gave half-maximal binding.
IgG isotyping
Rat muscle AChRs labeled with 4 nM biotinylated αBgt (Invitrogen, Carlsbad, CA) were incubated at 4°C overnight with EAMG sera at a dilution of 1/640. Subsequently, 100 μl/well were added to Reacti-Bind Streptavidin High Binding Capacity Coated Plates (Pierce, Rockford, IL) and incubated at room temperature for 4 h. After four washes, HRP conjugated goat antisera to rat IgG isotypes (Bethyl Laboratories, Montgomery, TX) diluted 1/1000 in phosphate buffered saline (PBS), pH7.4, were added for 1 h at 37°C. Bound antibodies were detected using the QuantaBlu Fluorogenic Peroxidase Substrate Kit (Pierce, Rockford, IL).
Statistics
Student’s two tailed t-test was used to determine the significance of differences between group means.
Results
Suppression of ongoing EAMG by i.p. administration of a mixture of human muscle AChR subunit constructs
Rats immunized repeatedly with these bacterially-expressed human muscle AChR subunit constructs in TiterMax adjuvant developed high titers of antibodies to rat muscle AChR, but did not develop EAMG.21 Oral treatment of rats with the subunit mixture prevented induction of EAMG, and modestly reduced the severity of ongoing EAMG.21 Nasal treatment with the subunit mixture was very potent at preventing EAMG, but ineffective at suppressing ongoing EAMG.
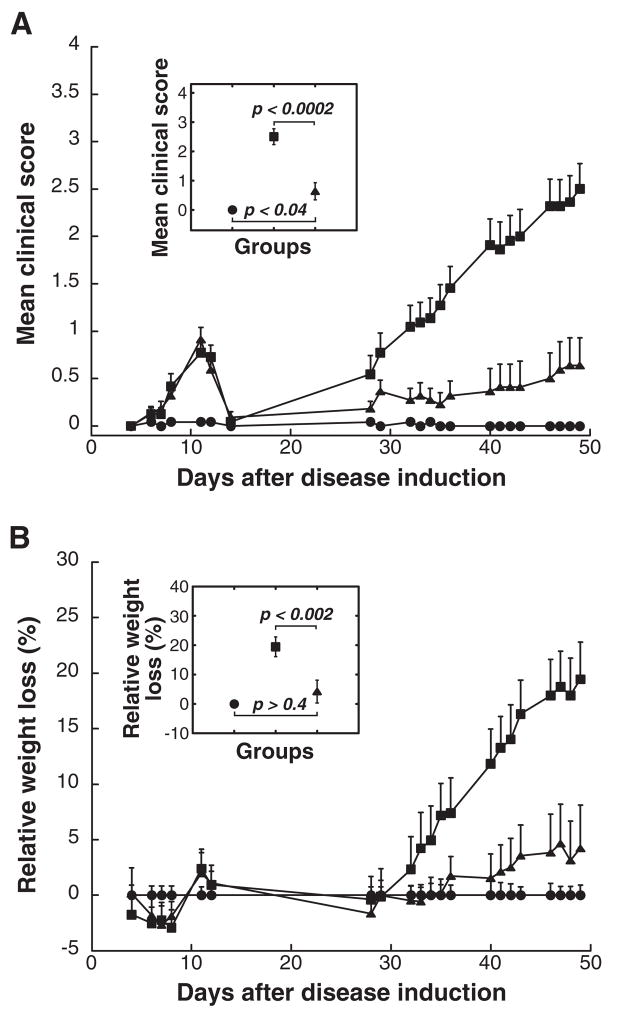
Administration of the subunit mixture i.p. suppressed ongoing EAMG more effectively than oral administration. Therapy was initiated on day 14, after the acute phase of EAMG. The acute phase provided a useful control showing that treated and untreated rats were equally affected prior to therapy. Treatment with 5 mg/dose of the subunit mixture substantially suppressed clinical symptoms (Fig 1). Seven weeks after induction of EAMG, the mean clinical score of rats treated with the subunit mixture was reduced to 0.64 compared to 2.5 for untreated EAMG rats (Fig 1A). In contrast, no substantial suppression of EAMG was observed after similar treatment with either an irrelevant protein, ovalbumin (OVA), or an α4 neuronal AChR subunit construct (Fig 2A). This indicates that suppression of EAMG was antigen-specific. The extracellular domain 1–209 of human muscle AChR α1 subunits (α1-ECD) was not effective (Fig 2A). Doses of 5 mg were more effective than 1 or 2 mg (Fig 3).
Figure 1. EAMG was substantially suppressed in rats treated i.p. with 5 mg doses of a mixture of human α1, β1, γ, δ, and ε subunits at weekly intervals starting on day 14 following disease induction.
All rats, except adjuvant control (closed circles) which received only adjuvant, were immunized with 35 μg of Torpedo californica AChR in TiterMax adjuvant at day 0. Treatments (i.p., 5 mg/dose) were initiated after the acute phase, 14 days after EAMG induction, and thereafter once a week for 5 weeks until the end of the experiments. The EAMG control rats received no treatment (closed squares). Data represent the mean ± SE of two independent experiments (n = 12 for each point, from 6 rat groups in each of two experiments). (A) The mean clinical scores of the rats treated with the subunit mixture (closed triangles) were significantly lower when compared to those of the EAMG control rats (closed squares) at all time points after day 30 (p < 0.01). (B) The effect of the treatment on clinical symptoms was corroborated by changes in the rats’ body weight. Relative weight loss was calculated as follows: % relative weight loss = 100 − (body weight on day X/average body weight of adjuvant control on day X) × 100. The insert indicates the statistical significance of the difference between groups on day 49 by the t-test.
