Abstract
Previous studies indicated that density-arrested cancer cells released an unidentified growth inhibitor whose secretion was prevented by overexpression of the lysosomal protease cathepsin D. Here, this growth inhibitor was purified by affinity chromatography and identified as the heat shock cognate 70 protein (Hsc70) based on its peptide microsequencing and specific antibody recognition. Among intracellular proteins, including other heat shock proteins, only constitutive Hsc70 was secreted in response to the high cell density. Moreover, Hsc70 secretion from cancer cells was generated by serum deprivation, whereas its cellular concentration did not change. Prevention of Hsc70 secretion by cathepsin D overexpression was associated with the formation of multilayer cell cultures, thus indicating a loss of contact inhibition. In addition, we demonstrated that supplementing the culture medium with purified Hsc70 inhibited cell proliferation in the nanomolar range. Conversely, removal of this extracellular Hsc70 from the medium by either retention on ADP-agarose or competition at the Hsc70 binding site restored cell proliferation. Hsc70 appears active in human breast cancer cells and hypersecreted by direct cathepsin D inhibition. These results suggest a new role of this secreted Hsc70 chaperone in cell proliferation that might account for the higher tumor growth of cancer cells overexpressing cathepsin D.
Keywords: Animals; Blotting, Western; Breast Neoplasms; metabolism; pathology; physiopathology; Cathepsin D; genetics; metabolism; Cell Count; Cell Line; Cell Line, Tumor; Cell Proliferation; Chromatography, High Pressure Liquid; Culture Media, Serum-Free; pharmacology; Electrophoresis, Polyacrylamide Gel; Female; HSC70 Heat-Shock Proteins; genetics; metabolism; secretion; Humans; Microscopy, Electron, Scanning; Neoplasms; metabolism; pathology; physiopathology; RNA Interference; Recombinant Proteins; pharmacology; Signal Transduction; drug effects; physiology
Keywords: cancer, growth inhibition, Hsc70, secretion, extracellular, cathepsin D
Introduction
Stress-related proteins are known as heat-shock proteins (Hsp) and are divided into families according to their average molecular weight. The 70-kDa Hsp family is composed of heat-inducible proteins (Hsp70), which are expressed under cellular stress conditions, and heat shock cognate proteins (Hsc70), which are constitutively expressed without any stress stimulation (Dworniczak & Mirault, 1987). These ATP-dependent proteins are involved in important cellular functions. Hsc70 is most efficient when associated with heat shock factor cochaperones (Morimoto, 1998), and could also bind to nascent polypeptides (Beckmann et al., 1990) and to polypeptides containing abnormal amino acids, dissociate clathrin from clathrin coats (Rothman & Schmid, 1986), and promote lysosomal degradation of cytosol proteins (Chiang et al., 1989). Hsc70 contains signal peptides which allow its nucleolar or its cytoplasmic localization (Tsukahara et al. 2004). Hsc70 is expressed in non-malignant mammary cells as well as breast cancer cells (Soulier et al. 1993; Vargas-Roig et al. 1998; Kao et al. 2003) and the overexpression of Hsp/hsc70 in chemoresistant cancer cells (Ciocca et al. 1992, 2003; Lazaris et al. 1997; Vargas-roig et al. 1997, 1998) has prompted studies about possible clinical markers of these proteins (Ellegde et al. 1994; Nylandsted et al., 2002; Thanner et al. 2003; Ciocca & Calderwood, 2005; Torronteguy et al., 2006)). While the heat shock protein 70 family is primarily implicated in several intracellular functions, the presence of extracellular Hsp70 was evidenced on tumor cell surfaces (Multhoff et al., 1995; Melendez et al., 2006), suggesting an immunological role through, for instance, the stimulation of effector cells of the innate immune system (Udono & Srivastava, 1993; Srivastava, 2005). Stressed rat cells (Hightower et al. 1989) and human cells like myocardium (Zou et al 2008), HeLa (Saito et al. 2005), erythroleukemic (Barreto et al 2003), and neural cells (Tytell et al. 2005) together with synapses (Chen et al. 2007), can release Hsc70. However, little is known for unstressed breast cancer.
Pioneer studies (Abercrombie & Heaysman, 1954; Eagle & Levine, 1967) have shown that contact inhibition of cell growth, i.e. the mechanism by which cell-cell contacts mediate antiproliferative signals, plays a fundamental role in regulating homeostasis in normal tissues. In contrast, the loss of contact inhibition is one trait of cancer cells, which can also grow to higher saturation densities and form multilayered foci (Abercrombie, 1979). This cellular behavior contributes to disruption of natural tissue barriers, and favors tumor spreading.
We previously demonstrated that the growth of cancer cells to higher density was promoted when these cells overexpressed cathepsin D (cath D) (Garcia et al., 1990; Glondu et al. 2002). Furthermore, we have shown that these effects are due to modulation of extracellular growth inhibitor(s) produced by high density cells, but the nature of the inhibitor(s) is still unknown (Liaudet et al., 1995). In the present report, we demonstrate that Hsc70 is the main protein secreted by density-arrested 3Y1-Ad12 cancer cells and is associated with the antiproliferative activity of conditioned medium. The extracellular release of Hsc70 is not the result of cell lysis but appears positively regulated by either serum deprivation or cell density, or negatively regulated by cath D overexpression. In addition, human breast cancer cells also secreted Hsc70, and this secretion is enhanced by adding the cath D inhibitor pepstatin A or by cath D silencing. Finally, the growth inhibition obtained at nano molar concentrations of purified secreted Hsc70 in 3Y1-Ad12 cancer cells or recombinant Hsc70 in human cell lines strongly supports the hypothesis that decreased Hsc70 secretion may be involved in the loss of contact inhibition and high density growth of cancer cells.
Results
Purification and identification of an extracellular protein associated with growth inhibition
Conditioned culture media from 3Y1-Ad12 cells arrested at confluency were used to purify factor(s) associated with growth inhibition. Protein components were separated by HPLC and the elution profile was recorded with an UV detector (Figure 1A). Each fraction was evaluated for antiproliferative activity in rat mammary adenocarcinoma RBA cells (Cohen et al., 1974) test which was previously shown to be a better antiproliferative test than that with 3Y1-Ad12 cells (Liaudet et al., 1995). The antiproliferative activity appeared to be concentrated as a single peak around fraction 48, which corresponded to an apparent molecular weight of 240 kDa in a gel filtration column (arrows in Figure 1A). This activity was also found as a single peak in another HPLC condition performed on a Mono Q anion-exchange column with an elution carried out with a salt concentration of 0.2 M NaCl (data not shown).
