Abstract
Does early gesture use predict later productive and receptive vocabulary in children with pre- or perinatal unilateral brain lesions (PL)? Eleven Children with PL were categorized into 2 groups based on whether their gesture at 18 months was within or below the range of typically developing (TD) children. Children with PL whose gesture was within the TD range developed a productive vocabulary at 22 and 26 months and a receptive vocabulary at 30 months that were all within the TD range. In contrast, children with PL below the TD range did not. Gesture was thus an early marker of which children with early unilateral lesions would eventually experience language delay, suggesting that gesture is a promising diagnostic tool for persistent delay.
Children with pre- or perinatal unilateral brain lesions (PL) exhibit marked plasticity for language functions. Even when their lesions affect classic language areas, children typically do not exhibit the aphasias that adults with similar lesions display (e.g., Bates & Dick, 2002; Feldman, 2005; Levine, Kraus, Alexander, Suriyakham, & Huttenlocher, 2005; Reilly, Levine, Nass, & Stiles, in press; Stiles, Reilly, Paul, & Moses, 2005; Woods & Teuber, 1978). However, children with PL often exhibit delays in both productive and receptive language (e.g., Bates, Thal, & Janowsky, 1992; Chilosi, Cipriani, Bertuccelli, Pfanner, & Cioni, 2001; Feldman, Holland, Kemp, & Janosky, 1992; Marchman, Miller, & Bates, 1991; Thal et al., 1991). Importantly, these delays are transient for some children with PL but persistent for others (Bates et al., 1997; Feldman et al., 1992; Thal et al., 1991; Vicari, Albertoni, Chilosi, Cipriani, Cioni, & Bates, 2000). The question we ask here is whether early child gesture can be used to predict subsequent vocabulary development in children with PL, just as it can for typically developing children (Rowe & Goldin-Meadow, 2009a). If so, gesture may be a potential early diagnostic indicator of which children with PL will exhibit subsequent language delays and which will catch up and thus fall within the normative range.
The relation between early gesture and vocabulary growth has been extensively studied in typically developing children. Many children begin by communicating through gesture and over time replace gesture with speech (Bates, Benigni, Bretherton, Camaioni, & Volterra, 1979; Bates & Dick, 2002; Iverson, Capirci, & Caselli, 1994). Early gesture use not only precedes subsequent vocabulary development, it also predicts subsequent vocabulary production and comprehension (Goldin-Meadow & Butcher, 2003; Iverson & Goldin-Meadow, 2005; O’Reilly, Painter, & Bornstein, 1997; Rowe & Goldin-Meadow, 2009a,b; Rowe, Özçaliskan, & Goldin-Meadow, 2006a,b; Rowe, Özçaliskan, & Goldin-Meadow, 2008). For example, Iverson and Goldin-Meadow (2005) were able to predict which lexical items entered a child’s spoken vocabulary from looking at the child’s gestures several months earlier. Specifically, they found that children produced a deictic pointing gesture to refer to an object, person, or location (e.g., point at a ball) approximately 3 months before producing a word for that object, person, or location (e.g., the word “ball”). As a second example, Rowe and colleagues (2008) found that the number of different meanings children conveyed with their gestures at 14 months was significantly related to the size of their receptive vocabularies at 42 months (see also Rowe & Goldin-Meadow, 2009a,b). This relation held even after controlling for the number of different spoken words that the children produced at 14 months.
Previous research has examined gesture use in children with PL but has not directly related it to language delay. For example, Bates and her colleagues (1997) found that children with early right hemisphere lesions show delays in gesture between 10 and 17 months of age, based on parent responses on the MacArthur Communicative Development Inventory (see also Marchman et al., 1991). Are the children who exhibit delays in gesture the same children who exhibit delays in vocabulary development? We might expect delays in gesture use to go hand-in-hand with language delays simply because gesture and language form an integrated system, not only in adults (McNeill, 1992), but also in typically developing children at the early stages of language learning (Goldin-Meadow, 2003). If the gesture-language system is robust in the face of early unilateral brain injury, children whose language development is proceeding at a typical pace should display typical gesture, and children whose language is delayed should display delays in gesture. Moreover, early gesture should predict subsequent language development as it does in typically developing children.
It is, of course, possible that gesture and language do not form an integrated system in children with pre- and perinatal unilateral brain lesions. Early brain injury could disrupt the relation between language (both receptive and expressive) and gesture, leading to different patterns of gesture-language development than those observed in typically developing children. In addition, children with PL frequently have motor impairments involving the contralesional hand and arm (Huttenlocher, 2002; Levine, Huttenlocher, Banich, & Duda, 1987; Mercuri et al, 2004; Staudt, Grodd, Gerloff, Erb, Stitz, & Krägeloh-Mann, 2002). Such impairments might limit use of gesture in early language development and thus disrupt the gesture-language link in children with PL.
The goal of our study is to explore whether early child gesture predicts later vocabulary size in children with PL as it does in typically developing children. If so, not only will we have evidence that gesture and language form a tightly organized system that can withstand early injury to the brain, but we also will have uncovered an early marker that might be used to identify those children with PL who are at risk for subsequent language delay and who might benefit from early intervention.
Method
Participants
Participants were 11 children with PL (8 girls, 3 boys) studied longitudinally between 18 and 30 months. For 7 of the 11 children, we also have data about language comprehension based on parental responses on the MacArthur Communicative Development Inventory (MCDI) (Fenson et al., 1993). Lesion characteristics of the individual children are given in Table 1; for 10 of the 11 children, these characteristics were based on Magnetic Resonance Imaging (MRI), and for the remaining child, the characteristics were based on detailed medical notes from previous CT and MRI scans. Two pediatric neurologists reviewed all of the information available and agreed that each of the 11 children has a unilateral lesion. The lesions were either the result of cerebrovascular infarcts of the middle cerebral artery territory (CV lesions), primarily affecting inferior frontal and superior temporal regions, or the result of periventricular lesions (PV lesions), primarily affecting subcortical structures and white matter tracts. Periventricular lesions in very low birthweight, prematurely-born children have been the focus of much previous literature (although see Krägeloh-Mann & Horber, 2007, for a review of recent studies of PV lesion in full-term infants). Our sample is more restricted because we excluded children born earlier than 36 weeks gestation. Children were recruited through area health providers and parent support groups, and came from middle to upper-middle class Caucasian families. All children were being raised as monolingual English speakers.
Table 1.