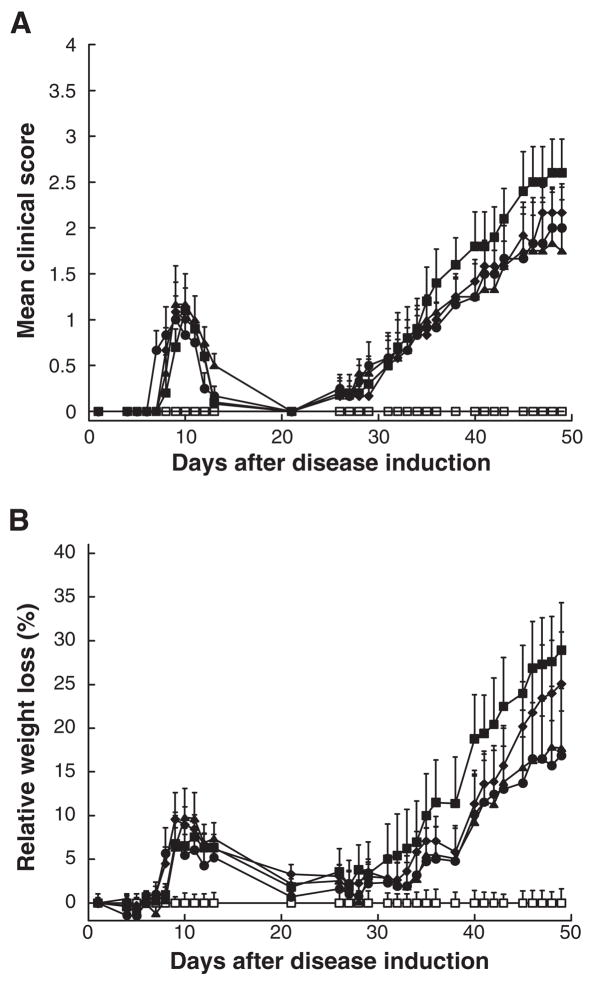
Figure 2. EAMG was not suppressed in rats treated i.p. with the same 5 mg doses of OVA, α4 AChR subunits, or α1-ECD on the same weekly dosage schedule starting on day 14.
All rats were immunized as described in Fig 1. Adjuvant control rats (open squares) received only adjuvant. Each group consisted of six rats. The EAMG control rats (closed squares) were the weakest. Control therapies of EAMG, also at 5 mg/dose i.p., including treatments with OVA (closed circle), human AChR α4 subunits (closed triangles), and α1-ECD (closed diamonds), were not statistically different from the EAMG control in clinical score (A) or weight loss (B). All groups showed similar acute phases with a peak around day 10.
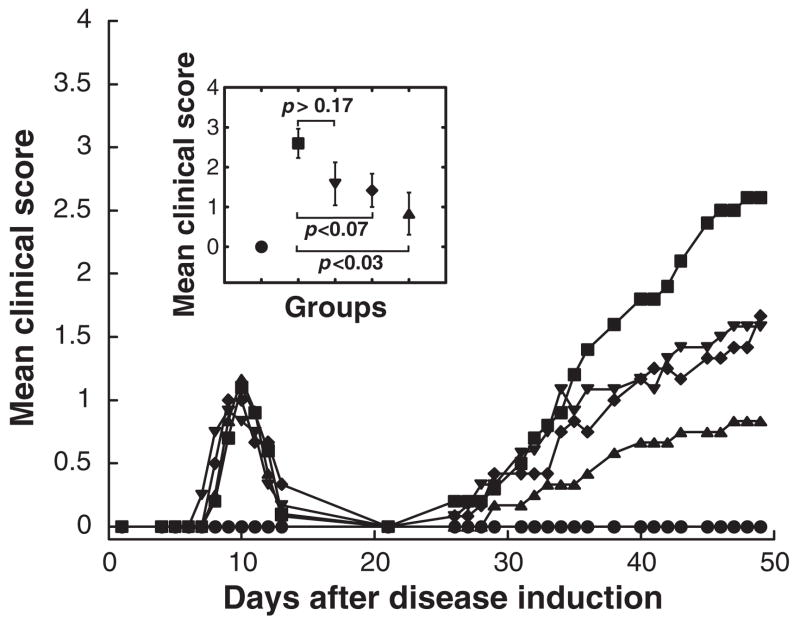
Figure 3. Treatment with the subunit mixture provided a significant dose-dependent therapeutic benefit with the 5 mg/dose being the most beneficial.
All rats were immunized as described in Fig 1. Adjuvant control rats (closed circles) received only adjuvant. Intraperitoneal treatments of EAMG consisted of 5 doses of 1 mg (closed reverse triangles), 2 mg (closed diamonds) or 5 mg subunit mixture (closed triangles) at weekly intervals starting on day 14. The insert indicates the statistical significance of the differences between the EAMG control group (closed squares) and groups treated with increasing doses of the subunit mixture on day 48 by the t-test.
Treatment with the AChR subunit mixture diverted autoantibody specificities towards pathologically irrelevant epitopes in the cytoplasmic domain
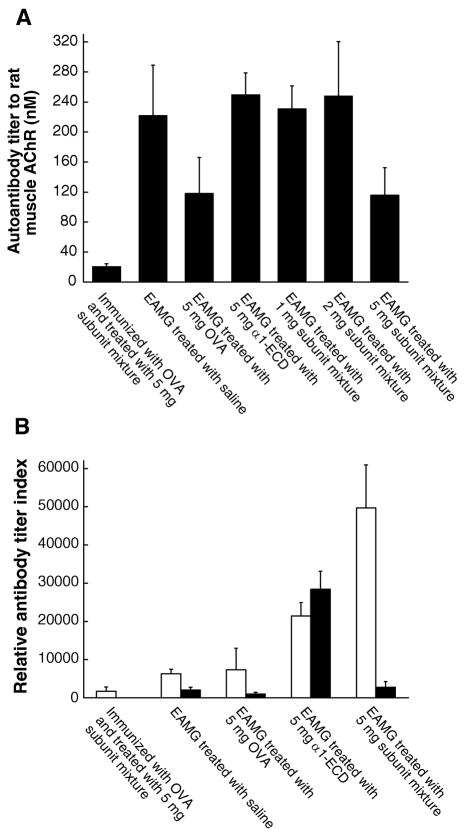
Rats treated with 5 mg doses of the subunit mixture had significantly decreased antibody titer to rat muscle AChR (116 nM as compared with 222 nM in EAMG rats in Fig 4A). Treatment with α1-ECD did not decrease the titer to rat AChR, consistent with its lack of effect on weakness. Treatment with 1 or 2 mg doses of the subunit mixture also did not reduce the titer to rat AChRs, although these treatments significantly reduced weakness (Fig 4A). Rats treated with 5 mg doses of OVA, which received no protection against EAMG, had the same level of autoantibody titer (118 nM) as those treated with 5 mg doses of the subunit mixture. Thus, measure of antibody titer to rat muscle AChR, including both antibodies to extracellular and cytoplasmic domains, did not precisely predict the clinical state of the rats. There must have been changes in specificities of autoantibodies from pathogenic to nonpathogenic epitopes, or in isotype from complement fixing to non-fixing.