Figure 1. Identification of the 70 kDa-protein as the heat shock cognate protein Hsc70.
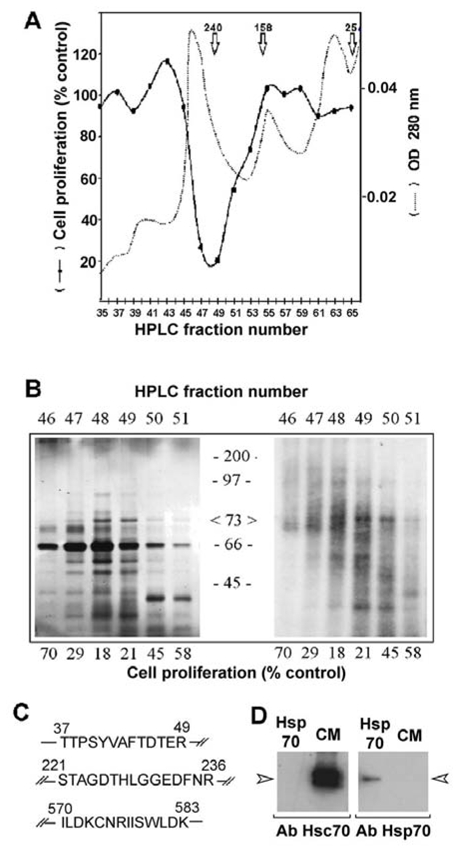
(A) Conditioned medium obtained from confluent 3Y1-Ad12 cells labelled with 35S-methionine was dialyzed, concentrated and purified on an HPLC system as described in the Materials and Methods. The UV elution profile was recorded at 280 nm (dashed lines). The antiproliferative activity of the collected fractions was measured by adding 75 μl of each odd fraction to triplicate culture wells containing 100,000 RBA cells growing in the log phase. The DNA content was evaluated after 5 days incubation and the values are expressed as a percentage of DNA recovered in control wells treated with buffer alone (solid lines). (B) HPLC fractions from the above experiment containing the antiproliferative activity were subjected to 12% SDS-PAGE separation without any reducing agent, and silver stained (left) or processed for fluorography of S35-labelled proteins (Brighty & Jassal). (C) Identification of rat Hsc70 by peptide microsequencing. The major 70–73 Kda protein was digested with trypsin as described in the Materials and Methods. Three different peptides were isolated by HPLC and sequenced. All sequences were identical to the rat 73 kDa heat shock cognate protein (Hsc70) and peptide 3 was specific to Hsc70 isoform 1. (D) Secreted proteins from conditioned medium (CM) or commercialized Hsp70 (Hsp70) were immunoblotted with Hsc70 or Hsp70 specific antibodies.
In order to identify the proteins associated with this antiproliferative activity, each active fraction was analyzed by sodium duodecyl sulphate polyacrylamide gel electrophoresis (SDS PAGE), and at least 15 proteins were detected by silver staining (Figure 1B). Some of these were serum contaminants since they were not detectable in the medium after 24 h of metabolic cell labeling with 35S-methionine. This serum contamination was illustrated by the presence of the most intense band at 66 kDa, which corresponded to bovine serum albumin. Two major neo-synthesized proteins with an apparent molecular weight of 30 and 73 kDa appeared to be correlated with the antiproliferative activity. More attention was given to the 73 kDa protein since its secretion was previously found to be decreased by cancer cells overexpressing cath D (Liaudet et al., 1995). To further characterize the 73 kDa proteins, this band was extracted from the gel after conditioned medium separation, and N-terminal sequencing was then performed on three trypsin digestion peptides using an Automatic Peptide Sequencer system (Figure 1C). The sequences were compared to protein databases and their analysis clearly revealed that these peptides corresponded to the 70 kDa heat shock protein sequence (O’Malley et al., 1985; Sorger & Pelham, 1987). Moreover, peptide 570-583 was able to differentiate the constitutive Hsc70 among other members of the highly conserved heat shock protein family owing to the presence of residues I570, N575, and I577. In addition, the sequence of this fragment also indicated that the secreted Hsc70 was the isoform 1 protein since isoform 2 did not contain residues encompassing the 570–583 sequence. Western blots with specific Hsp70 and Hsc70 antibodies confirmed that Hsc70 was the secreted protein (Figure 1D).
Hsc70 is secreted by high density cells but not shed by lysed cells
In order to determine whether Hsc70 was the result of secretion by cancer cells and not a cell lysis product, we compared the patterns of both the secreted and intracellular proteins of confluent cells on SDS-PAGE after metabolic labeling with 35S-methionine (Figure 2A). The relative protein patterns strongly differed between the extracellular and intracellular compartments. As examples, the 256 kDa protein was abundantly secreted but barely present within the cells, whereas the 135 kDa protein was poorly secreted. The amount of secreted 70 kDa band corresponded to 19% of the total amount of secreted proteins, whereas the intracellular amount of this band represented only 2% of the total labeled intracellular protein content in these cancer cells. In contrast, in cath D overexpressing cells, the secreted 70 kDa band is reduced to 0.1% of the total secreted proteins. From a macroscopic standpoint, we quantified the percentage of cell death in a trypan blue exclusion assay. Over the same assay period, only 1.6% of the total number of control and cath D overexpressing cells died. This suggests that release of the protein content by passive mechanisms is limited and identical in both situations (Figure 2B). In addition, we used the property of the heat shock protein family that binds ATP to subject the conditioned medium to ATP-agarose chromatography. The bound proteins were eluted prior to SDS-PAGE analysis and silver staining revelation (Figure 2C). The elution profile showed that the detected Hsc70 protein was the only excreted Hsc protein present in the medium. Fractions 4 to 6 contained the majority of the secreted Hsc70. Overall, these results indicated that the extracellular Hsc70 was the result of protein secretion in culture medium rather than being part of the general release of intracellular proteins following cell lysis.
Figure 2. Hsc70 from culture medium is secreted and is the only heat shock protein detectable.
