Abstract
Background
Chronic kidney disease becomes common with age and is characterized on renal biopsy by global glomerulosclerosis, tubular atrophy, interstitial fibrosis, and arteriosclerosis.
Objective
To see if the prevalence of these histological abnormalities in the kidney increases with age in healthy adults and whether histological findings are explained by age-related differences in kidney function or chronic kidney disease risk factors.
Design
Cross-sectional study
Setting
Mayo Clinic from 1999 to 2009
Patients
1203 Adult living kidney donors
Measurements
Core needle biopsy of the renal cortex obtained during surgical implantation of the kidney and medical record data of kidney function and risk factors obtained prior to donation.
Results
The prevalence of nephrosclerosis (two or more chronic histological abnormalities) was 2.7% (95% CI: 1.1-6.7%) for ages 18-29 y, 16% (12-20%) for ages 30-39 y, 28% (24-32%) for ages 40-49 y, 44% (38-50%) for ages 50-59 y, 58% (47-67%) for ages 60-69 y, and 73% (43-90%) for ages 70-77 y. Adjustment for kidney function and risk factor covariates did not explain the age-related increase in the prevalence of nephrosclerosis.
Limitations
Kidney donors are selected for health and lack the spectrum or severity of renal pathology in the general population.
Conclusions
Kidney function and chronic kidney disease risk factors fail to explain the strong association between age and nephrosclerosis in healthy adults.
Primary Funding Source
National Institutes of Health, US Public Health Service.
Background
Chronic kidney disease (CKD) is defined by a reduced estimated glomerular filtration rate (GFR) or elevated urine albumin.(1) Age is the strongest predictor of CKD,(2) and CKD is present among 47% of adults ages 70 y and older compared to 4% of adults ages 20-39 y.(1) The underlying cause of CKD is often unclear.(3) The cost, discomfort, morbidity, and lack of perceived benefit restrict use of renal biopsies. It is suspected that the age-related reduction in GFR is reflective of chronic histological abnormalities on renal biopsy.(4-9) The histological abnormalities of CKD are global glomerulosclerosis, tubular atrophy, interstitial fibrosis, and arteriosclerosis, which together can be described as “nephrosclerosis”.(10) These histological abnormalities are clearly associated with reduced GFR and risk of kidney failure among clinically diagnosed CKD patients and transplant recipients.(11-14) Mild nephrosclerosis is also known to occur with aging in autopsy series,(15-18) but whether nephrosclerosis occurs in older adults with normal kidney function and in the absence of CKD risk factors is unknown. Living kidney donors provide a unique opportunity to relate clinical characteristics to renal histology. Since donors are selected on good health, the spectrum and severity of renal pathology is limited, but this is advantageous to the study of aging in the absence of comorbidity. The purpose of this study was to see if the prevalence of histological abnormalities in the kidney increases with age in healthy adults, and whether histological findings are explained by age-related differences in kidney function or chronic kidney disease risk factors.
Methods
Study Population
This study was approved by the Mayo Clinic Institutional Review Board. Living kidney donors at the Mayo Clinic in Rochester, Minnesota between May 10, 1999 and February 4, 2009 in which a needle core biopsy of the allograft was obtained during implantation were identified. Donors who declined Minnesota research authorization were excluded. All kidney donors underwent a thorough evaluation with a pre-scheduled battery of tests. Younger adults were held to higher standards for health than older adults in the interpretation of specific test results and in the final assessment to approve or reject as kidney donors. Potential candidates were generally excluded if they had a measured GFR (iothalamate clearance) below the age-specific 5th percentile (19) or microalbuminuria (>30 mg per 24 hours). Patients with diabetes mellitus were excluded. Patients with glucose intolerance were excluded based on age-specific thresholds (110 mg/dl or lower). Persons with hypertension were permitted as kidney donors if they were controlled on minimal antihypertensive therapy (up to two agents if one was a thiazide diuretic). Serum lipids and uric acid levels were collected by protocol, but had a limited role in donor approval.