Figure 4. Treatments greatly increased titers of antibodies to the constructs used for therapy but reduced or had little effect on titer to rat muscle AChRs.
These are the rats used in Fig 2 and 3. Sera of individual rats from 6 rat groups were collected 7 weeks after the induction of EAMG. (A) Antibody titer to rat muscle AChR was evaluated by immunoprecipitation of AChRs labeled with 4 nM 125I-αBgt. Treatment with 5 mg doses of the subunit mixture reduced autoantibody titer by half, while treating with 1 or 2 mg doses had no significant effect on the titers. Control therapy with 5 mg doses of OVA, which resulted in no clinical benefit, also decreased antibody titer to rat muscle AChR by half. The other control therapy, with 5 mg doses of <1-ECD, had no effect on autoantibody titer. Thus, antibody titer to rat muscle AChR, including antibodies to extracellular and cytoplasmic domains, was not correlated with the clinical state of the rats. Rats immunized with OVA, but not Torpedo AChR, then treated i.p. with 5 mg doses of the AChR subunit mixture, developed very low titers to rat muscle AChRs (less than 10% of that of the EAMG control rats). (B) Antibody titers to the subunit mixture (open bar) and to the <1-ECD (closed bar) were evaluated by ELISA assays. Untreated EAMG, as expected, resulted in some titer to both antigens. Treatment of EAMG with i.p. OVA had little effect on these titers. Therapies with either the subunit mixture or the <1-ECD greatly increased antibody responses to the constructs used for therapy. Rats immunized with ovalbumin, but not Torpedo AChR, then treated i.p. with subunits, developed low titers to the subunit mix, but no detectable response to <1 extracellular domain. This indicates that those antibodies from rats treated with the subunit mixture were primarily directed against cytoplasmic domains.
Treatment with the subunit constructs produced large amounts of antibodies to those constructs (Fig 4B). Treatment of EAMG with i.p. α1-ECD greatly increased titer to α1-ECD (14 fold), and increased titer to the subunit constructs which included α1 extracellular domains (3.4 fold). Treatment with the subunit mixture greatly (7.9 fold) increased the response to the subunit constructs, with little effect on the response to α1-ECD. Antibodies from rats treated with α1-ECD reacted equally well with α1-ECD and the subunit constructs, since the α1 subunits among these subunit constructs also contained the α1 extracellular domain. However, antibodies from rats treated with the subunit mixture bound poorly to α1-ECD. This indicates that antibodies from rats treated with the denatured subunit mixture were mainly directed against cytoplasmic domains, as expected.10
Immunogenicity of the i.p. subunit mixture was tested by treating rats which received a control OVA immunization. Without initial immunization with Torpedo AChR, treatment with the subunit mixture produced markedly lower antibody titers to both rat muscle AChR (< 18%, Fig 4A) and the subunit constructs (< 4%, Fig 4B) than successfully treated EAMG. This indicates that the large antibody response provoked by treatment of rats with EAMG with the subunit mixture depended on boosting their response to the cytoplasmic domains already primed by immunization with Torpedo AChR.
The markedly decreased autoantibody titer to native rat AChR in rats treated with the subunit mixture (Fig 4A), combined with the greatly boosted antibody response to the subunit constructs in these rats (Fig 4B), indicates that the autoimmune response was predominantly redirected to denatured subunits rather than native AChR. This can be explained by the loss of highly immunogenic pathologically significant epitopes which are conformation-dependent (like the MIR7) in these subunit constructs and the exposure of pathologically irrelevant epitopes, which are inaccessible in native AChRs.
Sera from successfully treated rats were much less effective at passive transfer
Sera from rats treated with the subunit mixture had little ability to passively transfer EAMG when compared to those of untreated EAMG rats (Fig 5). Sera from successfully treated rats, for equal amounts of antibodies to rat muscle AChRs, contained fewer pathologically significant autoantibodies.
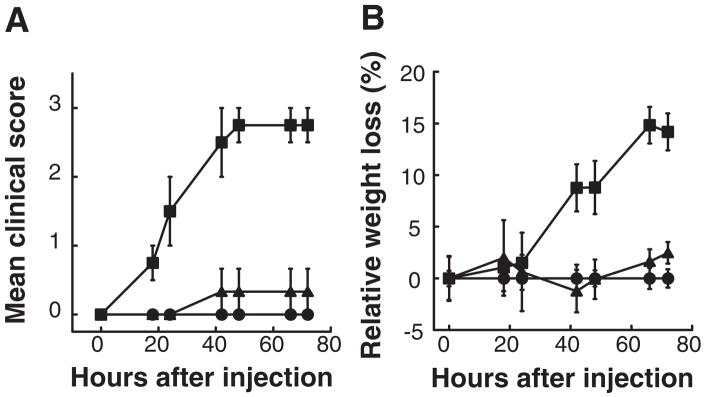
Figure 5. Sera from successfully treated rats were much less effective at passive transfer of EAMG.
Three rats per group were injected i.p. with pooled serum containing 70 pmol of autoantibodies to rat muscle AChR at time 0, and then were examined every 12–24 h for weight loss, muscular weakness and fatigability. This amounted to 0.79 ml of serum from rats treated with 5 mg doses of the subunit mixture (closed triangles) and 0.53 ml from untreated rats with EAMG (closed squares). 0.91 ml of serum from adjuvant control group was used as negative control (closed circles). Serum from rats treated with the subunit mixture had little ability to passively transfer EAMG when compared to that of untreated rats with EAMG (p < 0.04). The effect of injection of serum on clinical symptoms (A) was corroborated by changes in body weight of the rats (B).
Treatment with cytoplasmic domain constructs was more effective
If diversion of the autoimmune response towards cytoplasmic domains were the primary mechanism by which this therapeutic approach suppresses EAMG, the use of only cytoplasmic domains should be at least as effective as the use of whole subunit constructs.