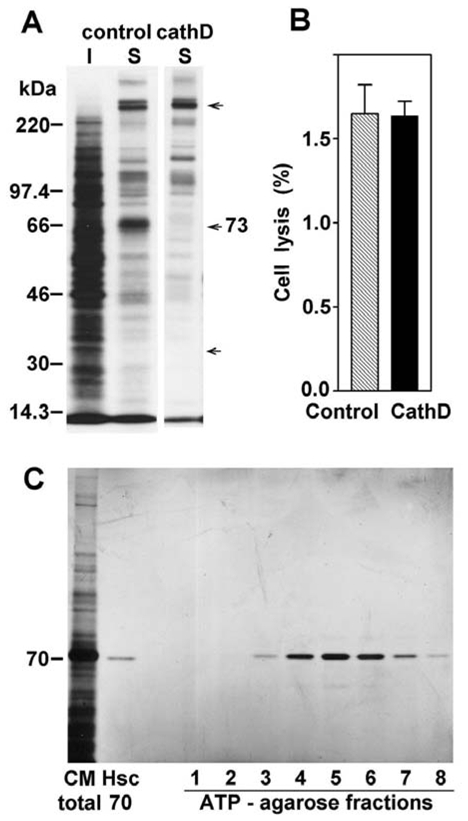
(A) 35-S-Met protein profile after SDS-PAGE separation and fluorography analysis for the intracellular proteins (I) or the secreted proteins (S) from control cells and secreted proteins from cath D overexpressing clones (Cath D S). Arrows represent examples of proteins present in only one compartment. (B) The % cell death was evaluated using trypan blue staining for control cells and Cath D transfected clones. (C) Hsc70 was purified by ATP-agarose gel chromatograghy from control cell conditioned medium, analyzed by SDS-PAGE, and silver stained. Commercial Hsc70 (100 ng) was used as control protein.
Contact inhibition and relationship with hsc70
We next examined the morphological changes between high density 3Y1-Ad12 controls and cath D overexpressing cells. Scanning electron microscopic studies revealed morphological alterations in confluent cells when comparing controls and clones stably transfected with cath D cDNA. Control cells exhibited a flattened morphology of monolayer cells at confluency with rare disseminated foci (Figure 3A). In contrast, transfected cells continued to proliferate with overlapping cell layers and achieved a higher density multilayer (Figure 3B) containing aggregated colonies. This loss of contact inhibition was even more pronounced when cultures were prolonged for over 10 days, as revealed by cellular DNA quantitation to monitor cell growth (Figure 3C). Control cells reached the plateau phase at around 18 μg DNA/well on day 6 and onwards, whereas transfected cells did not reach the plateau phase even on day 10 of the experimentation. The maximal density for the transfected cells reached 38 μg DNA/well. We secretion from control cells and from clones expressing cath D (Figure 3D). This ability of the transfected cells to proliferate and form multilayered colonies was linked to a marked decrease in Hsc70 secretion (Figure 3D). Cath D expressing cells secreted 60% less Hsc70 at confluency than the control cells. We have also tried to decrease Hsc70 secretion by RNA interference silencing in order to further demonstrate its implication in contact inhibition. However, we did not obtain a such genetic proof since cells became detached during the experiment and died (data not shown). This result was in accordance with data of Florin et al. (Florin et al. 2004) indicating that Hsc70 knockout cells were nonviable.
Figure 3. Loss of contact inhibition and repression of Hsc70 secretion induced by cath D.
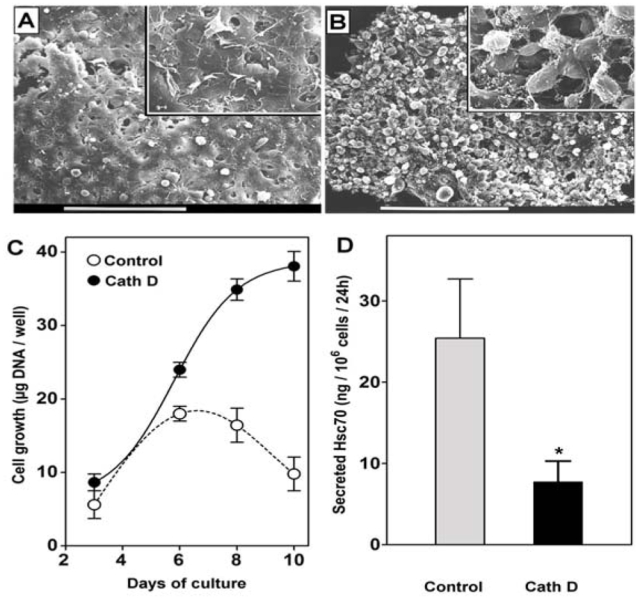
Control and cath D expressing cells were cultured for 6 days on glass coverslips and analyzed under a scanning electron microscope. Bars represent 90 nm. Inserts represent a 5-fold magnification. (A) A characteristic monolayer cell culture depicts confluent control cells. The high confluency did not allow observation of the cell contours. (B) Overlapping cell layers characterized the cath D transfected cells. Abundant foci were seen as spheroids. (C) Cell quantitation was performed by DNA content measurement using the DABA method. Two control cell lines (open circles) and two cath D transfected cells (filled circles) were monitored for a 10 day period. Results are means for two clones run in triplicate. (D) In the same experiments, Hsc70 content released for a 24 h period in the medium at day 6 was quantitated by Western blot and expressed as ng for 106 cells for 24 h (* p = 0.0148)
Regulation of Hsc70 secretion by cell density, serum deprivation and lysosomotrophic bases
The effects of different growth regulating parameters were explored on Hsc70 expression. Hsc70 levels were evaluated in both the intracellular and extracellular compartments of two cath D transfected clones and their corresponding controls after 24 h standard culture with increasing amounts of serum in the culture medium (Figure 4). No significant differences were observed with respect to the amount of intracellular Hsc70 in the controls and the cath D clones (Figure 4A). However, an increase in Hsc70 occurred within both cell types with serum removal from the culture medium. In contrast, secreted Hsc70 was markedly different (p = 0.0014) in clones expressing cath D as compared to control cells (Figure 4B). Control cells secreted large amounts of Hsc70, with a mean ranging from 24 to 36 ng/106 cells depending upon the percentage of serum in the culture medium, whereas cath D clones produced about 10-fold less Hsc70 than the controls (2.5 vs 24 ng/106 cells). For serum withdrawal, a slight increase in secreted Hsc70 was observed, and the transfected clones produced about 5-fold less Hsc 70 than the control cells. Hsc70 secretion was also influenced by the cell density, as shown in Figure 4C, and different effects were observed in the controls and the cath D expressing clones. In control cells, an increase in the cell density from 0.4 to 1.5 106 cells/cm2 is associated with enhancement of extracellular Hsc70 from 33 to 60 ng/106 cells. When clones expressed Cath D and formed multilayer cells, scarce amounts of extracellular Hsc70 could be recorded and no significant modulations of Hsc70 were observed throughout the tested density range. In the same culture conditions, we used the trypan blue assay to evaluate the percentage of cell death. For control cells, as well as for the transfected clones, the mean cell death did not exceed 2.4% of total cells (data not shown), as expected for these cultured cells.