Donor Characteristics
Donor characteristics and test results were obtained from the medical record prior to donation. If multiple test results were present, the result closest to kidney donation was analyzed. Medical records were manually reviewed to obtain test results completed outside the Mayo Clinic. The kidney function tests obtained were serum creatinine, estimated GFR by the Modification of Diet in Renal Disease study equation,(20) measured GFR by cold iothalamate clearance,(19) and 24-h urine protein and albumin. The serum creatinine assay was standardized (20) in October 2006 and prior serum creatinine levels were adjusted by subtracting 0.14 mg/dl (the mean change in donor serum creatinine levels after standardization). CKD was defined by an estimated GFR <60 ml/min/1.73 m2 or a 24-h urine albumin >30 mg.(21) An ambulatory blood pressure monitor obtained systolic blood pressure and diastolic blood pressure readings every 10 minutes during the day and every 20 minutes during the night over an 18-h period. The mean overall blood pressure, mean active blood pressure (4 PM to 9 PM), and mean nocturnal blood pressure (12 AM to 5 AM) were analyzed. Hypertension was defined by use of anti-hypertensive therapy. Family history of end-stage renal disease (ESRD) was defined by the recipient being related to the donor. Serum lipids, glucose, and uric acid were obtained from a fasting blood draw.
Kidney Histology
Implantation renal biopsies were a routine part of the transplantation surgery intended to provide baseline histology for the recipient. After vascular and ureteral anastomoses of the allograft in the recipient, biopsies were obtained with an 18-gauge Bard Monopty biopsy gun with a 1.7cm specimen slot (Bard Peripheral Vascular Inc. Tempe, AZ). Formalin-fixed, paraffin-embedded sections stained with hematoxylin and eosin, periodic acid-Schiff, methenamine silver, and Masson's trichrome were examined by renal pathologists. Since biopsies were assigned to the recipient, pathologists were blinded to most donor characteristics. However, donor-recipient relationships (e.g., parent-child) were documented in the recipient's chart, and in theory, could have been used to approximate donor age.
Biopsy reports were manually abstracted for chronic histological abnormalities. Reports usually provided a Banff 1997 classification score for chronic/sclerosing lesions (12) and if not, a score was inferred from the descriptive text (none=0, mild=1, moderate=2). Scores for interstitial fibrosis were 0 if less than 5% of cortex, 1 if 6-25% of cortex, and 2 if 26-50% of cortex. Scores for tubular atrophy were 0 if no atrophic tubules, 1 if up to 25% were atrophic, and 2 if 26-50% were atrophic. Scores for arteriosclerosis were 0 if no narrowing, 1 if up to 25% luminal narrowing, and 2 if 26-50% luminal narrowing, and 3 if >50% luminal narrowing for the most severely affected vessel. The proportion global glomerulosclerosis among glomeruli present was classified as 0 if 0%, 1 if up to 25%, and 2 if 26-50%. Biopsy quality (total length of biopsy cores, number of glomeruli, and presence of medulla) was abstracted from pathology reports.
Validation and Reproducibility of Kidney histology
To address the concern that pathologists could have estimated the donor age from the donor-recipient relationship, thereby introducing a diagnostic suspicion bias, biopsy slides were re-read (L.D.C.) in a blinded manner and in a random order for the 19 oldest and 19 youngest donors. To evaluate the reproducibility of chronic histological abnormalities on repeat biopsies, recipients of ABO incompatible kidneys were identified. These transplant recipients are at increased risk for subclinical acute rejection, and thus, underwent a percutaneous repeat biopsy within 3 weeks of transplantation.
Statistical Analysis
Donors with only medulla (no cortex) on renal biopsy were excluded. Donor characteristics and chronic histological abnormalities overall and by age groups (18-29, 30-39, 40-49, 50-59, 60-69, and 70-77 years) were described. Chronic histological abnormalities were dichotomized between a score of 0 versus 1 or higher and logistic regression models assessed whether these histological abnormalities co-segregated. A sclerosis score was defined as the sum of abnormalities between glomerulosclerosis, tubular atrophy, interstitial fibrosis, and arteriosclerosis (0 to 4). As there is no universal histological definition of ‘nephrosclerosis,’(7) this study defined nephrosclerosis a priori as the lowest sclerosis score threshold with a prevalence <5% in the 18-29 y age group. Reproducibility of nephrosclerosis was assessed with an agreement statistic (kappa) in the subset with repeat biopsies. Logistic regression models identified characteristics associated with nephrosclerosis. The multivariable model included age, sex, 24-h urine albumin, measured GFR, nocturnal systolic and diastolic blood pressure, hypertension, family history of ESRD, body mass index, total cholesterol, glucose, and uric acid. A p-value <.05 was considered statistically significant. Analyses were completed using JMP 8.0 and SAS 9.1 (SAS Institute Inc, Cary, NC).