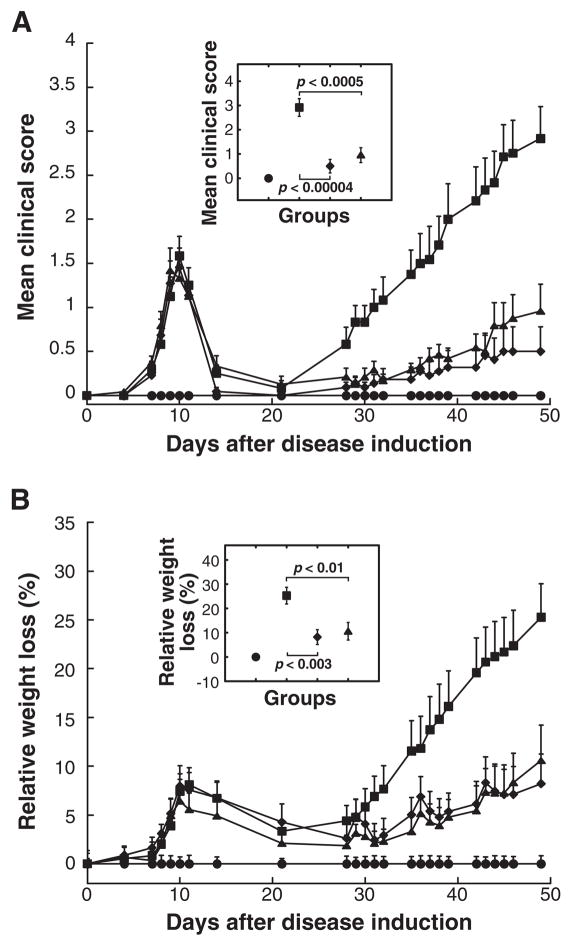
Treatment with a mixture of the cytoplasmic domain constructs (Table 1) was more potent in suppressing ongoing EAMG than treatment with the subunit mixture by measures of clinical state, weight loss, death rate, and EAMG incidence (Fig 6 and Table 2). Seven weeks after disease induction, all twelve untreated EAMG rats got sick, and six died of EAMG (mean clinical score 2.92). In the group treated with the subunit mixture, five out of twelve were healthy and none died (mean clinical score 1.05). In the group treated with the cytoplasmic domain mixture, eight out of eleven did not show any symptoms of EAMG, and none died (mean clinical score 0.50). Untreated EAMG rats lost 25% of body weight, as compared to an 11% loss in those treated with subunit mixture or an 8% loss in those treated with the cytoplasmic domain mixture. Treatment with 5 mg doses of the cytoplasmic domain mixture was the most beneficial, but a dose of only 0.5 mg of cytoplasmic domains prevented all death from EAMG (0/6 deaths in treated rats, versus 4/6 deaths in untreated rats).
Figure 6. Treatment with only cytoplasmic domains was even more effective in suppressing ongoing EAMG than using both extracellular and cytoplasmic domains.
All rats, except adjuvant control (closed circles), were immunized with 70 μg of Torpedo AChR in TiterMax adjuvant at day 0. This is twice the dose used in Fig 1–5. Treatments of EAMG consisted of 5 i.p. doses of 5 mg of the subunit mixture (closed triangles) or a mixture of the cytoplasmic domain constructs in the weight ratio 1:1 (closed diamonds) at weekly intervals starting on day 14. Data represent the mean ± SE of two independent experiments (n = 12 for each point, from 6 rat groups in each of two experiments). (A) The mean clinical scores of both the rats treated with the subunit mixture (closed triangles) and those treated with the cytoplasmic domain mixture were significantly lower when compared to those of the untreated rats (closed squares) at all time points after day 29 (p < 0.01 and p< 0.005, respectively). (B) The effect of the treatment on clinical symptoms was corroborated by changes in body weight. The rats treated with the cytoplasmic domain mixture had less weakness and weight loss than those treated with the subunit mixture. The insert indicates the statistical significance of the differences between untreated EAMG group (closed square) and groups treated with either the subunit mixture or the cytoplasmic domain mixture on day 49 by the t-test. Detailed features of the rats in these experiments are presented in Table 2.
Table 2.
Detailed clinical score data for the experiments shown in Fig 6.
| Phase of EAMG | Group | Clinical scores (affected rats/total in group) | EAMG Incidence | |||||
|---|---|---|---|---|---|---|---|---|
| 0–1 | 1–2 | 2–3 | 3–4 | Deaths | Mean | |||
| Acute (day 10) (before treatment) | EAMG control | 1/12 | 4/12 | 6/12 | 1/12 | 0/12 | 1.63 | 11/12 |
| Treated with extracellular and cytoplasmic domains | 2/12 | 4/12 | 5/12 | 1/12 | 0/12 | 1.63 | 10/12 | |
| Treated with cytoplasmic domains | 0/11 | 7/11 | 3/11 | 1/11 | 0/11 | 1.59 | 11/11 | |
| Chronic (day 49) (after treatment) | EAMG control | 0/12 | 3/12 | 2/12 | 1/12 | 6/12 | 2.92 | 12/12 |
| Treated with extracellular and cytoplasmic domains | 5/12 | 4/12 | 2/12 | 1/12 | 0/12 | 0.96 | 7/12 | |
| Treated with cytoplasmic domains | 8/11 | 2/11 | 0/11 | 1/11 | 0/11 | 0.50 | 3/11 | |
Treatment with the cytoplasmic domain mixture was accompanied by a shift of AChR-specific antibody isotypes
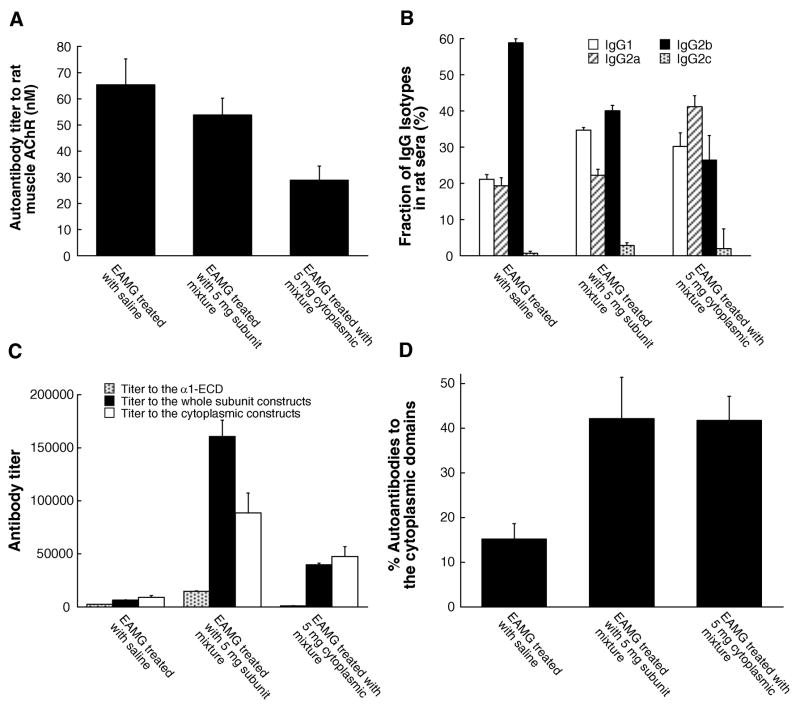
There was a 55% decrease in antibodies to native rat muscle AChR in rats treated with the cytoplasmic domain mixture (29 nM as compared with 65 nM in untreated EAMG rats at day 49). Rats treated with the subunit mixture had slightly decreased antibody titer to rat muscle AChR (54 nM) (Fig 7A).