Figure 4. Effects of serum deprivation, cell density and weak base treatments on Hsc70 secretion.
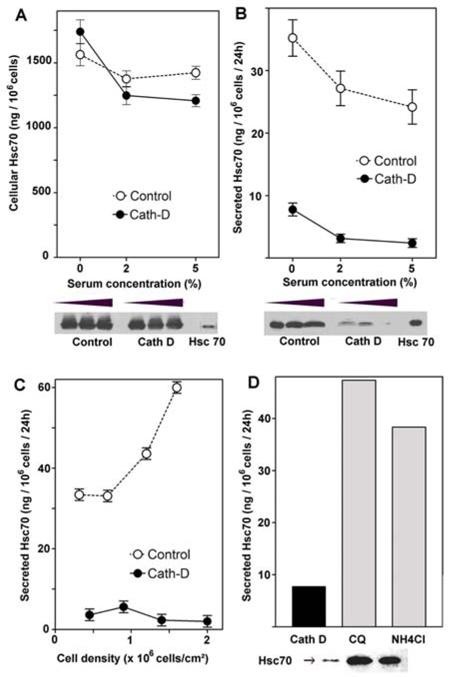
Control cells (open circle) and Cath D expressing clones (filled circle) were cultured for 2 days in the presence of the indicated percentage of foetal calf serum. Hsc70 concentrations within the cells (A) and in the corresponding culture media (B) were evaluated by Western blot using Hsc70 (100 ng/track) as protein loading control. Hsc70 secretion was significantly different (p = 0.0014) between control and over-expressing cath D cells. (C) Effects of cell density on Hsc70 secretion was analyzed in the range from 0.5 to 2 106 cells/cm2. (p=0.0056) (D) Regulation of Hsc70 secretion by cath D and lysosomotropic bases. Cath D cells were cultured for 5 days in serum-free medium, either untreated (control) or treated with 50 μM chloroquine (CQ) or 0.2 mM ammonium chloride (NH4Cl). Hsc70 was assayed in the culture medium and expressed as ng/106 cells/24 h, and Western blot of the corresponding conditioned media was shown.
To further confirm that the cath D enzyme was involved in Hsc70 secretion, we neutralized cath D maturation in acidic compartments by using weak bases such as chloroquine or ammonium chloride, as previously described by Liaudet (Liaudet et al., 1995). As shown by Western blotting, when such bases were added to the medium, secreted Hsc70 was significantly increased from 7.7 ng/106 cells in cath D cells to 47 ng and 38 ng/106 cells in chloroquine and ammonium chloride treated cells, respectively (Figure 4D). These results suggest that the acidity of cellular organelles, which is necessary for cath D maturation and activity, is required to prevent Hsc70 secretion. It is known that a pH increase induces a conformational change in cath D since the N terminal becomes positioned in the active site cleft at neutral pH, masking both active D33 and D231 residues to the substrate, as revealed by crystal structure (Baldwin et al., 1993; Metcalf & Fusek, 1993).
Cath D expression did not affect the responsiveness to extracellular Hsc70
To verify the hypothesis that Hsc70 secretion and Hsc70-mediated growth inhibition are independent mechanisms, we complemented the culture medium with 35S-methionine labeled conditioned media from control and cath D cells (Figure 5A). As expected, the cellular binding of the 70 kDa protein was observed only in the presence of a conditioned medium (CM) obtained from control cells which secrete Hsc70. Interestingly, the amount of 70 kDa protein bound to cath D cells was strictly identical to that retained by the control cells. In addition, conditioned medium from control cells inhibited proliferation of cath D recipient cells to the same extent as for control cells (Figure 5B). These data indicated that cath D expression did not affect neither the Hsc70 cell uptake nor the Hsc70-induced growth inhibition
Figure 5. Cath D expression and responsiveness to secreted Hsc70.
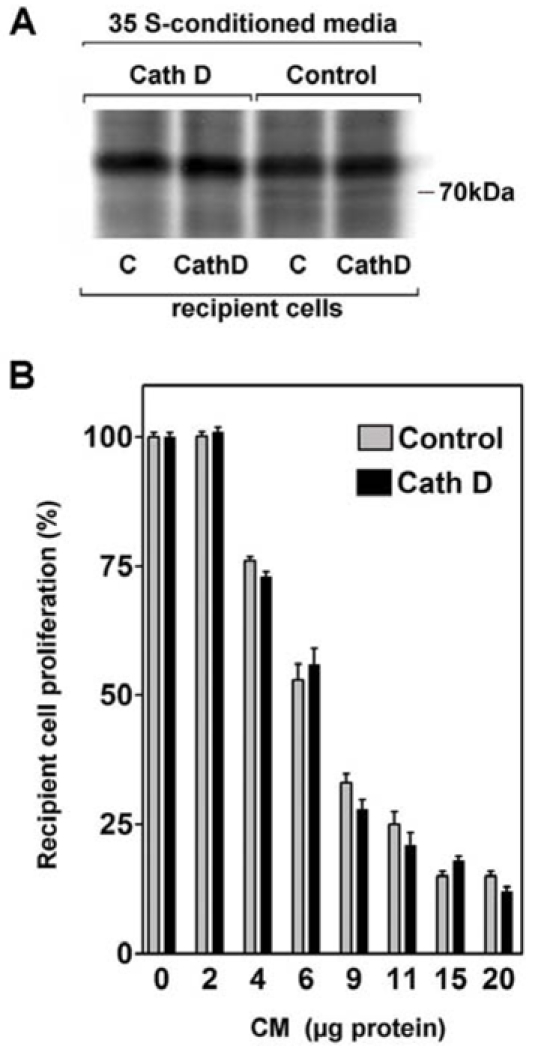
(A) Confluent control cells or Cath D expressing clones were cultured in the presence of 35S-methionine for 24 h. The corresponding conditioned media were then added in the presence of 1 mM non radioactive methionine, to confluent control (C) or cath D recipient cells. After a 24 h incubation and two washes, the cell proteins were subjected to SDS-PAGE and fluorography. (B) Proliferation of control or cath D cells cultured in the presence of the indicated concentration of proteins recovered from conditioned media of control density-arrested cells.