Lesion Characteristics for Individual Children With PL
| ID | Sex | Hemisphere | Size | Type | Location |
|---|---|---|---|---|---|
| 1 | Female | Left | Large | Cerebrovascular Infarct | F, T, P, O, subcortical |
| 2 | Female | Right | Large | Cerebrovascular Infarct | F, T, P, subcortical |
| 3 | Male | Right | Small | Periventricular | subcortical |
| 4 | Female | Left | Large | Cerebrovascular Infarct | F, T, P, subcortical |
| 5 | Male | Left | Medium | Cerebrovascular Infarct | F, T, P, subcortical |
| 6 | Male | Left | Small | Periventricular | F, T, subcortical |
| 7 | Female | Left | Large | Cerebrovascular Infarct | F, T, P, O subcortical |
| 8 | Female | Left | Large | Cerebrovascular Infarct | F, T, P |
| 9 | Female | Right | Large | Cerebrovascular Infarct | F, T, P, O, subcortical |
| 10 | Female | Left | Small | Periventricular | T, subcortical |
| 11 | Female | Left | Small | Periventricular | details not available |
Note. F=Frontal, T=Temporal, P=Parietal, O=Occipital, subcortical=involves subcortical areas.
Lesions were characterized by type (CV or PV), laterality (left or right), and size (small, medium, or large). Lesion size was determined using the following criteria: small lesions affected only one lobe or minimally affected subcortical regions; medium lesions affected more than one lobe or extended into more than one subcortical region; large lesions affected three or four lobes, and often affected subcortical regions and the thalamus (large lesions were typically cerebrovascular infarcts). Four children with PL had small lesions, 1 had a medium lesion, and 6 had large lesions; we collapsed children with small and medium lesions into a single group based on previous findings indicating that the language development of these groups tend to pattern together (Brasky, Nikolas, Meanwell, Levine, & Goldin-Meadow, 2005). Four of the children had periventricular lesions (PV) and 7 had cerebrovascular infarcts (CV). Eight children had left hemisphere lesions and 3 had right hemisphere lesions.
To situate the gesture and speech development of children with PL within a normative sample, we also observed 53 typically developing (TD) children (26 girls, 27 boys) between 18 and 26 months whose gesture and speech development have been previously described (Rowe et al., 2006, 2008; Rowe & Goldin-Meadow, 2009a,b). All TD children were being raised as monolingual English speakers and had no known medical conditions. The families of the TD children reflected the demographic diversity of the Chicago area in terms of family income and ethnicity and thus contained families of low, middle, and high SES. In contrast, the families of the children with PL were skewed toward the high end of the SES continuum. Because children from higher SES families tend to have larger vocabularies than children from lower SES families (Hart & Risley, 1995), the SES difference between the two groups would work against our finding language delays in the children with PL. Further, findings from Rowe, Özçaliskan, and Goldin-Meadow (2008) suggest that early gesture predicts subsequent vocabulary comprehension in TD children regardless of SES. Thus, the SES difference between the two groups is not likely to affect the pattern of results.
Procedures
All children were observed in their homes for 90 minutes while interacting naturally with their primary caregivers at 18, 22, and 26 months. Parents were asked to perform their everyday activities and interact with their children as they typically would. The PPVT-3 (Dunn & Dunn, 1997), a normed test of vocabulary comprehension, was given to the children at 30 months.
Coding Speech and Gesture
All child speech produced during each videotaped session was transcribed. Sounds that were reliably used to refer to entities, properties, or events (e.g., ‘doggie’, ‘nice’, ‘broken’), along with onomatopoeic sounds (e.g., ‘meow’, ‘choo-choo’) and conventionalized evaluative sounds (e.g., ‘oopsie’, ‘uh-oh’), were counted as words. We established reliability for our speech transcripts by having a second individual transcribe 20% of the videotapes; reliability was achieved when the second coder agreed with the first on 95% of the transcription decisions.
Any communicative hand movement that did not involve direct manipulation of objects (e.g., twisting a jar open) or a ritualized game (e.g., patty cake) was considered a gesture. Each gesture was classified as deictic, iconic, or conventional: (1) Deictic gestures were those that indicated concrete objects, people, or locations. We considered these objects/people/locations to be the referents of the deictic gestures (e.g., pointing to a dog referred to a dog, holding up a bottle referred to a bottle). (2) Iconic gestures were those that depicted the attributes or actions of an object via hand or body movements (e.g., moving the index finger in circles to convey a ball rolling). (3) Conventional gestures were those whose forms and meanings are prescribed by the culture (e.g., nodding the head to mean yes, extending an open palm next to a desired object to mean give). We assessed reliability for gesture on a subset of the videotaped sessions coded by an independent coder. Agreement between coders was 88% (k = .76; N = 763) for identifying gestures and 91% (k = .86; N = 375) for assigning meanings to gestures.
Measures
We calculated the number of unique spoken words (speech types) that each child produced during a single session and used this measure as an index of the child’s productive vocabulary in speech at each age. Several decisions were made as to what constituted a word type. Morphologically inflected variants of words (e.g., run, running) were considered a single type. Words produced in imitation of the mother were included in the corpus of child word types, as were words that the child produced in the context of book reading. We included imitations because children typically only imitate linguistic structures that are within their spontaneous language reach (Slobin & Welsh, 1967). Further, the children with PL produced a very small number of imitations (M = 1.09, accounting for only 1.53% of their total speech types), as did the TD children (M = 1.42, accounting for 1.9% of their total speech types). Thus, it is likely that including imitations has little effect on the results. The number of word types produced by each child at each session served as our measure of productive vocabulary.
The PPVT-3 (Dunn & Dunn, 1997), administered at 30 months, served as our measure of children’s receptive vocabulary. We chose this measure because it is widely used, sensitive to individual differences, standardized, and can be used reliably with children as young as 30 months.
We calculated the number of unique gesture meanings (gesture types) that each child produced at each session. We counted gestures that conveyed different meanings as unique gesture types. The meaning assigned to a deictic gesture was the object, person, or place to which the deictic gesture referred. Note that, under this system, each pointing gesture indicating a different entity is considered a unique type. For example, if a child pointed to a ball and a cup, the child would be given credit for two gesture types in his repertoire, ball and cup. This procedure for attributing meaning to early child gesture has been used in previous studies and, importantly, results in systematic patterns. For example, when this system is used to code early child communications in TD children, we are able to predict the size of a child’s later receptive vocabulary (Rowe & Goldin-Meadow, 2009a,b) and the onset and nature of the child’s two-word speech (Goldin-Meadow & Butcher, 2003; Iverson & Goldin-Meadow, 2005; Özçaliskan & Goldin-Meadow, 2005; see Goldin-Meadow & Mylander, 1984, and Iverson & Goldin-Meadow, 2005, for further justification of the coding system, and details of how meanings were assigned to gestures).
Results
Gesture Use From 18 Months to 26 Months in Children With PL
We first investigated the spontaneous use of gesture in children with PL at 18, 22, and 26 months. When looking at overall amount of gesturing, we find that most of the gestures that the children with PL produced at all three ages were deictic gestures (average across ages = 72%), as were the gestures produced by the TD children (average across ages = 75%) and there was not a significant difference between children with PL and TD children on this measure (F = 1.05, p = .31). Also, children with PL did not differ significantly from TD children in total number of gestures produced at 18- or 22-months of age (18-months: t = 1.39, p = .17; 22-months: t = 0.32, p = .75). But there was a marginally significant difference between the groups at 26-months (t = 1.86, p = .07), with children with PL producing an average of 75 gestures (SD = 38.35) and TD children producing an average of 113 gestures (SD = 63.96) during the 90-minute session.