Figure 7. Successful suppression of EAMG greatly increased antibody titers to the constructs used for therapy, and increased the fraction of autoantibodies to rat muscle AChR cytoplasmic domains, while modestly reducing titer to rat muscle AChR, and slightly changing autoantibody isotypes.
These are rats used in Fig 6. Sera of individual rats from 6 rat groups were collected 7 weeks after the induction of EAMG. (A) These sera were assayed for their immunoprecipitation titer to 125I-αBgt labeled rat muscle AChRs. Treatment with the subunit mixture decreased the titer to rat muscle AChR slightly (18%). Treatment with the cytoplasmic domain mixture substantially reduced the response (56%). (B) Anti-AChR IgG isotypes were determined by ELISA assays. These data represent the proportion of each IgG isotype in rat serum. Both therapies increased the proportion of IgG1 and decreased IgG2b. (C) These sera were also assayed by ELISA for reaction with subunit constructs containing both extracellular and cytoplasmic sequences, containing only the α1 extracellular domain, or only cytoplasmic domain sequences. Treatments with either the subunit mixture or the cytoplasmic domain mixture provoked large amounts of antibodies to the constructs used for therapy. Antibodies from the treated rats were primarily directed against the cytoplasmic domains. In particular, unlike treating with the subunit mixture, treatment with the cytoplasmic domain mixture not only avoided provoking antibody response to the α1 extracellular domain, but actually reduced antibodies to the α1 extracellular domains as compared with both the untreated EAMG rats and, especially, rats treated with the subunit mixture. (D) The fraction of AChR-specific autoantibodies which were directed against cytoplasmic domains was also assayed by inhibition of rat serum binding to 125I αBgt labeled rat muscle AChR by 50 nM of the cytoplasmic domain constructs. The proportion of autoantibodies to the cytoplasmic domain is calculated as follows: % Abs to cytoplasmic domain = (1 - titer in the presence of the cytoplasmic constructs/titer in the presence of OVA) × 100. Both treatments significantly increased the fraction of autoantibodies specific for cytoplasmic domains.
Therapy moderately shifted the AChR-specific IgG isotype profile in sera (Fig 7B). Limited changes in proportions of IgG1 and IgG2 isotypes (14% in the rats treated with the subunit mixture and 9% in those treated with the cytoplasmic mixture) were not sufficient to account for the extent of suppression of EAMG in the treated rats (see Supplementary Text for further details).
Treatment with the cytoplasmic domain mixture shifted autoantibody specificities toward cytoplasmic domains
Treatment with the subunit mixture greatly increased titers to the whole subunit mixture (25 fold) and somewhat less (10 fold) to the cytoplasmic domain mixture (Fig 7C). The increase in autoantibodies to subunit constructs, in excess of the response to cytoplasmic domains, must be largely directed at buried pathologically irrelevant epitopes because the titer to native AChRs decreased. The extent of increase in response to α1 extracellular domains was less (6 fold). Thus, the response was greatly increased to the cytoplasmic domains, but the subunit constructs had the liability of also increasing the response to extracellular domains, which could form pathologically relevant epitopes.
Treatment with the cytoplasmic domain mixture increased titer only to the subunit and cytoplasmic domain mixture, and reduced the titer to the α1-ECD by 57% with respect to untreated EAMG (from titer 2480 to 1070) (Fig 7C). Thus, in rats treated only with cytoplasmic domains, the autoantibody response was diverted from the extracellular domain in untreated EAMG rats to the cytoplasmic domain.
Competitive inhibition of binding of rat sera to native muscle AChR by the cytoplasmic constructs gave an estimate of the minimum amounts of autoantibodies directed to cytoplasmic domains. Successful therapy significantly increased the fraction of autoantibodies specific for cytoplasmic domains (Fig 7D).
Therapy with the cytoplasmic domains reduced pathologically significant autoantibodies
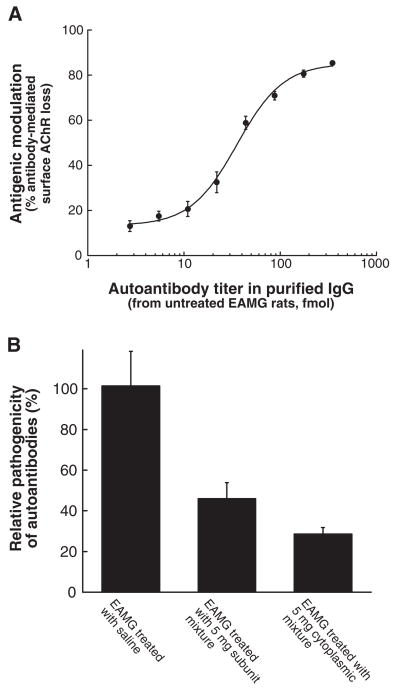
The amount of pathologically significant autoantibodies capable of causing antigenic modulation was analyzed on TE671 cells (Fig 8). Treatment with the subunit mixture caused 54% loss of pathologically significant autoantibodies, while treatment with the same doses of the cytoplasmic domain mixture resulted in a 71% loss. These large losses of pathogenic autoantibody specificities can be explained by diversion of the autoimmune response toward pathologically irrelevant cytoplasmic epitopes.
Figure 8. Sera from successfully treated rats had reduced ability to cause antigenic modulation of AChR in TE671 cells.