Involvement of extracellular Hsc70 in antiproliferative cell activity
To assess the antiproliferative activity of this protein, we supplemented the culture medium with an ATP-agarose purified Hsc70 from serum-free conditioned medium, as described in Figure 2C. Increasing nanomolar amounts of Hsc70 were then added to log phase proliferating cells. As shown in Figure 6A, up to 2 nM purified Hsc70 markedly decreased cell proliferation in a dose-dependent manner. Thereafter, higher amounts of Hsc70 did not significantly influence cell proliferation. Hsc70 decreased cell proliferation to 24% (i.e. 76% inhibition) as compared to an inactive fraction used as control. To determine whether or not the binding site of extracellular Hsc70 was required for its antiproliferative activity, we used an RNase A protein bounding Hsc70 at position 450–462 through its KFERQ sequence (Agarraberes et al., 1997) (residues 7-11 from RNase A). The RNAse A from up to 1 μg/ml totally reversed the inhibitory effect of conditioned medium from confluent cells (CM) (Figure 6B). This indicated that the binding of Hsc70 to proteins containing KFERQ was probably required to avoid proliferation. Moreover, the addition of the S-peptide (Chiang et al., 1989) (corresponding to the 1–20 N-terminal amino acids of RNAse A) prevented much of the activity of the conditioned medium thereby suggesting that extracellular Hsc70 played a major role in the growth inhibition (Figure 6C). On the contrary, the additional growth inhibition observed after serum withdrawal was not totally prevented by S-peptide, indicating that serum may contain an additional factor. Moreover, retention of Hsc70 by ADP-agarose partially prevented the antiproliferative effect of CM (Figure 6D). Altogether, these data indicated an antiproliferative activity of extracellular Hsc70 requiring the binding to the substrate containing the KFERQ sequence.
Figure 6. Effect of purified Hsc70 and Hsc70 competitors on cell growth.
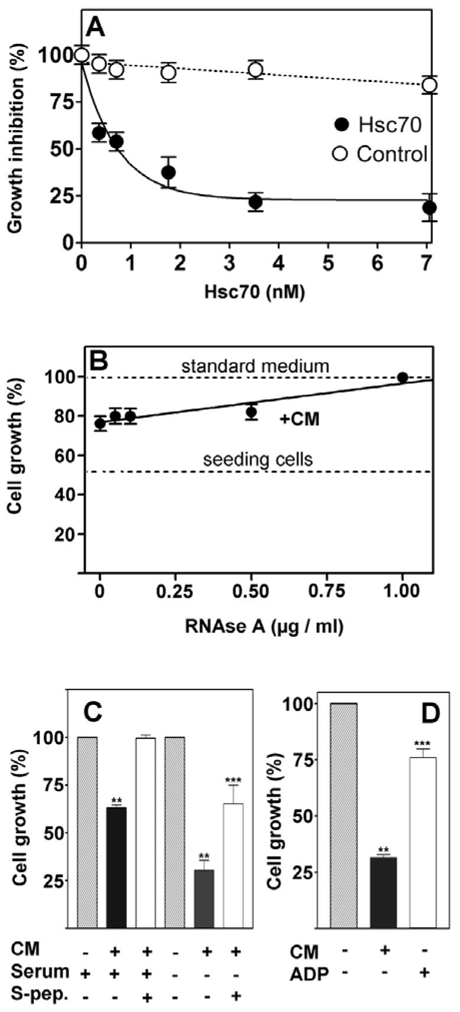
(A) Increasing amounts of Hsc70 purified from confluent control cells as described in Figure 2C were added to recipient exponential cell cultures. Cell growth was monitored by their DNA content after 5 days treatment (filled circles). Identical volumes of ATP-eluted fraction that did not contain hsc70 was used as control (open circles). (B) Increasing amounts of RNAse A were added to the control cell conditioned medium (CM) at the indicated concentration. Cell growth was the result of the cellular DNA content determined by DABA assay. Dashed lines represent the seeding cells at the beginning of the experiment, and the cell growth in standard medium with 2% serum (without CM). The data are mean results of three independent experiments run in triplicate. (C) 1 μg/ml S-peptide was added to the culture medium with 5% (+) or with 0.5% (−) serum. Cell growth was measured as previously mentioned. (D) Cell growth was inhibited by the control cell CM and this effect was reversed by an initial batchwise treatment with ADP-agarose (CM+ADP) to remove Hsc70. (** p<0.001 vs untreated control, *** p<0.001 vs CM).
Effect of Hsc70 on human breast cancer cell lines and cath D modulation
Extracellular Hsc70 also modulated the growth of human cancer cells (Figure S1). Recombinant Hsc70 but not Hsp70 decreased by 25% and 20% the proliferation of MCF7 (Figure S1-A) and MDA-MB-231 (Figure S1-B) cells, respectively. The treatment with pepstatin A significantly increased Hsc70 secretion (Figure S2-A) in both cell lines. Moreover, by silencing cath D, an increase in Hsc70 secretion was observed in both cell lines. (Figure S2-B).
Discussion
This study provides evidences of the contribution of Hsc70 in cancer cell growth regulation through secretion of this protein in the extracellular environment. We isolated this extracellular protein for its antiproliferative activity and identified it as the heat shock cognate 70 protein through microsequencing of trypsin fragments. The sequence of the fragment at the amino-terminal end of the protein is characteristic of the Hsp70/Hsc70 family within the ATP-binding site of the protein (Munro & Pelham, 1986; Sorger & Pelham, 1987). Retention of the protein on an ATP-affinity chromatography column suggests that a functional ATP binding site is present on the molecule. In addition, the carboxy-terminal end of the protein fragment allowed us to clearly identify this protein as the rat Hsc70 isoform 1, since this Cterm sequence is specific to the Hsc70 isoform 1 and is missing within isoform 2 (Tsukahara et al., 2000), in addition to the monoclonal antibody recognition which discriminates an antigenic determinant localized only within the specific isoform 1 protein. This 646 residue protein was highly conserved during mammalian evolution and differs only by the N579S amino-acid change between human and mouse or rat (O’Malley et al., 1985) whereas the hsp70-gene family is characterized by a large diversity (Brocchieri et al., 2008). In addition, the identification of this protein as being Hsc70 was confirmed by the fact that an Hsp70 antibody was not able to recognize this protein. This protein was eluted in a gel filtration system as a single active peak corresponding to approximately 290 kDa, indicating that the protein may exist as a multimeric (possibly tetrameric) species. Such self-association has been described via size-exclusion chromatography when an Hsc70 concentration of over 4 μM was loaded onto a fast pressure liquid chromatography column (Fouchaq et al., 1999).