Results
There were 1221 living kidney donors identified with an implantation biopsy of the allograft. After excluding 18 donors with biopsies of only medulla, there were 1203 donors used in the analysis. The number of needle core biopsies obtained from each donor allograft was one in 87% and two or more in 13%. The mean ± SD total length of the core biopsies for each donor was 1.6 ± 0.4 cm. Biopsies had a mean ± SD of 20 ± 9 glomeruli and were described as cortex in 61%, cortex and medulla in 30%, and renal parenchyma in 9%. Of the 1203 kidney donors, test results were available for 24-h urine albumin in 94%, measured GFR in 94%, 18-h ambulatory blood pressure in 90%, BMI in 99%, and blood tests in at least 97%. Among these kidney donors, 93% were white; other characteristics by age group are described in Table 1. Measured GFR was the characteristic with the strongest correlation to age (r=-0.42, p<0.001) in this population.
Table 1.
Characteristics of kidney donors, overall and by age group, presented as mean ± SD or frequency (%)
| Characteristic | Overall n=1203 | 18-29 y n=148 | 30-39 y n=303 | 40-49 y n=392 | 50-59 y n=257 | 60-69 y n=92 | 70-77 y n=11 | Trend Test p-value† |
|---|---|---|---|---|---|---|---|---|
| Age, y | 44 ± 11 | 25 ± 3 | 35 ± 3 | 45 ± 3 | 54 ± 3 | 64 ± 3 | 72 ± 2 | |
| Male |
510 (42%) |
74 (50%) |
143 (47%) |
153 (39%) |
93 (36%) |
43 (47%) |
4 (36%) |
.038 |
| Kidney Function | ||||||||
| 24-h urine protein, mg | 46 ± 28 | 44 ± 24 | 42 ± 23 | 46 ± 29 | 47 ± 31 | 52 ± 29 | 62 ± 58 | 0.002 |
| 24-h urine albumin, mg | 6.2 ± 9.2 | 6.6 ± 7.8 | 6.1 ± 6.2 | 6.2 ± 9.7 | 5.4 ± 11 | 6.8 ± 8.7 | 14 ± 25 | 0.96 |
| Serum creatinine, mg/dl | 0.89 ± 0.16 | 0.88 ± 0.15 | 0.90 ± 0.15 | 0.90 ± 0.17 | 0.88 ± 0.16 | 0.91 ± 0.16 | 0.89 ± 0.12 | 0.57 |
| Estimated GFR, ml/min/1.73 m2 | 81 ± 14 | 94 ± 14 | 84 ± 13 | 79 ± 13 | 77 ± 12 | 74 ± 10 | 70 ± 7 | <0.001 |
| Measured.GFR, ml/min/1.73 m2 | 104 ± 17 | 114 ± 19 | 110 ± 18 | 103 ± 15 | 97 ± 14 | 90 ± 12 | 86 ± 11 | <0.001 |
| Chronic kidney disease* |
55 (4.8%) |
2 (1.4%) |
8 (2.8%) |
12 (3.2%) |
22 (8.9%) |
10 (12%) |
1 (11%) |
<0.001 |
| Blood Pressure | ||||||||
| 18-h mean systolic BP, mmHg | 119 ± 9 | 118 ± 8 | 118 ± 9 | 118 ± 9 | 120 ± 10 | 124 ± 11 | 126 ± 9 | <0.001 |
| 18-h mean active systolic BP, mmHg | 122 ± 10 | 121 ± 9 | 122 ± 10 | 121 ± 10 | 123 ± 11 | 128 ± 12 | 129 ± 12 | <0.001 |
| 18-h mean nocturnal systolic BP, mmHg | 108 ± 10 | 108 ± 8 | 107 ± 9 | 107 ± 10 | 108 ± 10 | 110 ± 11 | 112 ± 10 | 0.18 |
| 18-h mean diastolic BP, mmHg | 73 ± 7 | 70 ± 6 | 72 ± 6 | 73 ± 7 | 74 ± 7 | 74 ± 8 | 76 ± 8 | <0.001 |
| 18-h mean active diastolic BP , mmHg | 75 ± 8 | 73 ± 7 | 75 ± 7 | 76 ± 8 | 76 ± 8 | 76 ± 10 | 79 ± 10 | <0.001 |
| 18-h mean nocturnal diastolic BP, mmHg | 63 ± 8 | 60 ± 7 | 62 ± 7 | 63 ± 8 | 64 ± 8 | 64 ± 8 | 66 ± 10 | <0.001 |
| Hypertension (on therapy) |
71 (5.9%) |