IgG antibodies were purified by Protein G Sepharose affinity chromatography from pooled serum from rats used in Fig 6. (A) Serially diluted IgG antibodies purified from the untreated EAMG rat serum pool were incubated for 4 hours with TE671 cells in 12-well tissue culture plates (Corning, Lowell, MA) in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Then antigenic modulation, measured as loss of αBgt binding sites, was determined after 3 hours incubation with 4 nM 125I-<Bgt.39 Background radioactivity was determined using a 100-fold excess of unlabeled <Bgt. IgG purified from normal rat serum was used as negative control to determine the total amount of surface AChRs. All measurements were in triplicate. The percent loss of surface AChR by antigenic modulation was calculated as follows: % loss of surface AChR = (1-Δcpm in the presence of antibodies/Δcpm in the presence of normal IgGs) ×100. Antigenic modulation was dose-dependent within tested antibody concentration range. (B) Based on the dose-dependence curve, the relative pathogenicity of the autoantibodies from the treated groups was calculated by the equation: % relative pathogenicity of the autoantibodies = (Anti-AChREAMG/Anti-AChRtreated) × 100. Anti-AChRtreated corresponds to the autoantibody titer of IgG from the treated groups used in antigenic modulation. Anti-AChREAMG corresponds to the autoantibody titer of IgG from the EAMG control rat serum, which cause an equivalent extent of antigenic modulation in TE671 cells. Treatment with 5 mg doses of the subunit mixture caused a 54% loss of pathologically significant autoantibodies, while treating with the same doses of the cytoplasmic domain mixture caused a 71% loss of pathologically significant autoantibodies.
Discussion
Treatment of autoimmune diseases usually involves non-specific immunosuppressants, which may suppress desirable responses and cause serious side effects.11, 12, 31 An ideal specific immunosuppressive therapy would suppress only responses to pathologically significant epitopes. Autoantigen therapy risks exacerbation rather than improvement of the disease.17, 26, 32 Therapies which depend on a particular epitope might be successful in a model disease using an inbred animal and lost on translation to the disease in an outbred human population, or the animal model might not adequately model the human disease.33
Here we demonstrated that i.p. injection of bacterially-expressed human AChR cytoplasmic domain constructs is effective therapy for ongoing EAMG by diverting the autoimmune response from pathologically significant epitopes on the extracellular domains of muscle AChRs to pathologically irrelevant epitopes in their cytoplasmic domains. Treatment with constructs from α1, β1, γ, δ, and ε subunits is robust because many epitopes are involved. Treatment with only cytoplasmic domains is incrementally more potent than constructs containing extracellular sequences by many measures: clinical state, weight loss, death rate, EAMG incidence, reduction in titer to muscle AChR, and reduction in antigenic modulation. This is because there is no possibility of competing stimulation of responses to pathologically significant extracellular epitopes like the MIR, only diversion to pathologically irrelevant epitopes. Treatment with only cytoplasmic domains is safer because no potentially pathologically significant sequences are used, thereby avoiding the potential for damaging epitope spreading.
Explanation of the mechanisms by which i.p. treatment with denatured AChR subunits reduces the pathological autoantibody response involves four concepts: 1) The therapeutic immunogen must be contiguous with the disease-inducing immunogen (or perhaps have been in the initial depot with it), because irrelevant (OVA) or distantly related (α4) immunogens were not therapeutically effective. 2) There must be an endogenous limit on the total autoantibody response which causes a net decrease in response to pathological epitopes when the response to irrelevant epitopes is boosted with more antigen. 3) Large antibody responses to therapeutic immunogen given i.p. were observed only in rats already primed through immunization with native AChR in adjuvant. 4) T helper lymphocytes stimulated near the depot of AChR and adjuvant which induced EAMG might be responsible for: a) the boosted response to therapeutic antigen in rats with EAMG compared to adjuvant controls, b) part of the limitation on the total autoantibody response, and c) the requirement for muscle AChR antigen for therapy. In our experiments Torpedo AChR was used to induce EAMG in rats (through crossreaction of a tiny fraction of the total antibody response to Torpedo AChR), and therapy involved human AChR sequences (similar but not identical to rat AChR sequences). Human MG, induced by and directed against human AChR, might be treated with human AChR sequences more potently than is EAMG.
The mechanism of suppression of EAMG by i.p. injection of bacterially-expressed AChR constructs is quite different from those proposed for oral or nasal treatment, which involved changes in T regulatory lymphocytes to account for decreases in amount or affinity of autoantibodies to native muscle AChRs.23, 25, 32, 34, 35
Therapy with the cytoplasmic domain mixture was accompanied by a 55% decrease in total autoantibody titer to native rat muscle AChR and an additional 71% loss of pathologically relevant autoantibodies, resulting in a net 87% loss of pathogenicity. Concomitantly, titer to the cytoplasmic constructs used for therapy increased 12 fold. Thus, the net decrease in pathogenic autoantibody specificities reflected diversion of antibody specificity from conformation-dependent epitopes on the extracellular surface to epitopes on the cytoplasmic domain and other epitopes which are not accessible in vivo, rather than a net suppression of muscle AChR-related antibody response.
Immunization with native Torpedo AChR produced 70–80% of anti-AChR antibodies directed at extracellular epitopes. Antibodies to extracellular epitopes are only 10–20% of total when substituting denatured AChR for native AChR.10 This suggests that conformation-dependent extracellular sequences (e.g. the MIR7) are much more immunogenic than cytoplasmic sequences in native AChR. After denaturation of highly antigenic pathologically significant extracellular epitopes, cytoplasmic sequences are the most immunogenic by default. Immunization with either purified subunits from electric organ AChR or recombinant AChR extracellular fragments can induce EAMG, if sufficiently high doses are given.36, 37 Thus, the virtue of the use of AChR subunit cytoplasmic domains is obvious. Successful therapy with the cytoplasmic domain mixture indicates that the extracellular sequences were not required for effective therapy.
Specific immunosuppression of EAMG with bacterially-expressed human AChR cytoplasmic domains is a very promising approach. It specifically diverts the existing autoimmune response away from pathologically relevant epitopes, thus potently, robustly, and safely suppresses the pathogenic autoimmune response in EAMG. Autoantibodies to the cytoplasmic region of human AChR were detected in MG sera.38 This implies the existence of activated T and B cells specific for cytoplasmic epitopes in MG patients. This basic approach should be applicable to specific immunosuppression of MG. This approach to specific immunosuppressive therapy through diversion of the autoantibody response to pathologically irrelevant cytoplasmic domains should be applicable to other antibody-mediated autoimmune diseases.
Supplementary Material
Acknowledgments
This work was supported by grants to Jon Lindstrom from the National Institutes of Health (NS11323 and NS052463).