Hsc70, like many other heat shock proteins, remains intracellular and the detection of a such protein in the extracellular compartment might be the consequence of either cell lysis or cell death, which is always possible in cell cultures, or in contrast due to active release by the cells. According to the first hypothesis, the protein pattern between the two compartments would be qualitatively similar, considering the extremely higher abundance of some major intracellular proteins. This hypothesis was not confirmed by our data, since the absence of major intracellular proteins in the extracellular compartment suggests that the existence of Hsc70 out of cells is due to an active secretion. Hsc70 does not present the sequence leader to be secreted through the classical pathway. However, the use of a general lysosomal inhibitor, such as ammonium chloride which alters the lysosomal pH, functions as an enhancer of Hsc70 secretion. It should be stressed that the opposite effect has been reported for Hsp70 from prostate cancer cells, where an increase in the intralysosomal pH inhibited Hsp70 secretion (Mambula & Calderwood, 2006). Taken together, these results suggest that the routes of Hsp70 and Hsc70 secretion differ. These observations could be linked to the fact that, although they have very similar sequences, an increasing body of data indicate that these two proteins have different physiological functions (Dressel et al., 2003; Rohde et al., 2005; Goldfarb et al., 2006; Tutar et al., 2006). Another possible alternative route for Hsc70 secretion is via exosomes (Agarraberes et al., 1997; Thery et al., 1999; Geminard et al., 2001; Clayton et al., 2005). In our model, however, we were unable to demonstrate the presence of Hsc70 within a 100 000 × g pellet isolated from conditioned medium (data not shown).
Moreover, we also found that Hsc70 secretion was increased by two events that negatively regulated cell growth, i.e. serum starvation and cell confluence. These events trigger numerous cellular modifications in cancer cells, including intracellular regulator overexpression (Marchesini et al., 2004; Anagnostopoulou et al., 2006). The modulation of Hsc70 secretion by cell confluence was abolished when cells overexpressed moderate levels of cath D. Previous data have shown that overexpression of cath D in 3Y1-Ad12 rat cancer cells was sufficient to increase both the metastatic phenotype of the cells and their capacity to proliferate to a higher cell density and form colonies in soft agar. In breast cancer patients, cath D overexpression can be measured in tumor tissues, and several clinical meta-analyses have correlated the cytosolic concentration of cath D in primary tumors with an increased risk of metastasis (Bossard et al. 2003). It should be noted that the present results were obtained for cath D levels below the median concentration noted for primary breast cancers (70 pmol/mg cytosol protein) (Garcia et al., 1996). An attractive hypothesis would be that Hsc70 serves as a substrate for cath D overexpressing cells within acidic endolysosomes, and thus the pH of this organelle would be neutralized by lysosomotropic agents inhibiting cath D activity and increasing Hsc70 secretion. The antagonistic effect of cath D overexpression on Hsc70 secretion indicates that these two proteins might be localized in the same organelle. In this regard, Mambula et al. (Mambula et al., 2007) suggested that endolysosomes contain both Hsc70 and cath D proteins. Although the mechanism of action of cath D is not known, the reversal of cath D action by lysosomotropic compounds in 3Y1-Ad12 cells, by pharmacological inhibitor pepstatin A in the human breast cancer MCF7 and MDA-MB-231 cell lines, or the siRNA silencing, suggests that the enzyme might proteolyse Hsc70, or another protein that is required for Hsc70 secretion. Our unsuccessful attempts to hydrolyse Hsc70 by cath D has advocate for a cath D indirect effect on Hsc70 secretion. However, an interaction between cath D and Hsc70 cannot be excluded since a sequence equivalent to the classical KFERQ motif (Chiang & Dice, 1988; Terlecky & Dice, 1993) for Hsc70 binding was found at position 109 (KvERQ) in the N-termimus of cath D, corresponding to an external domain of this protein, as revealed by crystal structure analysis (Baldwin et al., 1993; Metcalf & Fusek, 1993).
Finally, we demonstrated that secreted Hsc70 inhibited cell proliferation in the nanomolar concentration range in rat cells. This effect corresponded to most of the antiproliferative activity of the conditioned medium. Nanomolar concentrations of recombinant Hsc70 also modulated the breast cancer cell proliferation, however, to a lesser extent, indicating that Hsc70 could be part of a more complex structure to fully reduce growth. The prevention of the Hsc70 antiproliferative activity due to competitors at the Hsc70 binding site also suggests that this binding site is involved in the antiproliferative response. Moreover, we also showed that cellular binding to Hsc 70 and its subsequent antiproliferative response were clearly unchanged within all clones overexpressing cath D. This indicates that the Hsc70 inhibition of cell proliferation is independent of cath D overexpression, whereas its secretion is not. These findings suggest that the routing pathways for Hsc70 secretion and uptake are quite distinct. In conclusion, we provide the first evidence that the heat shock cognate protein 70 is secreted by confluent cells, and that this extracellular protein plays an active role in the contact inhibition of cancer cells. Moreover, the decrease of this secreted chaperone might account for the higher growth of cancer cells overexpressing cath D.
Materials and Methods
Cell line and culture
Rat embryo cell lines transformed with adenovirus type 12 (3Y1-Ad12), RBA rat mammary adenocarcinoma cells (Cohen et al., 1974) were maintained in RPMI supplemented with 5% FCS.
Stable transfectants of 3Y1-Ad12 expressing human cath D, or controls transfected with the vector alone, were cultured with 400 μg/ml geneticin as previously described (Liaudet et al., 1995).
Protein labeling
For 35S-methionine labeling, cells were incubated for 24 h at 37°C in 1 ml methionine-free DMEM supplemented with 200 μCi/ml 35S-methionine (>1000 Ci/mol; Amersham). The secreted proteins were analyzed by SDS PAGE, followed by fluorography.