0 (0.0%) |
6 (2.0%) |
18 (4.6%) |
26 (10%) |
18 (20%) |
3 (27%) |
<0.001 |
| Other CKD Risk Factor | ||||||||
| Family history of ESRD | 699 (58%) | 106 (72%) | 192 (63%) | 238 (61%) | 115 (45%) | 43 (47%) | 5 (45%) | <0.001 |
| Body mass index, kg/m2 | 2 ± 5 | 27 ± 5 | 28 ± 6 | 28 ± 6 | 28 ± 5 | 28 ± 5 | 26 ± 4 | 0.31 |
| Body mass index > 30 kg/m2 | 333 (30%) | 33 (22%) | 101 (33%) | 124 (32%) | 71 (28%) | 33 (36%) | 1 (9.1%) | 0.55 |
| Total cholesterol, mg/dl | 197 ± 37 | 170 ± 30 | 192 ± 38 | 200 ± 36 | 210 ± 34 | 203 ± 38 | 202 ± 36 | <0.001 |
| Triglycerides, mg/dl | 127 ± 95 | 113 ± 85 | 130 ± 73 | 132 ±127 | 123 ± 74 | 127 ± 71 | 100 ± 42 | 0.90 |
| High density cholesterol, mg/dl | 57 ± 16 | 51 ± 12 | 53 ± 14 | 57 ± 16 | 62 ± 18 | 60 ± 17 | 66 ± 15 | <0.001 |
| Low density cholesterol, mg/dl | 116 ± 35 | 99 ± 36 | 114 ± 38 | 120 ± 35 | 123 ± 30 | 116 ± 35 | 116 ± 31 | <0.001 |
| Glucose, mg/dl | 95 ± 9 | 91 ± 8 | 94 ± 8 | 95 ± 9 | 98 ± 8 | 99 ± 9 | 97 ± 7 | <0.001 |
| Uric acid, mg/dl | 5.2 ± 1.4 | 5.3 ± 1.4 | 5.3 ± 1.5 | 5.0 ± 1.3 | 5.0 ± 1.3 | 5.5 ± 1.4 | 5.6 ± 1 | 0.64 |
Chronic kidney disease defined by an estimated GFR <60 ml/min/1.73 m2 or a 24-h urine albumin >30 mg. Physicians relied more on measured GFR than estimated GFR at the time of donor selection.
Trend test was the p-value for Pearson's correlation between the characteristic and age (continuous).
SD = standard deviation, BP = blood pressure. GFR = glomerular filtration rate
The prevalence of any global glomerulosclerosis was 48%, any tubular atrophy was 22%, interstitial fibrosis >5% was 4.9%, and any arteriosclerosis was 32%. These abnormalities co-segregated; there was an association for all pair wise comparisons (p<.001 for each pair) that was preserved with age-adjustment (p<.001 for each pair), except between glomerulosclerosis and arteriosclerosis after age-adjustment (p=0.40). The individual and sum presence of these abnormalities (sclerosis score) increased with age, though abnormalities in the glomeruli, tubules, interstitium, and arteries generally affected less than 25% of these regions (Online Table 1). Nephrosclerosis, defined by two or more different histological abnormalities, increased with age (Table 2). Nephrosclerosis had a prevalence of only 2.7% (95% CI, 1.1% to 6.7%) in the youngest age-group but 28% (95% CI, 25% to 30%) overall. The prevalence of nephrosclerosis was similar between men and women (26% vs 29%, age-adjusted p=0.23). Excluding person with hypertension only slightly decreased the prevalence of nephrosclerosis. Adjusting for age, sex, 24-h urine albumin, measured GFR, nocturnal systolic and diastolic blood pressure, hypertension, family history of ESRD, body mass index, total cholesterol, glucose, and uric acid did not substantially alter the trend in increasing prevalence of nephrosclerosis associated with age in crude analyses.
Table 2.