We next turn to our measure of gesture use: gesture types. We chose to use gesture types rather than total number of gestures because the gesture types measure captures the diversity of meanings each child conveyed in gesture and, in this sense, is a close analogue of our speech types measure. Moreover, number of gesture types was highly correlated with total number of gestures in our sample of children with PL: ρ = .96, p < 0.001 at 18 months; ρ = .93, p < 0.001 at 22 months; ρ = .96, p < 0.001 at 26 months (Spearman rank order correlations). Gesture types were predominantly deictic gestures for both children with PL and TD children (PL: 86% average across ages; TD: 82% average across ages). TD children and children with PL did not significantly differ in the proportion of gesture types produced with a deictic gesture (F = 1.18, p = .28).
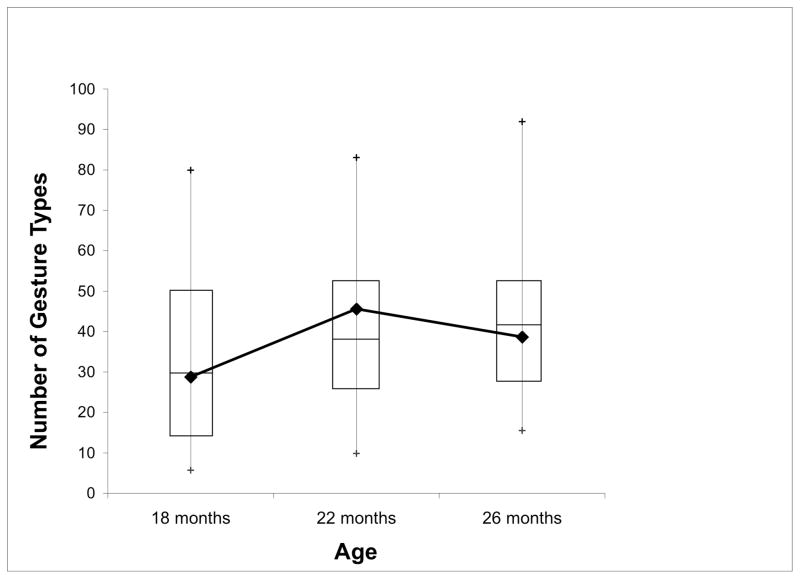
As a group, the children with PL produced approximately the same number of gesture types as the TD children. On average, the children with PL produced 28.73 gesture types at 18-months (SD = 21.89), 45.55 gesture types at 22-months (SD = 22.94), and 38.64 gesture types at 26-months (SD = 18.77). Figure 1 displays the mean number of gesture types that the children with PL produced in relation to the gesture type data from the TD children at each age. The boxes in the graph represent the inter-quartile range for the 53 TD children; the line in the middle of each box represents the median for the TD children, and the tails represent the 5th and 95th percentiles. Note that, as a group, the children with PL were close to the median for the TD children’s production of gesture types and t-tests confirmed that there were no significant differences between groups (18-months: t = 0.68, p = .50; 22-moths, t = −0.67, p = .50; 26-months: t = 0.66, p = .51).
Figure 1.
Mean number of gesture types that the children with PL produced at each observation session. The boxes in the graph represent the inter-quartile range for gesture types in the TD children; the line in the middle of each box represents the median for TD children and the tails represent the 5th and 95th percentiles.
There was, however, a great deal of variability within the group of children with PL. To explore this variability, we divided the children with PL into two groups based on their production of gesture types at 18 months: (1) Children in the LOW group fell below the 25th percentile for gesture production at 18 months in the TD group (25th percentile = 14.5 gesture types). Children in the HIGH group fell above the 25th percentile. Thus, we used the 25th percentile as a cut-off to identify children at the low end of the typical range (Thal et al., 1991). Based on these criteria, 5 of the 11 children with PL were classified into the LOW gesture group, and 6 were classified into the HIGH gesture group.
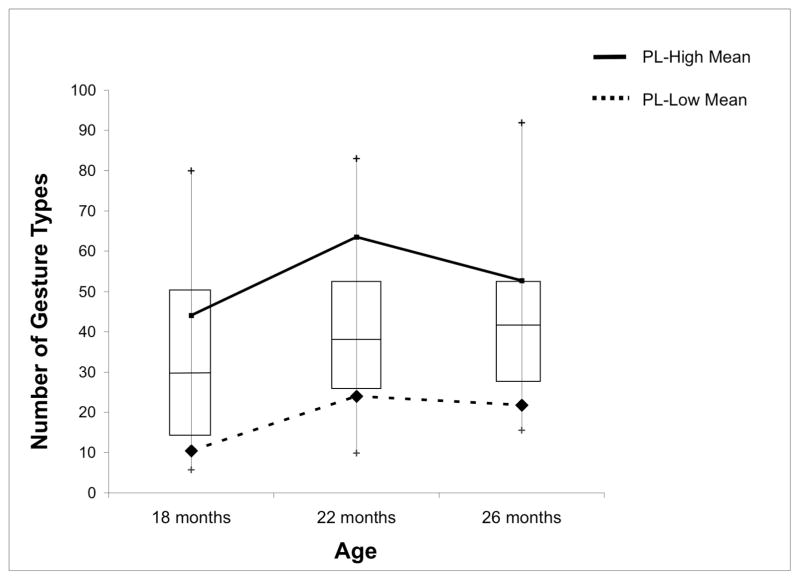
Importantly, there was stability in the children’s gesture use between 18 and 26 months: all 5 of the children who were assigned to the LOW gesture group on the basis of their 18-month performance fell below the 25th percentile cut-off on at least one of the other two sessions (22 and 26 months); 2 of these children fell below the cut-off on both sessions. Moreover, all 6 of the children assigned to the HIGH gesture group on the basis of their 18-month performance fell above the cut-off on both of the other two sessions. We ran permutation tests at each age (Hesterberg, Monaghan, Moore, Clipson, & Epstein, 2003) to determine whether the number of gesture types produced by the HIGH gesture group differed significantly from the number produced by the LOW gesture group. The HIGH gesture group not only produced significantly more gesture types than the LOW gesture group at 18 months (the age at which children were divided into groups, mean difference = 33.6, p = .006), but they also produced more gesture types at 22 months (mean difference = 39.5, p < .001) and at 26 months (mean difference = 30.87, p = .002) (see Figure 2). Thus, early differences in gesture use in the children with PL were stable over time.
Figure 2.
Mean number of gesture types produced by children with PL in the HIGH and LOW gesture groups at each observation session. Children with PL were divided into gesture groups based on their production of gesture types at 18 months. The boxes in the graph represent the inter-quartile range for gesture types in the TD children; the line in the middle of each box represents the median for TD children and the tails represent the 5th and 95th percentiles.
Does Gesture Production at 18 months Predict Later Productive Vocabulary?