References
- 1.Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006;116:2843–2854. doi: 10.1172/JCI29894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindstrom JM. Acetylcholine receptors and myasthenia. Muscle Nerve. 2000;23:453–477. doi: 10.1002/(sici)1097-4598(200004)23:4<453::aid-mus3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Vincent A, Palace J, Hilton-Jones D. Myasthenia gravis. Lancet. 2001;357:2122–2128. doi: 10.1016/S0140-6736(00)05186-2. [DOI] [PubMed] [Google Scholar]
- 4.Patrick J, Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973;180:871–872. doi: 10.1126/science.180.4088.871. [DOI] [PubMed] [Google Scholar]
- 5.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzartos SJ, Barkas T, Cung MT, et al. Anatomy of the antigenic structure of a large membrane autoantigen, the muscle-type nicotinic acetylcholine receptor. Immunol Rev. 1998;163:89–120. doi: 10.1111/j.1600-065x.1998.tb01190.x. [DOI] [PubMed] [Google Scholar]
- 7.Luo J, Taylor P, Losen M, et al. Main immunogenic region structure promotes binding of conformation-dependent myasthenia gravis autoantibodies, nicotinic acetylcholine receptor conformation maturation, and agonist sensitivity. J Neurosci. 2009 doi: 10.1523/JNEUROSCI.2833-09.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzartos SJ, Seybold ME, Lindstrom JM. Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci USA. 1982;79:188–192. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beroukhim R, Unwin N. Three-dimensional location of the main immunogenic region of the acetylcholine receptor. Neuron. 1995;15:323–331. doi: 10.1016/0896-6273(95)90037-3. [DOI] [PubMed] [Google Scholar]
- 10.Froehner SC. Identification of exposed and buried determinants of the membrane-bound acetylcholine receptor from Torpedo californica. Biochemistry. 1981;20:4905–4915. doi: 10.1021/bi00520a016. [DOI] [PubMed] [Google Scholar]
- 11.Keesey JC. Clinical evaluation and management of myasthenia gravis. Muscle Nerve. 2004;29:484–505. doi: 10.1002/mus.20030. [DOI] [PubMed] [Google Scholar]
- 12.Phillips LH. The epidemiology of myasthenia gravis. Semin Neurol. 2004;24:17–20. doi: 10.1055/s-2004-829593. [DOI] [PubMed] [Google Scholar]
- 13.Bartfeld D, Fuchs S. Specific immunosuppression of experimental autoimmune myasthenia gravis by denatured acetylcholine receptor. Proc Natl Acad Sci U S A. 1978;75:4006–4010. doi: 10.1073/pnas.75.8.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krolick KA, Yeh TM, Edlund SA. Lewis rats given antibodies against denatured acetylcholine receptor become resistant to induction of experimental autoimmune myasthenia gravis. Cell Immunol. 1996;172:10–20. doi: 10.1006/cimm.1996.0209. [DOI] [PubMed] [Google Scholar]
- 15.Liblau R, Tisch R, Bercovici N, McDevitt HO. Systemic antigen in the treatment of T-cell-mediated autoimmune diseases. Immunol Today. 1997;18:599–604. doi: 10.1016/s0167-5699(97)01171-7. [DOI] [PubMed] [Google Scholar]
- 16.Weiner HL. Oral tolerance for the treatment of autoimmune diseases. Annu Rev Med. 1997;48:341–351. doi: 10.1146/annurev.med.48.1.341. [DOI] [PubMed] [Google Scholar]
- 17.Xiao BG, Link H. Mucosal tolerance: a two-edged sword to prevent and treat autoimmune diseases. Clin Immunol Immunopathol. 1997;85:119–128. doi: 10.1006/clin.1997.4432. [DOI] [PubMed] [Google Scholar]
- 18.Barchan D, Souroujon MC, Im SH, et al. Antigen-specific modulation of experimental myasthenia gravis: nasal tolerization with recombinant fragments of the human acetylcholine receptor α-subunit. Proc Natl Acad Sci U S A. 1999;96:8086–8091. doi: 10.1073/pnas.96.14.8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drachman DB, Okumura S, Adams RN, McIntosh KR. Oral tolerance in myasthenia gravis. Ann N Y Acad Sci. 1996;778:258–272. doi: 10.1111/j.1749-6632.1996.tb21134.x. [DOI] [PubMed] [Google Scholar]
- 20.Karachunski PI, Ostlie NS, Okita DK, Conti-Fine BM. Prevention of experimental myasthenia gravis by nasal administration of synthetic acetylcholine receptor T epitope sequences. J Clin Invest. 1997;100:3027–3035. doi: 10.1172/JCI119857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindstrom J, Peng X, Kuryatov A, et al. Molecular and antigenic structure of nicotinic acetylcholine receptors. Ann N Y Acad Sci. 1998;841:71–86. doi: 10.1111/j.1749-6632.1998.tb10910.x. [DOI] [PubMed] [Google Scholar]
- 22.Ma CG, Zhang GX, Xiao BG, et al. Suppression of experimental autoimmune myasthenia gravis by nasal administration of acetylcholine receptor. J Neuroimmunol. 1995;58:51–60. doi: 10.1016/0165-5728(94)00187-s. [DOI] [PubMed] [Google Scholar]
- 23.Shi FD, Li H, Wang H, et al. Mechanisms of nasal tolerance induction in experimental autoimmune myasthenia gravis: identification of regulatory cells. J Immunol. 1999;162:5757–5763. [PubMed] [Google Scholar]
- 24.Wang ZY, Huang J, Olsson T, et al. B cell responses to acetylcholine receptor in rats orally tolerized against experimental autoimmune myasthenia gravis. J Neurol Sci. 1995;128:167–174. doi: 10.1016/0022-510x(94)00235-g. [DOI] [PubMed] [Google Scholar]
- 25.Maiti PK, Feferman T, Im SH, et al. Immunosuppression of rat myasthenia gravis by oral administration of a syngeneic acetylcholine receptor fragment. J Neuroimmunol. 2004;152:112–120. doi: 10.1016/j.jneuroim.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Im SH, Barchan D, Souroujon MC, Fuchs S. Role of tolerogen conformation in induction of oral tolerance in experimental autoimmune myasthenia gravis. J Immunol. 2000;165:3599–3605. doi: 10.4049/jimmunol.165.7.3599. [DOI] [PubMed] [Google Scholar]