Prevalence of nephrosclerosis by age group among 1203 living kidney donors at the Mayo Clinic
| Age group |
Crude prevalence |
Crude prevalence after excluding persons on therapy for hypertension |
|---|---|---|
| % (95% CI) | % (95% CI) | |
| 18-29 y | 2.7% (1.1% to 6.7%) | 2.7% (1.1% to 6.7%) |
| 30-39 y | 16% (12% to 20%) | 15% (12% to 20%) |
| 40-49 y | 28% (24% to 32%) | 26% (22% to 31%) |
| 50-59 y | 44% (38% to 50%) | 42% (36% to 49%) |
| 60-69 y | 58% (47% to 67%) | 55% (44% to 66%) |
| 70-77 y | 73% (43% to 90%) | 75% (41% to 93%) |
| Overall | 28% (25% to 30%) | 26% (24% to 29%) |
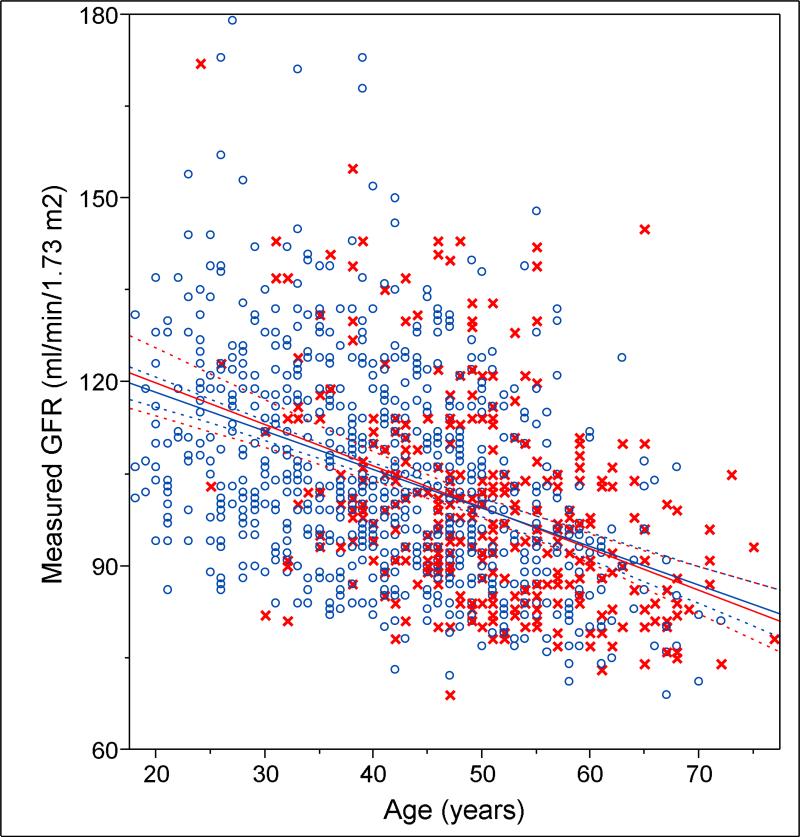
Characteristics associated with nephrosclerosis after age-sex-adjustment were 24-h urine albumin (p=0.010), nocturnal systolic and diastolic blood pressure (p=0.019 and p=0.002), and hypertension (p=0.020). To put these in perspective, a 32 mg increase in urine albumin, a 21 mmHg increase in nocturnal diastolic blood pressure, a 35 mmHg increase in nocturnal systolic blood pressure, and treated hypertension had the same association with nephrosclerosis as an 8-year increase in age. After excluding 18 patients with 24-h urine albumin >30 mg, nephrosclerosis still had an age-sex-adjusted association with nocturnal systolic and diastolic blood pressure (p=0.015 and p=0.002) and hypertension (p=0.008). After age-sex-adjustment, other characteristics (see Table 1) including CKD (p=.75), were not associated with nephrosclerosis in this population selected on health. GFR showed a 6.3 ml/min/1.73 m2 decline with each age decade (p<.001), but there was no evidence of effect modification by nephrosclerosis with this decline (Figure 1). Adjustment for biopsy quality had no substantive effect on associations with nephrosclerosis.
Figure 1.
The relationship between glomerular filtration rate (GFR) and age in persons with nephrosclerosis (red x) and in persons without nephrosclerosis (blue o). Regression of glomerular filtration rate onto age in persons with nephrosclerosis (red line) is similar to persons without nephrosclerosis (blue line). Dashed curves represent the 95% confidence interval.
The prevalence of nephrosclerosis in the oldest compared to youngest donors was 15/19 versus 0/19 by the original pathology reports and identically 15/19 versus 0/19 by the blinded re-read. There were 53 donors for whom the recipient underwent a repeat biopsy a mean 5 days after the transplant surgery. There was modest evidence of reproducibility for nephrosclerosis (kappa=0.35, p=.007). There were fewer glomeruli on the percutaneous repeat biopsy compared to the intraoperative initial biopsy (mean 15 vs 21, p<.001).