We next investigated whether gesture use at 18 months can be used to predict later speech use. On average, the children with PL produced 15.36 (SD = 14.64) speech types at 18 months, 56.91 (SD = 36.44) at 22 months, and 123.36 (SD = 77.23) at 26 months. Thus, as a group, the children with PL increased the number of different words they produced over time. However, there was once again a great deal of variability within the group. Can this variability be related to gesture use at 18 months?
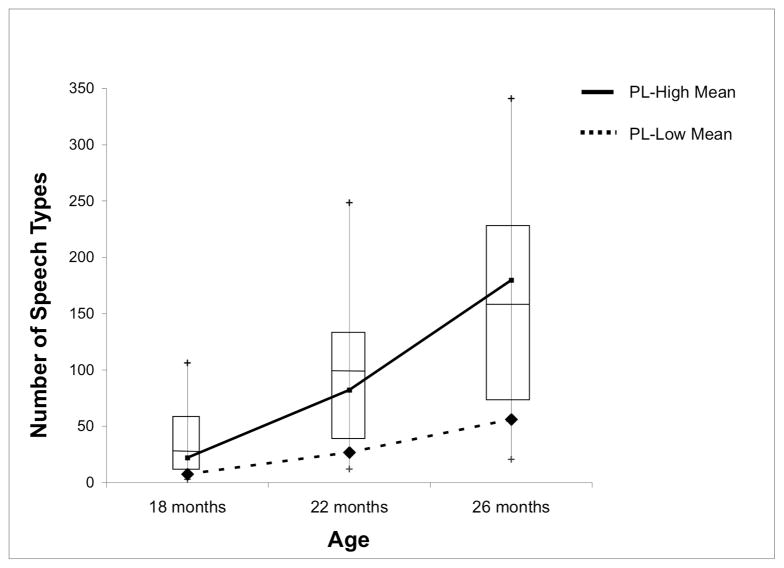
Figure 3 presents the mean number of speech types children in the LOW and HIGH gesture groups produced at each age, displayed in relation to the speech type data from the TD children. We ran permutation tests at 18 months to determine whether the number of speech types produced by the HIGH gesture group differed significantly from the number produced by the LOW gesture group. At 18 months, the age used to divide the children into LOW and HIGH gesture groups, the HIGH group produced slightly more speech types than the LOW group, but the difference was not reliable (mean difference = 14.6, p = .112). At 18 months, all 5 LOW gesture children fell below the TD 25th percentile for speech types, but 3 of the 6 HIGH gesture children did as well (Fisher Exact Probability = .12). In other words, at 18 months, it was not possible to reliably distinguish the two groups on the basis of their speech use, only their gesture use.
Figure 3.
Mean number of speech types produced by children with PL in the HIGH and LOW gesture groups at each observation session. Children with PL were divided into gesture groups based on their production of gesture types at 18 months. The boxes in the graph represent the inter-quartile range for speech types in the TD children; the line in the middle of each box represents the median for the TD children and the tails represent the 5th and 95th percentiles.
The interesting question, however, is whether gesture use at 18 months presages later speech use. We ran permutation tests at 22 and 26 months to determine whether the number of speech types produced by the HIGH gesture group differed significantly from the number produced by the LOW gesture group, and found that they did. The HIGH gesture group reliably produced more speech types than the LOW gesture group at 22 months (mean difference = 55.2, p = .003) and at 26 months (mean difference = 123.5, p = .005). Moreover, at 22 months, all 5 LOW gesture children continued to fall below the TD 25th percentile for speech types but none of the 6 HIGH gesture children did (Fisher Exact Probability = .002). At 26 months, 4 of the 5 LOW gesture children fell below the TD 25th percentile for speech types, compared to none of the 6 HIGH gesture children (Fisher Exact Probability = .01). Although the sample is small, we can use gesture production at 18 months to predict which children will be below the TD 25th percentile in speech production at 22 and 26 months with some precision: At 22 months, positive predictive value for LOW gesture children to be below the TD 25th percentile was 1.00, and sensitivity was 1.00; at 26 months, positive predictive value was .80 and sensitivity was 1.00. We used categorical analyses (rather than regression analyses) to test whether later speech production can be predicted from early gesture use because our data do not meet the assumptions for linear regression. However, it is worth noting that 18-month gesture does significantly correlate with productive vocabulary at both 22 months and 26 months (22 months: ρ = .81, p = .002; 26 months: ρ = .81, p = .003, Spearman rank order correlations).
Does Gesture Production at 18 months Predict Later Receptive Vocabulary?
We have found thus far that differences in gesture production in children with PL at 18 months predict later differences in productive vocabulary. We next ask whether early gesture use also predicts later differences in receptive vocabulary, that is, performance on the PPVT-3. In addition to extending our language probes from production to comprehension, using the PPVT-3 has the advantage of allowing us to assess children’s performance on a standardized measure of language development. Moreover, the PPVT-3 relies on a different data collection technique from our speech production measure, one that is less tightly tied to our gesture measure. Our speech and gesture production measures both come from spontaneous communication during a 90-minute period. In contrast, the PPVT-3 is a normed and validated receptive vocabulary test in which the child is given a word and must select the picture he thinks the word refers to out of a distractor set. The PPVT-3 thus allows us to test the robustness of the relation between gesture and early language.
The children with PL had an average PPVT-3 standard score of 84.64 (SD = 21.82). This score is approximately one standard deviation below the average score for this test (M = 100, SD = 15) (Dunn & Dunn, 1997). Thus, as a group, the children with PL exhibited relatively low vocabulary comprehension. However, there was great variability in the vocabulary comprehension scores of the children with PL, ranging from a low of 45 to a high of 113. The standard deviation for the group of children with PL was 21.8, considerably higher than the standard deviation of 15 in the PPVT-3 norms. Thus, some children with PL were performing well within the average range on the PPVT-3, while others were falling far below that range.
Can we predict performance on the PPVT-3 at 30 months from the gestures the children produced a year earlier, at 18 months? On average, children in the LOW gesture group (M = 66.8, SD = 16.65) scored significantly lower on the PPVT-3 than children in the HIGH gesture group (M = 99.5, SD = 12.15), (U = 1.00, p = .009, Mann-Whitney). Moreover, all 5 LOW gesture children fell below the 25th percentile for the PPVT-3, whereas only 2 of the 6 HIGH gesture children did (Fisher Exact Probability = .045). Thus, positive predictive value for LOW gesture children to be below the 25th percentile on the PPVT-3 was 1.00, and sensitivity was .71.