- 27.Lindstrom J, Luo J, Kuryatov A. Myasthenia gravis and the tops and bottoms of AChRs: antigenic structure of the MIR and specific immunosuppression of EAMG using AChR cytoplasmic domains. Ann N Y Acad Sci. 2008;1132:29–41. doi: 10.1196/annals.1405.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindstrom JM, Lennon VA, Seybold ME, Whittingham S. Experimental autoimmune myasthenia gravis and myasthenia gravis: biochemical and immunochemical aspects. Ann N Y Acad Sci. 1976;274:254–274. doi: 10.1111/j.1749-6632.1976.tb47691.x. [DOI] [PubMed] [Google Scholar]
- 29.Lennon VA, Lindstrom JM, Seybold ME. Experimental autoimmune myasthenia gravis: cellular and humoral immune responses. Ann N Y Acad Sci. 1976;274:283–299. doi: 10.1111/j.1749-6632.1976.tb47693.x. [DOI] [PubMed] [Google Scholar]
- 30.Protti MP, Manfredi AA, Horton RM, et al. Myasthenia gravis: recognition of a human autoantigen at the molecular level. Immunol Today. 1993;14:363–368. doi: 10.1016/0167-5699(93)90237-F. [DOI] [PubMed] [Google Scholar]
- 31.Alshekhlee A, Miles JD, Katirji B, et al. Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology. 2009;72:1548–1554. doi: 10.1212/WNL.0b013e3181a41211. [DOI] [PubMed] [Google Scholar]
- 32.Yi HJ, Chae CS, So JS, et al. Suppression of experimental myasthenia gravis by a B-cell epitope-free recombinant acetylcholine receptor. Mol Immunol. 2008;46:192–201. doi: 10.1016/j.molimm.2008.08.264. [DOI] [PubMed] [Google Scholar]
- 33.Wiendl H, Hohlfeld R. Therapeutic approaches in multiple sclerosis: lessons from failed and interrupted treatment trials. BioDrugs. 2002;16:183–200. doi: 10.2165/00063030-200216030-00003. [DOI] [PubMed] [Google Scholar]
- 34.Im SH, Barchan D, Fuchs S, Souroujon MC. Suppression of ongoing experimental myasthenia by oral treatment with an acetylcholine receptor recombinant fragment. J Clin Invest. 1999;104:1723–1730. doi: 10.1172/JCI8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi FD, Bai XF, Li HL, et al. Nasal tolerance in experimental autoimmune myasthenia gravis (EAMG): induction of protective tolerance in primed animals. Clin Exp Immunol. 1998;111:506–512. doi: 10.1046/j.1365-2249.1998.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lennon VA, Lambert EH, Leiby KR, et al. Recombinant human acetylcholine receptor α-subunit induces chronic experimental autoimmune myasthenia gravis. J Immunol. 1991;146:2245–2248. [PubMed] [Google Scholar]
- 37.Lindstrom J, Einarson B, Merlie J. Immunization of rats with polypeptide chains from torpedo acetylcholine receptor causes an autoimmune response to receptors in rat muscle. Proc Natl Acad Sci U S A. 1978;75:769–773. doi: 10.1073/pnas.75.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzartos SJ, Remoundos M. Detection of antibodies directed against the cytoplasmic region of the human acetylcholine receptor in sera from myasthenia gravis patients. Clin Exp Immunol. 1999;116:146–152. doi: 10.1046/j.1365-2249.1999.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mamalaki A, Trakas N, Tzartos SJ. Bacterial expression of a single-chain Fv fragment which efficiently protects the acetylcholine receptor against antigenic modulation caused by myasthenic antibodies. Eur J Immunol. 1993;23:1839–1845. doi: 10.1002/eji.1830230816. [DOI] [PubMed] [Google Scholar]
- 40.Balasa B, Deng C, Lee J, et al. The Th2 cytokine IL-4 is not required for the progression of antibody-dependent autoimmune myasthenia gravis. J Immunol. 1998;161:2856–2862. [PubMed] [Google Scholar]
- 41.Balasa B, Sarvetnick N. Is pathogenic humoral autoimmunity a Th1 response? Lessons from (for) myasthenia gravis. Immunol Today. 2000;21:19–23. doi: 10.1016/s0167-5699(99)01553-4. [DOI] [PubMed] [Google Scholar]
- 42.Saoudi A, Bernard I, Hoedemaekers A, et al. Experimental autoimmune myasthenia gravis may occur in the context of a polarized Th1- or Th2-type immune response in rats. J Immunol. 1999;162:7189–7197. [PubMed] [Google Scholar]
- 43.Link J, Soderstrom M, Ljungdahl A, et al. Organ-specific autoantigens induce interferon-γ and interleukin-4 mRNA expression in mononuclear cells in multiple sclerosis and myasthenia gravis. Neurology. 1994;44:728–734. doi: 10.1212/wnl.44.4.728. [DOI] [PubMed] [Google Scholar]
- 44.Yi Q, Lefvert AK. Idiotype- and anti-idiotype-reactive T lymphocytes in myasthenia gravis. Evidence for the involvement of different subpopulations of T helper lymphocytes. J Immunol. 1994;153:3353–3359. [PubMed] [Google Scholar]
- 45.De Baets M, Stassen M, Losen M, et al. Immunoregulation in experimental autoimmune myasthenia gravis--about T cells, antibodies, and endplates. Ann N Y Acad Sci. 2003;998:308–317. doi: 10.1196/annals.1254.033. [DOI] [PubMed] [Google Scholar]
- 46.Medgyesi GA, Fust G, Gergely J, Bazin H. Classes and subclasses of rat immunoglobulins: interaction with the complement system and with staphylococcal protein A. Immunochemistry. 1978;15:125–129. doi: 10.1016/0161-5890(78)90052-4. [DOI] [PubMed] [Google Scholar]
- 47.Gomez CM, Richman DP. Monoclonal anti-acetylcholine receptor antibodies with differing capacities to induce experimental autoimmune myasthenia gravis. J Immunol. 1985;135:234–241. [PubMed] [Google Scholar]
- 48.Tzartos S, Hochschwender S, Vasquez P, Lindstrom J. Passive transfer of experimental autoimmune myasthenia gravis by monoclonal antibodies to the main immunogenic region of the acetylcholine receptor. J Neuroimmunol. 1987;15:185–194. doi: 10.1016/0165-5728(87)90092-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.