Discussion
Among healthy adults, nephrosclerosis occurred with older age and was not explained by age-related differences in kidney function or CKD risk factors. The prevalence of nephrosclerosis increased linearly from 2.7% for ages 18-29 y to 73% for ages 70-77 y. This increase in the prevalence of nephrosclerosis with age was preserved after standardizing the kidney function and CKD risk factors to the mean values of the youngest age group. These findings reveal a subclinical age-related nephropathy that is not detected by existing clinical tests other than a renal biopsy.
Traditionally, the term “nephrosclerosis” has implied that narrowing in arteries and arterioles led to ischemia resulting in glomerulosclerosis and chronic tubulointerstitial injury.(22) In this study, nephrosclerosis was defined by a histological pattern of sclerosis and atrophy without inferring an etiology. Critical telomere shortening (23), increased cell cycle inhibition,(24) inflammation with accelerated apoptosis (25), and reduction and simplification of vessels (26) may all contribute to age-related nephrosclerosis. The atrophy of nephrons that characterizes nephrosclerosis could explain the decline in kidney mass from young adults to older adults.(27, 28)
Surprisingly, the decline in GFR with normal aging was not explained by nephrosclerosis. Factors that govern GFR at levels higher than needed for prevention of uremic illness are largely unknown.(29) The indexing of GFR to body surface area (1.73 m2) may not account for age-related changes in physiological demand for GFR.(30) Five percent of kidney donors met estimated GFR and urine albumin criteria for CKD.(21) These criteria for CKD may not be sufficient as they were not associated with nephrosclerosis in this healthy population. Inaccuracy of serum creatinine-based estimated GFR (31, 32) is part of the problem, as all donors had a measured GFR > 60 ml/min/1.73 m2. The definition of CKD has largely been based on outcome studies,(33) but to the extent GFR and age are correlated, one cannot distinguish whether an outcome is linked to GFR or another age-related process (such as nephrosclerosis). The lack of association between the age-related decline in GFR and nephrosclerosis is supportive of proposed age-specific thresholds (5th percentiles) for GFR to improve kidney donor selection (32) and CKD classification (34, 35).
The only characteristics associated with nephrosclerosis independent of age and sex in this healthy population were urine albumin, nocturnal blood pressure, and treated hypertension. These findings support the interpretation of urinary albumin as a marker of underlying renal pathology.(36) The term “hypertensive nephrosclerosis” is occasionally used to describe patients with CKD attributed to hypertension even in the absence of a renal biopsy. However, these findings show that hypertension associates with nephrosclerosis in adults with normal GFR and urine albumin. Nephrosclerosis may be the underlying pathway by which urine albumin variation, even in the normal range, predicts the development of hypertension.(37)
There were several potential limitations to this study. Living kidney donors are selected based on good health, and lack the spectrum and severity of renal pathology present in the general population. Thus, the absence of an association in healthy adults should not be extended to the general population. The reproducibility (precision) of nephrosclerosis on a renal biopsy is limited, due to the small amount of tissue obtained to minimize bleeding and injury to the kidney. The implication is that, to some extent, the prevalence estimates reflect the probability of detecting nephrosclerosis with each renal biopsy in a person of a certain age. Biopsies varied with respect to the number of glomeruli, tubules, and vessels present and this affected detection of abnormalities. However, adjusting for biopsy quality factors did not change the findings.
In conclusion, an age-related nephrosclerosis occurs in healthy adults, and is not detected by age differences in CKD risk factors, urine albumin, or GFR. Independent of age, nephrosclerosis associated with hypertension, nocturnal blood pressure and urine albumin, but not GFR. As such, age-specific thresholds for GFR based on healthy adults are appropriate for living kidney donor selection and CKD screening. Future studies are needed to determine whether nephrosclerosis predicts adverse outcomes. Novel tests that detect nephrosclerosis are needed to be able to even assess whether prevention or treatment strategies are warranted.
Supplementary Material
Online Table 1. Frequency (%) of chronic histological abnormalities by age group among 1203 kidney donors
Grant support
National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK078229).
Footnotes
Current author addresses: All authors at Mayo Clinic, 200 1st St SW, Rochester, MN, 55905.