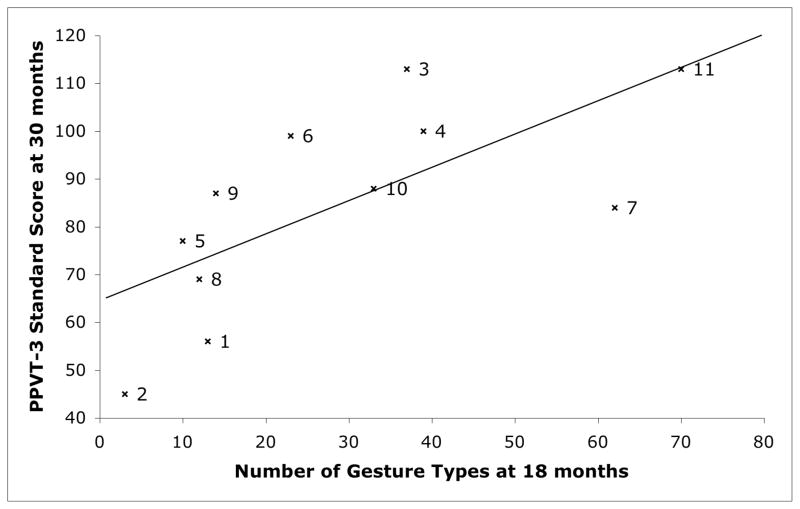
We also explored the relation between 18-month gesture and 30 month receptive vocabulary using a continuous measure of gesture production. Figure 4 presents a scatter-plot of the relation between gesture types at 18 months (x-axis) and receptive vocabulary (PPVT-3) at 30 months (y-axis); each point represents an individual child. We conducted Spearman rank order correlations between the number of gesture types a child produced at 18 months and that child’s PPVT-3 standard score at 30 months, and found a significant relation between the two measures (ρ = 0.80, p = .003). Importantly, the number of speech types a child produced at 18 months did not significantly correlate with that child’s PPVT-3 standard score at 30 months (ρ = .34, p = .31), presumably because there was very little variation in number of speech types these children produced at 18 months.
Figure 4.
The relation between the number of gesture types produced at 18 months and vocabulary comprehension score on the PPVT-3 at 30 months in children with PL. Each x represents an individual. The labels correspond to the ID numbers in Table 1. The five leftmost points (1, 2, 5, 8, 9) represent children in the LOW gesture group. The regression line on the graph is from model 1.
We then used a linear regression model to explore whether gesture production at 18 months predicts vocabulary comprehension at 30 months (see Table 2). The data meet the assumptions of linear regression required for these analyses: there is a linear relation, the residuals are normally distributed and all are within +/−2, and there is no evidence that homeoscedasticity is violated. We found that number of gesture types at 18 months significantly predicted PPVT-3 scores at 30 months, accounting for 48.1% of the variance (model 1). When we included number of child speech types at 18 months in the regression model, number of gesture types at 18 months continued to significantly predict 30-month word comprehension on the PPVT-3 (model 2); in fact, it was the only significant predictor and model 2 accounted for 52.8% of the variance in children’s PPVT-3 scores. Thus, early child gesture predicts later receptive vocabulary even when controlling for early child speech.
Table 2.
Regression Models Predicting PPVT-3 Scores at 30 Months Using Gesture Types and Speech Types at 18 Months in Children With PL (n = 11)
| Vocabulary Comprehension (child PPVT-3 at 30 months) B(se) | ||
|---|---|---|
| Model 1 | Model 2 | |
| Intercept | 64.77** (8.50) | 61.05** (9.57) |
| Child gesture types at 18 months | 0.69* (0.24) | 0.65* (0.25) |
| Child speech types at 18 months | 0.33 (0.37) | |
| R-squared statistic (%) | 48.1 | 52.8 |
p < .05.
p < .01.
We have found that, for children with PL, variability in early gesture use predicts later variability in receptive vocabulary. But is gesture use the only measure that predicts later receptive vocabulary in these children? It is likely that the children also varied in their ability to comprehend spoken language at the early time point. Variability in early language comprehension might then be as good as, or an even better, predictor of later variability in receptive vocabulary than early gesture use. To explore this possibility, we used the 14-month MCDI language comprehension score, which was available on 7 of the 11 children (the parents of 2 of the 4 missing children did not return the MCDI; the other 2 children did not begin our study until 18 months and the age-appropriate version of the MCDI for 18-month-olds does not contain a language comprehension measure). As expected, we found that 14-month MCDI language comprehension scores were related to 14-month gesture production measures (ρ = .75, p = .05). However, 14-month MCDI language comprehension scores showed only a small, and not significant, correlation with 30-month PPVT scores (ρ = .25, p = .59). In contrast, 14-month gesture production for these 7 children showed a higher, and marginally significant, correlation with 30-month PPVT (ρ = .63, p = .07). Consistent with these findings, in analyses of the 53 TD children who served as the normative base for the current study, Rowe and Goldin-Meadow (2009b) found that 14-month language comprehension, as measured by the CDI, did not relate to children’s 42-month PPVT scores, whereas 14-month gesture production did. Although early gesture and early language comprehension skills have both been found to predict later language production in previous work on late-talkers (Thal & Tobias, 1992), the power of the two predictors has not been directly compared. Our findings suggest that early gesture may be the more powerful predictor in children with PL and in typically developing children (Rowe & Goldin-Meadow, 2009b).
Lesion Characteristics and Gesture
We have found that early gesture use predicts later productive and receptive vocabulary in children with PL. Does gesture use vary with lesion characteristics? The small number of children in our sample of children with PL, combined with their diversity in lesion location, size, and type, makes it difficult to explore this question (see Rowe, Levine, Fisher, and Goldin-Meadow, 2009, for discussion). We therefore used non-parametric chi-square analyses to compare the distribution of children in the LOW vs. HIGH gesture groups as a function of each of the three lesion characteristics: Lesion Type (CV or PV), Lesion Size (Small or Large), and Hemisphere (Right or Left). However, we stress that the findings must be considered tentative given our sample size of 11 children.
We found a significant effect for lesion type (χ2 = 5.24, df = 1, p = .02): all 4 children with PV lesions fell into the HIGH gesture group (none in the LOW gesture group), compared to only 2 of 7 children with CV infarcts (5 in the LOW gesture group). We found a trend for lesion size (χ2 = 2.396, df = 1, p = .12): 1 of the 5 children with small lesions fell into the LOW gesture group and 4 fell into the HIGH; in contrast, 4 of the 6 children with large lesions fell into the LOW gesture group and 2 fell into the HIGH. We did not find a significant result for lesion hemisphere (χ2 = 0.749, df = 1, p = .39): 3 of the 8 children with left hemisphere lesions fell into the LOW gesture group and 5 fell into the HIGH, compared to 2 and 1, respectively, of the 3 children with right hemisphere lesions (but note that there were only 3 children in the right hemisphere lesion group).
Overall, children with small (as opposed to large) lesions and children with PV lesions (as opposed to CV infarcts) were more likely to produce gestures at a typical rate at 18 months. In turn, these children were also more likely to develop vocabulary at a typical rate than those with CV infarcts and those with large lesions, as evidenced by the significant relation between 18-month gesture and 22- and 26-month productive vocabulary and between 18-month gesture and 30-month receptive vocabulary. These findings are consistent with our previous studies including larger samples of children with PL (Rowe et al., 2008).
Discussion
Consistent with previous studies, we found that children with PL experience language delays that vary in terms of their severity and their persistence over time. Our study goes beyond previous studies of children with PL in that we observed gesture as well as language use in the children. We not only found great variability in gesture use at 18 months in children with PL, but we also found that this variability was related to later vocabulary levels––the children who produced fewer gesture types at 18 months were just the children who exhibited lower scores on productive and receptive vocabulary measures at 30 months. Most dramatically, child gesture at 18 months predicted child receptive vocabulary at 30 months, even when controlling for child speech types at 18 months.
Our findings extend previous research conducted on other child clinical groups, which report relationships between gesture and language (Capone & McGregor, 2004). For example, Hill, Bishop, and Nimmo-Smith (1998) found gesture deficits in children with specific language impairment. As another example, Thal and Tobias (1992) observed communicative gesture use in 18- to 28-month old late talkers, none of who had been diagnosed with brain injury. The late talkers whose productive language had normalized at the one-year follow-up had, at the earlier ages, produced significantly more communicative gestures than the late talkers whose productive language delays persisted at the 1-year follow-up. A tight relation between gesture and language has also been reported in adult Broca’s and Wernicke’s aphasics (McNeill, 1992; Cicone, Wapner, Foldi, Zurif, & Gardner, 1979). Taken together, these findings indicate that the relation between gesture and language is robust, found not only in samples of TD children but also in a variety of clinical populations.
Our findings have both theoretical and practical implications. In theoretical terms, the findings provide further evidence that the integrated gesture-language system found in typically developing children may be fundamental to the language learning process. Indeed, the diversity of our sample of children with PL underscores the robustness of this relation. Despite the fact that the children with PL varied widely in the location, size, and type of lesion they sustained, their early gestures were a reliable index of their later productive and receptive language skills. Although a larger sample is clearly needed to fully understand the relation between gesture and language, the fact that gesture and language remain linked in a population whose language functions are being carried out by a wider variety of neural substrates than is typical suggests that early gesture may be inextricably linked to the language learning process.
The theoretical question raised by our data is––why does gesture predict subsequent vocabulary development? Gesturing and word learning may draw upon a common underlying cognitive skill, for example, the ability to produce and understand symbols (cf. Werner & Kaplan, 1963; Bates et al, 1979; Bretherton & Bates, 1984; McNeill, 1992). Gesturing and word learning may also be affected in similar ways by more general skills, including overall cognitive functioning (as measured by IQ tests, Levine et al., 1987) or general motor abilities (particularly given recent speculation about the importance of motor movements in language processing, e.g. Nishitani, Schürmann, Amunts, & Hari, 2005; Pulvermüller, 2005; Rizzolatti & Arbib, 1998). Indeed, previous findings have linked motor and language skills in typically developing individuals (Bates & Dick, 2002; Iverson & Thelen, 1999) and in clinical populations for whom some form of delay in language production is central (Bishop & Edmundson, 1987; Corriveau & Goswami, 2009; Hill, 2001; Visscher, Houwen, Scherder, Moolenaar, & Hartman, 2007). Individuals with PL, including many of the children in our sample, have hemiparesis of varying severity (Huttenlocher, 2002; Levine, Huttenlocher, Banich, & Duda, 1987; Mercuri et al, 2004; Staudt, Grodd, Gerloff, Erb, Stitz, & Krägeloh-Mann, 2002; Staudt, Gerloff, Grodd, Holthausen, Niemann, & Krägeloh-Mann, 2004; Steenbergen & Gordon, 2006). Although it has not been shown that the severity of their symptoms varies systematically with their language skills, severity of hemiparesis has been shown to vary with IQ scores (see, for example, Feldman, Janosky, Scher, & Wareham, 1994; Levine et al., 1987).
To summarize thus far, gesturing may predict subsequent vocabulary development because it is affected by cognitive or motor skills in the same way that word learning is. If so, gesture will be able to serve as a good index of vocabulary growth, but it will play no role in bringing that growth about. It is possible, however, that gesture goes beyond reflecting skill in vocabulary learning to actually play a role in causing vocabulary learning (Goldin-Meadow, 2003). Consider, for example, a child who points at a bird. Mother responds by saying, “yes, that’s a bird,” thus providing a timely word-learning model for the child (see Goldin-Meadow, Goodrich, Sauer, & Iverson, 2007). In other words, gesture may be playing a role in vocabulary learning by allowing children to elicit input targeted to their level and, in this way, shape their own learning environments. Gesture could also play a role in vocabulary learning more directly by providing children with the opportunity to refer to objects whose names they are not yet able to express in speech, and to do so in a communicative context. Repeated use of gestures to refer to particular objects in an act of communication could pave the way for later acquisition of the words that refer to these particular objects (cf., Iverson & Goldin-Meadow, 2005). If so, gesture may be playing a role in the process of change by affecting learners themselves.
Our findings also have practical implications. The findings suggest that early gesture provides a way to identify children with persistent versus transient language difficulties at a time when all of the children are saying very little. Consistent with previous research with children with PL, 8 of the 11 children in our sample of children with PL fell below the 25th percentile of TD children on vocabulary production at 18 months, but only 5 children continued to be below the 25th percentile at 22 months. The striking result of our study is that these 5 were the same 5 children who were low gesture producers at 18 months. Thus, early gesture may provide clinicians with a way to identify children who may end up having persistent language difficulties before those difficulties appear in the children’s speech. This early identification may then be useful in guiding intervention practices that may be particularly effective when employed early in development. Larger samples are clearly needed to explore gesture’s use as a diagnostic indicator in clinical practice (for example, to determine what the cut-off in gesture use ought to be to reliably distinguish children with transient vs. persistent language delays). However, our study suggests that early child gesture may be a useful indicator of subsequent language delay, and thus motivates future research with both children with PL and children without PL who experience language delays.
Our results also raise the possibility that encouraging children to gesture may prove to be an effective intervention. That is, early gesture may not only precede early language but may facilitate its development. Studies of typically developing elementary school children have shown that the knowledge children convey uniquely in their gestures when explaining their solutions to mathematical equivalence problems is a harbinger of the knowledge that they will soon acquire (Alibali & Goldin-Meadow, 1993; Perry, Church, & Goldin-Meadow, 1988). More importantly, children who were told to gesture during a mathematical equivalence lesson retained more from the lesson than children who were told not to gesture (Cook, Mitchell, & Goldin-Meadow, 2008; see also Broaders, Cook, Mitchell, & Goldin-Meadow, 2007; Goldin-Meadow, Cook, & Mitchell, 2009), suggesting that the act of gesturing itself facilitates learning. Research on the language development of typically developing children suggests that gesture may play a similar role in language learning. For example, Goodwyn, Acredolo, and Brown (2000) find that encouraging children to gesture (via parent modeling) facilitates subsequent language development. Thus, gesture might serve not only as a tool for diagnosing language delay but also as a tool for early intervention.
In summary, we have found that that the relation between early gesture and later language, reported in typically developing children, is also found in children with early unilateral brain injuries. The tight relation between early gesture and subsequent language development thus appears to be a robust aspect of language development. Our findings further suggest that early delays in gesture production can be used to identify those children with unilateral pre- or perinatal lesions whose language learning is likely to be delayed in the future. If so, we may be able to offer these children interventions while their language-learning trajectory is most malleable.