Reproducibility Research Statement: Statistical code is not available. Data set is not available.
Contributor Information
Andrew D Rule, Division of Nephrology and Hypertension, Division of Epidemiology, Mayo Clinic.
Hatem Amer, Division of Nephrology and Hypertension, Mayo Clinic.
Lynn D Cornell, Division of Anatomic Pathology, Mayo Clinic.
Sandra J Taler, Division of Nephrology and Hypertension, Mayo Clinic.
Fernando G Cosio, Division of Nephrology and Hypertension, Mayo Clinic.
Walter K Kremers, Division of Biostatistics, Mayo Clinic.
Stephen C Textor, Division of Nephrology and Hypertension, Mayo Clinic.
Mark D Stegall, Division of Transplantation Surgery, Mayo Clinic.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007;298(17):2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Bang H, Vupputuri S, Shoham DA, et al. SCreening for Occult REnal Disease (SCORED): a simple prediction model for chronic kidney disease. Arch Intern Med. 2007;167(4):374–81. doi: 10.1001/archinte.167.4.374. [DOI] [PubMed] [Google Scholar]
- 3.Couser WG. Chronic kidney disease the promise and the perils. J Am Soc Nephrol. 2007;18(11):2803–5. doi: 10.1681/ASN.2007080964. [DOI] [PubMed] [Google Scholar]
- 4.Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419–28. [PMC free article] [PubMed] [Google Scholar]
- 5.Choudhury D, Moshe L. Aging and Kidney Disease. In: Brenner BM, editor. Brenner and Rector's The Kidney. 8th ed. Saunders, an imprint of Elsevier Inc.; Philadelphia: 2007. pp. 681–693. [Google Scholar]
- 6.Silva FG. The aging kidney: a review--part II. International Urology & Nephrology. 2005;37(2):419–32. doi: 10.1007/s11255-004-0874-5. [DOI] [PubMed] [Google Scholar]
- 7.Silva FG. The aging kidney: a review -- part I. International Urology & Nephrology. 2005;37(1):185–205. doi: 10.1007/s11255-004-0873-6. [DOI] [PubMed] [Google Scholar]
- 8.Eknoyan G. Chronic kidney disease definition and classification: no need for a rush to judgment. Kidney Int. 2009;75(10):1015–8. doi: 10.1038/ki.2009.53. [DOI] [PubMed] [Google Scholar]
- 9.Stevens LA, Coresh J, Levey AS. CKD in the elderly--old questions and new challenges: World Kidney Day 2008. Am J Kidney Dis. 2008;51(3):353–7. doi: 10.1053/j.ajkd.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Tracy RE, Ishii T. What is ‘nephrosclerosis’? lessons from the US, Japan, and Mexico. Nephrol Dial Transplant. 2000;15(9):1357–66. doi: 10.1093/ndt/15.9.1357. [DOI] [PubMed] [Google Scholar]
- 11.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. American Journal of Kidney Diseases. 1992;20(1):1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 12.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–23. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 13.Howie AJ, Ferreira MA, Adu D. Prognostic value of simple measurement of chronic damage in renal biopsy specimens. Nephrol Dial Transplant. 2001;16(6):1163–9. doi: 10.1093/ndt/16.6.1163. [DOI] [PubMed] [Google Scholar]
- 14.Takebayashi S, Kiyoshi Y, Hisano S, et al. Benign nephrosclerosis: incidence, morphology and prognosis. Clin Nephrol. 2001;55(5):349–56. [PubMed] [Google Scholar]
- 15.Darmady EM, Offer J, Woodhouse MA. The parameters of the ageing kidney. J Pathol. 1973;109(3):195–207. doi: 10.1002/path.1711090304. [DOI] [PubMed] [Google Scholar]
- 16.Kasiske BL. Relationship between vascular disease and age-associated changes in the human kidney. Kidney Int. 1987;31(5):1153–9. doi: 10.1038/ki.1987.122. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan C, Pasternack B, Shah H, Gallo G. Age-related incidence of sclerotic glomeruli in human kidneys. Am J Pathol. 1975;80(2):227–34. [PMC free article] [PubMed] [Google Scholar]
- 18.Tracy RE, Berenson GS, Cueto-Garcia L, Wattigney WA, Barrett TJ. Nephrosclerosis and aortic atherosclerosis from age 6 to 70 years in the United States and Mexico. Virchows Arch A Pathol Anat Histopathol. 1992;420(6):479–88. doi: 10.1007/BF01600252. [DOI] [PubMed] [Google Scholar]
- 19.Rule AD, Gussak HM, Pond GR, et al. Measured and estimated GFR in healthy potential kidney donors. American Journal of Kidney Diseases. 2004;43(1):112–9. doi: 10.1053/j.ajkd.2003.09.026. [erratum appears in Am J Kidney Dis. 2004 Dec;44(6):1126].