Acknowledgments
This research was supported by P01HD40605 from NICHD and a grant from the Brain Research Foundation. We thank the participating families for sharing their child’s language development with us; K. Brasky, L. Chang, E. Croft, K. Duboc, J. Griffin, S. Gripshover, K. Harden, L. King, C. Meanwell, E. Mellum, M. Nikolas, J. Oberholtzer, C. Rousch, L. Rissman, B. Seibel, M. Simone, K. Uttich, and J. Wallman for help in collecting and transcribing the data; Seyda Özçaliskan for gesture coding; J. Fisher, P. Huttenlocher, S. Small and M. Staudt for coding brain scans; Meredith Rowe for statistical advice; Kristi Schonwald and Jason Voigt for administrative and technical assistance.
References
- Alibali M, Goldin-Meadow S. Gesture-speech mismatch and mechanisms of learning: what the hands reveal about a child’s state of mind. Cognitive Psychology. 1993;25:468–523. doi: 10.1006/cogp.1993.1012. [DOI] [PubMed] [Google Scholar]
- Bates E, Benigni L, Bretherton I, Camaioni L, Volterra V. The emergence of symbols. New York: Academic Press; 1979. [Google Scholar]
- Bates E, Dick F. Language, gesture, and the developing brain. Developmental Psychobiology. 2002;40:293–310. doi: 10.1002/dev.10034. [DOI] [PubMed] [Google Scholar]
- Bates E, Thal D, Janowsky J. Early language development and its neural correlates. In: Segalowitz S, Rapin I, editors. Handbook of neuropsychology: Vol. 7. Child neuropsychology. Amsterdam: Elsevier; 1992. pp. 69–110. [Google Scholar]
- Bates E, Thal D, Trauner D, Fenson J, Aram D, Eisele J, et al. From first words to grammar in children with focal brain injury. Developmental Neuropsychology. 1997;13:275–343. [Google Scholar]
- Bishop DVM, Edmundson A. Specific language impairment as a maturational lag - evidence from longitudinal data on language and motor development. Developmental Medicine and Child Neurology. 1987;29:442–459. doi: 10.1111/j.1469-8749.1987.tb02504.x. [DOI] [PubMed] [Google Scholar]
- Brasky K, Nikolas M, Meanwell C, Levine SC, Goldin-Meadow Language development in children with unilateral brain injury: Effects of lesions size. Poster session presented at the Symposium on Research in Child Language Disorders; Madison, Wisconsin. 2005. Jun, [Google Scholar]
- Bretherton I, Bates E. The development of representation from 10 to 28 months. In: Emde R, Harmon R, editors. Continuities and discontinuities of development. New York: Plenum Press; 1984. pp. 229–261. [Google Scholar]
- Broaders S, Cook SW, Mitchell Z, Goldin-Meadow S. Making children gesture brings out implicit knowledge and leads to learning. Journal of Experimental Psychology: General. 2007;136:539–550. doi: 10.1037/0096-3445.136.4.539. [DOI] [PubMed] [Google Scholar]
- Capone N, McGregor K. Gesture development: A review for clinical and research practices. Journal of Speech, Language, and Hearing Research. 2004;47:173–186. doi: 10.1044/1092-4388(2004/015). [DOI] [PubMed] [Google Scholar]
- Chilosi A, Cipriani P, Bertuccelli B, Pfanner L, Cioni G. Early cognitive and communication development in children with focal brain lesions. Journal of Child Neurology. 2001;16:309–316. doi: 10.1177/088307380101600502. [DOI] [PubMed] [Google Scholar]
- Cicone M, Wapner W, Foldi N, Zurif E, Gardner H. The relation between gesture and language in aphasic communication. Brain and Language. 1979;8:324–349. doi: 10.1016/0093-934x(79)90060-9. [DOI] [PubMed] [Google Scholar]
- Cook SW, Mitchell Z, Goldin-Meadow S. Gesturing makes learning last. Cognition. 2008;106:1047–1058. doi: 10.1016/j.cognition.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriveau KH, Goswami U. Rhythmic motor entrainment in children with speech and language impairments: Tapping to the beat. Cortex. 2009;45:119–130. doi: 10.1016/j.cortex.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Dunn L, Dunn L. Peabody Picture Vocabulary Test. 3. Circle Pines, MN: American Guidance Service; 1997. PPVT-3. [Google Scholar]
- Feldman HM. Language learning with an injured brain. Language Learning and Development. 2005;1:265–288. [Google Scholar]
- Feldman HM, Holland AL, Kemp SS, Janosky JE. Language development after unilateral brain injury. Brain and Language. 1992;42:89–102. doi: 10.1016/0093-934x(92)90058-m. [DOI] [PubMed] [Google Scholar]
- Feldman HM, Janosky JE, Scher MS, Wareham NL. Language abilities following prematurity, periventricular brain injury, and cerebral palsy. Journal of Communication Disorders. 1994;27:71–90. doi: 10.1016/0021-9924(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Thal D, Bates E, Hartung JP, et al. The MacArthur Communicative Development Inventories: User’s guide and technical manual. Baltimore: Brookes; 1993. [Google Scholar]
- Goldin-Meadow S. Hearing gesture: How our hands help us think. Cambridge, MA: Harvard University Press; 2003. [Google Scholar]
- Goldin-Meadow S, Butcher C. Pointing toward two-word speech in young children. In: Kita S, editor. Pointing: Where language, culture, and cognition meet. Hillsdale, NJ: Erlbaum; 2003. pp. 85–107. [Google Scholar]
- Goldin-Meadow S, Cook SW, Mitchell ZA. Gesturing gives children new ideas about math. Psychological Science. 2009;20:267–272. doi: 10.1111/j.1467-9280.2009.02297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin-Meadow S, Goodrich W, Sauer E, Iverson J. Young children use their hands to tell their mothers what to say. Developmental Science. 2007;10:778–785. doi: 10.1111/j.1467-7687.2007.00636.x. [DOI] [PubMed] [Google Scholar]
- Goldin-Meadow S, Mylander C. Gestural communication in deaf children: the effects and noneffects of parental input on early language development. Monographs of the Society for Research in Child Development. 1984;49 [PubMed] [Google Scholar]
- Goodwyn S, Acredolo L, Brown C. Impact of symbolic gesturing on early language development. Journal of Nonverbal Behavior. 2000;24:81–103. [Google Scholar]
- Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. Baltimore: Brookes; 1995. [Google Scholar]
- Hesterberg T, Monaghan S, Moore DS, Clipson A, Epstein R. Bootstrap methods and permutation tests. In: Moore DS, McCabe GP, editors. Introduction to the practice of statistics. 5. 2003. pp. 14-1–14-70. [Google Scholar]
- Hill EL. Non-specific nature of specific language impairment: a review of the literature with regard to concomitant motor impairments. International Journal of Language and Communication Disorders. 2001;36:149–171. doi: 10.1080/13682820010019874. [DOI] [PubMed] [Google Scholar]
- Hill EL, Bishop DVM, Nimmo-Smith I. Representational gestures in developmental coordination disorder and specific language impairment: Error-types and the reliability of ratings. Human Movement Sciences. 1998;17:655–678. [Google Scholar]
- Huttenlocher P. Neural plasticity: the effects of environment on the development of the cerebral cortex. Cambridge, MA: Harvard University Press; 2002. [Google Scholar]
- Iverson J, Capirci O, Caselli MC. From communication to language in two modalities. Cognitive Development. 1994;9:23–43. [Google Scholar]
- Iverson J, Goldin-Meadow S. Gesture paves the way for language development. Psychological Science. 2005;16:367–371. doi: 10.1111/j.0956-7976.2005.01542.x. [DOI] [PubMed] [Google Scholar]
- Iverson JM, Thelen E. Hand, mouth, and brain: the dynamic emergence of speech and gesture. Journal of Consciousness Studies. 1999;6:19–40. [Google Scholar]
- Krägeloh-Mann I, Horber V. The role of magnetic resonance imaging in elucidating the pathogenesis of cerebral palsy: A systematic review. Developmental Medicine & Child Neurology. 2007;49:144–151. doi: 10.1111/j.1469-8749.2007.00144.x. [DOI] [PubMed] [Google Scholar]
- Levine SC, Huttenlocher P, Banich MT, Duda E. Factors affecting cognitive functioning of hemiplegic children. Developmental Medicine and Child Neurology. 1987;29:27–35. doi: 10.1111/j.1469-8749.1987.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Levine SC, Kraus R, Alexander E, Suriyakham L, Huttenlocher P. IQ decline following early unilateral brain injury: A longitudinal study. Brain and Cognition. 2005;59:114–123. doi: 10.1016/j.bandc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Marchman V, Miller R, Bates E. Babble and first words in children with focal brain injury. Applied Psycholinguistics. 1991;12:1–22. [Google Scholar]
- McNeill D. Hand and Mind. Chicago: University of Chicago Press; 1992. [Google Scholar]
- Mercuri E, Barnett A, Rutherford M, Guzzetta A, Haataja L, Cioni G, et al. Neonatal cerebral infarction and neuromotor outcome at school age. Pediatrics. 2004;113:95–100. doi: 10.1542/peds.113.1.95. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Schürmann M, Amunts K, Hari R. Broca’s region: From action to language. Physiology. 2005;20:60–69. doi: 10.1152/physiol.00043.2004. [DOI] [PubMed] [Google Scholar]
- Özçaliskan S, Goldin-Meadow S. Gesture is at the cutting edge of early language development. Cognition. 2005;96:B101–B113. doi: 10.1016/j.cognition.2005.01.001. [DOI] [PubMed] [Google Scholar]
- O’Reilly AW, Painter KM, Bornstein MH. Relations between language and symbolic gesture development in early childhood. Cognitive Development. 1997;12:185–197. [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nature Reviews Neuroscience. 2005;6:576–582. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Perry M, Church RB, Goldin-Meadow S. Transitional knowledge in the acquisition of concepts. Cognitive Development. 1988;3:359–400. [Google Scholar]
- Reilly J, Levine SC, Nass R, Stiles J. Brain plasticity: Evidence from children with prenatal brain injury. In: Reed J, Warner J, editors. Child Neuropsychology. Oxford, England: Blackwell; in press. [Google Scholar]
- Rizzolatti G, Arbib M. Language within our grasp. Trends in Neurosciences. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rowe M, Goldin-Meadow S. Differences in early gesture explain SES disparities in child vocabulary size at school entry. Science. 2009a;323:951–953. doi: 10.1126/science.1167025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M, Goldin-Meadow S. Early gesture selectively predicts later language learning. Developmental Science. 2009b;12:182–187. doi: 10.1111/j.1467-7687.2008.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe ML, Levine SC, Fisher J, Goldin-Meadow S. Does linguistic input play the same role in language learning for children with and without early brain injury? Developmental Psychology. 2009;45:90–102. doi: 10.1037/a0012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M, Özçaliskan S, Goldin-Meadow S. The added value of gesture in predicting vocabulary growth. In: Bamman D, Magnitskaia T, Zaller C, editors. Proceedings of the 30th Annual Boston University Conference on Language Development. Somerville, MA: Cascadilla Press; 2006. pp. 501–512. [Google Scholar]
- Rowe M, Özçaliskan S, Goldin-Meadow S. Learning words by hand: Gesture’s role in predicting vocabulary development. First Language. 2008;28:185–203. doi: 10.1177/0142723707088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobin DI, Welsh CA. Elicited imitation as a research tool in developmental psycholinguistics. 1967. (ERIC Document Reproduction Service No. ED012892) [Google Scholar]
- Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krägeloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Annals of Neurology. 2004;56:854–863. doi: 10.1002/ana.20297. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krägeloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: A TMS and fMRI study. Brain. 2002;125:2222–2237. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- Steenbergen B, Gordon A. Activity limitation in hemiplegic cerebral palsy: evidence for disorders in motor planning. Developmental Medicine & Child Neurology. 2006;48:780–783. doi: 10.1017/S0012162206001666. [DOI] [PubMed] [Google Scholar]
- Stiles J, Reilly J, Paul B, Moses P. Cognitive development following early brain injury: evidence for neural adaptation. Trends in Cognitive Sciences. 2005;9:136–143. doi: 10.1016/j.tics.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Thal D, Marchman V, Stiles J, Aram D, Trauner D, Nass R, et al. Early lexical development in children with focal brain injury. Brain and Language. 1991;40:491–527. doi: 10.1016/0093-934x(91)90145-q. [DOI] [PubMed] [Google Scholar]
- Thal D, Tobias S. Communicative gestures in children with delayed onset of oral expressive vocabulary. Journal of Speech and Hearing Research. 1992;35:1281–1289. doi: 10.1044/jshr.3506.1289. [DOI] [PubMed] [Google Scholar]
- Vicari S, Albertoni A, Chilosi A, Cipriani P, Cioni G, Bates E. Plasticity and reorganization during language development in children with early brain injury. Cortex. 2000;36:31–46. doi: 10.1016/s0010-9452(08)70834-7. [DOI] [PubMed] [Google Scholar]
- Visscher C, Houwen S, Scherder EJA, Moolenaar B, Hartman E. Motor profile of children with developmental speech and language disorders. Pediatrics. 2007;120:E158–E163. doi: 10.1542/peds.2006-2462. [DOI] [PubMed] [Google Scholar]
- Werner H, Kaplan B. Symbol formation: An organismic-developmental approach to language and the expression of thought. New York: Wiley; 1963. [Google Scholar]
- Woods B, Teuber H. Changing patterns of childhood aphasia. Annals of Neurology. 1978;3:273–280. doi: 10.1002/ana.410030315. [DOI] [PubMed] [Google Scholar]