- 20.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clinical Chemistry. 2007;53(4):766–72. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 21.Kidney Disease Outcome Quality Initiative K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 2):S1–246. [PubMed] [Google Scholar]
- 22.Alpers CE. The Kidney. In: Kumar V, Abul AK, Fausto N, Aster JC, editors. Robins and Cotran Pathologic Basis of Disease. Robins and Cotran Pathologic Basis of Disease: Saunders, an imprint of Elsevier Inc.; 2009. [Google Scholar]
- 23.Melk A, Kittikowit W, Sandhu I, et al. Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int. 2003;63(6):2134–43. doi: 10.1046/j.1523-1755.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 24.Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int. 2004;65(2):510–20. doi: 10.1111/j.1523-1755.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 25.Thomas SE, Anderson S, Gordon KL, Oyama TT, Shankland SJ, Johnson RJ. Tubulointerstitial disease in aging: evidence for underlying peritubular capillary damage, a potential role for renal ischemia. J Am Soc Nephrol. 1998;9(2):231–42. doi: 10.1681/ASN.V92231. [DOI] [PubMed] [Google Scholar]
- 26.Takazakura E, Sawabu N, Handa A, Takada A, Shinoda A, Takeuchi J. Intrarenal vascular changes with age and disease. Kidney Int. 1972;2(4):224–30. doi: 10.1038/ki.1972.98. [DOI] [PubMed] [Google Scholar]
- 27.Tauchi H, Tsuboi K, Okutomi J. Age changes in the human kidney of the different races. Gerontologia. 1971;17(2):87–97. doi: 10.1159/000211811. [DOI] [PubMed] [Google Scholar]
- 28.Wald H. The weight of normal adult human kidneys and its variability. Arch Pathol Lab Med. 1937;23:493–500. [Google Scholar]
- 29.Meyer TW, Hostetter TH. Uremia. N Engl J Med. 2007;357(13):1316–25. doi: 10.1056/NEJMra071313. [DOI] [PubMed] [Google Scholar]
- 30.Daugirdas JT, Meyer K, Greene T, Butler RS, Poggio ED. Scaling of measured glomerular filtration rate in kidney donor candidates by anthropometric estimates of body surface area, body water, metabolic rate, or liver size. Clin J Am Soc Nephrol. 2009;4(10):1575–83. doi: 10.2215/CJN.05581008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poggio ED, Rule AD. A critical evaluation of chronic kidney disease--should isolated reduced estimated glomerular filtration rate be considered a ‘disease’? Nephrol Dial Transplant. 2009;24(3):698–700. doi: 10.1093/ndt/gfn704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poggio ED, Rule AD, Tanchanco R, et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int. 2009;75(10):1079–87. doi: 10.1038/ki.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckardt KU, Berns JS, Rocco MV, Kasiske BL. Definition and classification of CKD: the debate should be about patient prognosis--a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53(6):915–20. doi: 10.1053/j.ajkd.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M. Age- and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int. 2007;72(5):632–7. doi: 10.1038/sj.ki.5002374. [DOI] [PubMed] [Google Scholar]
- 35.Winearls CG, Glassock RJ. Dissecting and refining the staging of chronic kidney disease. Kidney Int. 2009;75(10):1009–14. doi: 10.1038/ki.2009.49. [DOI] [PubMed] [Google Scholar]
- 36.de Jong PE, Curhan GC. Screening, monitoring, and treatment of albuminuria: Public health perspectives. Journal of the American Society of Nephrology. 2006;17(8):2120–6. doi: 10.1681/ASN.2006010097. [DOI] [PubMed] [Google Scholar]
- 37.Brantsma AH, Bakker SJ, de Zeeuw D, de Jong PE, Gansevoort RT. Urinary albumin excretion as a predictor of the development of hypertension in the general population. J Am Soc Nephrol. 2006;17(2):331–5. doi: 10.1681/ASN.2005111153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Table 1. Frequency (%) of chronic histological abnormalities by age group among 1203 kidney donors