Growth inhibition
The growth inhibitory activity of conditioned media, HPLC fractions or purified Hsc70 protein, was measured on recipient low-density cells (RBA rat mammary cancer cells or 3Y1-Ad12 rat embryo cancer cells) growing for 5 days in the log phase in the presence of 2% FCS. Controls were performed with similar volumes of medium, or buffer used in the same conditions. The DNA content was determined in triplicate wells as previously described (Liaudet et al., 1995).
Purification of 70 kDa protein and microsequencing
Confluent 3Y1-Ad12 cells were washed and incubated for a further 24 h in serum-free medium. The media were dialyzed against distilled water, adjusted with 20 mM Tris (pH 7.5) and 1 mM CHAPS prior to concentration by lyophilization. The proteins were purified in a procedure involving ion-exchange chromatography with an elution performed with a 0–0.5 M NaCl gradient. The antiproliferative activity of each fraction was then evaluated in an RBA-mammary cell test which has previously shown to be a better antiproliferative test than that involving 3Y1-Ad12 cells. Fractions containing the inhibitory activity were pooled, then applied onto a gel filtration column using HPLC (Amersham-Pharmacia). The inhibitory activity of each HPLC fraction was determined as previously described. The proteins from positive fractions were pooled, lyophilized and separated on SDS-PAGE with low ammonium persulfate concentration, without heating or the addition of 2-mercaptoethanol. The major 70–73 Kda protein associated with growth inhibition was isolated from a non-reducing Coomassie blue stained SDS-PAGE, solubilized, then digested with trypsin for peptide isolation and sequencing in a Beckman automatic peptide sequencer.
High pressure liquid chromatography (HPLC)
HPLC was performed on a normal phase column using phosphate buffer as solvent. A linear gradient (1 ml/min, 30 min) was used and absorbance was measured at 280 nm. Fractions of 30 sec were collected, dried under nitrogen flow, then dissolved in 0.1 M phosphate buffer, and analyzed for the cell proliferation procedure.
ATP-agarose chromatography
Conditioned medium were prepared from high density control 3Y1-Ad12 cells after 24 h secretion in serum-free conditions. The conditioned medium was dialyzed, concentrated by lyophilisation and equilibrated in buffer D (20 mM NaCl, 15 mM 2-mercaptoethanol, 3 mM MgCl2, 20 mM Tris, pH 7.5) through a PD10 column. The concentrated medium was then loaded onto a 5 ml ATP-agarose gel column (Sigma). The column was washed successively with three volumes of i) buffer D, ii) buffer D supplemented with 0.5 M NaCl, and iii) buffer D. Finally, the retained proteins were eluted with 1 ml fractions of buffer D + 3 mM ATP. To test the antiproliferative activity, the fractions were washed three times with culture medium and concentrated twice with Centricon Biomax 5.
Scanning electron microscopy
Cells were grown on glass coverslips in phenol red-free DMEM with 5% DCC/FCS for 5 days. Cells were fixed with glutaraldehyde (3.75%) for 2 h and post-fixed with 1% osmium tetroxide (1% for 1 h in Millonig buffer at pH 7.3. Coverslips were rinsed in Millonig buffer and dehydrated through a graded series of alcohol and isoamyl acetate before critical-point drying. After mounting on an aluminum support, samples were coated with gold, and then examined with a JEOL Model JSM-35 scanning electron microscope at 15 kV.
Secreted Hsc70 quantitation
SDS-PAGE was performed with known amounts of commercial Hsc70 as standard tracks. After Western blot, the tracks were scanned and quantified using scion image for Windows (Scion Corp. Frederick, MA, USA).
Data analysis
Statistical significance was analysed using student’s t test from GraphPad Prism, version 3.00 for Windows (GraphPad Software Inc., San Diego, CA, USA). Values of p < 0.05 were considered significant.
Acknowledgments
We thank Jean Derancourt, (Centre de Recherches de Biochimie Macromoleculaire du CNRS, 1919 Route de Mende, 34293 Montpellier Cedex 5, France) for peptide fragments analysis. The authors also thank the Centre Régional d’Imagerie Cellulaire (Montpellier – France) for access to the scanning microscopy facilities. This work was supported by the Institut National de la Santé et de la Recherche Médicale, the Association pour la Recherche sur le Cancer, the Ligue contre le cancer, Comité de l’Hérault (fellowship to M.M.), and CNRS.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Supplementary information is available at http://www.nature.com/onc/
References
- Abercrombie M, Heaysman JE. Observations on the social behaviour of cells in tissue culture. II. Monolayering of fibroblasts. Exp Cell Res. 1954;6:293–306. doi: 10.1016/0014-4827(54)90176-7. [DOI] [PubMed] [Google Scholar]
- Abercrombie M. Contact inhibition and malignancy. Nature. 1979;281:259–62. doi: 10.1038/281259a0. [DOI] [PubMed] [Google Scholar]
- Agarraberes FA, Terlecky SR, Dice JF. An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation. J Cell Biol. 1997;137:825–34. doi: 10.1083/jcb.137.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulou A, Vultur A, Arulanandam R, Cao J, Turkson J, Jove R, et al. Differential effects of Stat3 inhibition in sparse vs confluent normal and breast cancer cells. Cancer Lett. 2006;242:120–32. doi: 10.1016/j.canlet.2005.10.047. [DOI] [PubMed] [Google Scholar]
- Baldwin ET, Bhat TN, Gulnik S, Hosur MV, Sowder RC, 2nd, Cachau RE, et al. Crystal structures of native and inhibited forms of human cathepsin D: implications for lysosomal targeting and drug design. Proc Natl Acad Sci U S A. 1993;90:6796–800. doi: 10.1073/pnas.90.14.6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto A, Gonzalez JM, Kabingu E, Asea A. Stress-induced release of Hsc70 from human tumors. Cell Immunol. 2003;222:97–104. doi: 10.1016/s0008-8749(03)00115-1. [DOI] [PubMed] [Google Scholar]
- Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–4. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Bossard N, Descotes F, Bremond AG, Bobin Y, De Saint Hilaire P, Golfier F, et al. Keeping data continuous when analyzing the prognostic impact of a tumor marker: an example with cathepsin D in breast cancer. Breast Cancer Res Treat. 2003;82:47–59. doi: 10.1023/B:BREA.0000003919.75055.e8. [DOI] [PubMed] [Google Scholar]
- Brighty DW, Jassal SR. The synthetic peptide P-197 inhibits human T-cell leukemia virus type 1 envelope-mediated syncytium formation by a mechanism that is independent of Hsc70. J Virol. 2001;75:10472–8. doi: 10.1128/JVI.75.21.10472-10478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocchieri L, Conway de Macario E, Macario AJ. hsp70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol Biol. 2008;8:19. doi: 10.1186/1471-2148-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Brown IR. Translocation of Constitutively Expressed Heat Shock Protein Hsc70 to Synapse-Enriched Areas of the Cerebral Cortex After Hyperthermic Stress. J Neurosci Res. 2007;85:402–409. doi: 10.1002/jnr.21124. [DOI] [PubMed] [Google Scholar]
- Chiang HL, Dice JF. Peptide sequences that target proteins for enhanced degradation during serum withdrawal. J Biol Chem. 1988;263:6797–805. [PubMed] [Google Scholar]
- Chiang HL, Terlecky SR, Plant CP, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246:382–5. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Fuqua SA, Lock-Lim S, Toft DO, Welch WJ, McGuire WL. Response of human breast cancer cells to heat shock and chemotherapeutic drugs. Cancer Res. 1992;52:3648–54. [PubMed] [Google Scholar]
- Ciocca DR, Rozados VR, Cuello Carrion FD, Gervasoni SI, Matar P, Scharovsky OG. Hsp25 and Hsp70 in rodent tumors treated with doxorubicin and lovastatin. Cell Stress Chaperones. 2003;8:26–36. doi: 10.1379/1466-1268(2003)8<26:hahirt>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci. 2005;118:3631–8. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- Dressel R, Grzeszik C, Kreiss M, Lindemann D, Herrmann T, Walter L, et al. Differential effect of acute and permanent heat shock protein 70 overexpression in tumor cells on lysability by cytotoxic T lymphocytes. Cancer Res. 2003;63:8212–20. [PubMed] [Google Scholar]
- Dworniczak B, Mirault ME. Structure and expression of a human gene coding for a 71 kd heat shock ‘cognate’ protein. Nucleic Acids Res. 1987;15:5181–97. doi: 10.1093/nar/15.13.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle H, Levine EM. Growth regulatory effects of cellular interaction. Nature. 1967;213:1102–6. doi: 10.1038/2131102a0. [DOI] [PubMed] [Google Scholar]
- Elledge RM, Clark GM, Fuqua SA, Yu YY, Allred DC. p53 protein accumulation detected by five different antibodies: relationship to prognosis and heat shock protein 70 in breast cancer. Cancer Res. 1994;54:3752–7. [PubMed] [Google Scholar]
- Fouchaq B, Benaroudj N, Ebel C, Ladjimi MM. Oligomerization of the 17-kDa peptide-binding domain of the molecular chaperone HSC70. Eur J Biochem. 1999;259:379–84. doi: 10.1046/j.1432-1327.1999.00053.x. [DOI] [PubMed] [Google Scholar]
- Garcia M, Derocq D, Pujol P, Rochefort H. Overexpression of transfected cathepsin D in transformed cells increases their malignant phenotype and metastatic potency. Oncogene. 1990;5:1809–14. [PubMed] [Google Scholar]
- Garcia M, Platet N, Liaudet E, Laurent V, Derocq D, Brouillet JP, et al. Biological and clinical significance of cathepsin D in breast cancer metastasis. Stem Cells. 1996;14:642–50. doi: 10.1002/stem.140642. [DOI] [PubMed] [Google Scholar]
- Geminard C, Nault F, Johnstone RM, Vidal M. Characteristics of the interaction between Hsc70 and the transferrin receptor in exosomes released during reticulocyte maturation. J Biol Chem. 2001;276:9910–6. doi: 10.1074/jbc.M009641200. [DOI] [PubMed] [Google Scholar]
- Glondu M, Liaudet-Coopman E, Derocq D, Platet N, Rochefort H, Garcia M. Down-regulation of cathepsin-D expression by antisense gene transfer inhibits tumor growth and experimental lung metastasis of human breast cancer cells. Oncogene. 2002;21:5127–34. doi: 10.1038/sj.onc.1205657. [DOI] [PubMed] [Google Scholar]
- Goldfarb SB, Kashlan OB, Watkins JN, Suaud L, Yan W, Kleyman TR, et al. Differential effects of Hsc70 and Hsp70 on the intracellular trafficking and functional expression of epithelial sodium channels. Proc Natl Acad Sci U S A. 2006;103:5817–22. doi: 10.1073/pnas.0507903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower LE, Guidon PT., Jr Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- Kao RH, Francia G, Poulsom R, Hanby AM, Hart IR. Application of differential display, with in situ hybridization verification, to microscopic samples of breast cancer tissue. Int J Exp Pathol. 2003;84:207–12. doi: 10.1111/j.1365-2613.2003.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaris A, Chatzigianni EB, Panoussopoulos D, Tzimas GN, Davaris PS, Golematis B. Proliferating cell nuclear antigen and heat shock protein 70 immunolocalization in invasive ductal breast cancer not otherwise specified. Breast Cancer Res Treat. 1997;43:43–51. doi: 10.1023/a:1005706110275. [DOI] [PubMed] [Google Scholar]
- Liaudet E, Derocq D, Rochefort H, Garcia M. Transfected cathepsin D stimulates high density cancer cell growth by inactivating secreted growth inhibitors. Cell Growth Differ. 1995;6:1045–52. [PubMed] [Google Scholar]
- Malusecka E, Zborek A, Krzyzowska-Gruca S, Krawczyk Z. Immunohistochemical detection of the inducible heat shock protein hsp70: a methodological study. J Histochem Cytochem. 2006;54:183–90. doi: 10.1369/jhc.5A6748.2005. [DOI] [PubMed] [Google Scholar]
- Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–57. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- Mambula SS, Stevenson MA, Ogawa K, Calderwood SK. Mechanisms for Hsp70 secretion: Crossing membranes without a leader. Methods. 2007;43:168–75. doi: 10.1016/j.ymeth.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini N, Osta W, Bielawski J, Luberto C, Obeid LM, Hannun YA. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J Biol Chem. 2004;279:25101–11. doi: 10.1074/jbc.M313662200. [DOI] [PubMed] [Google Scholar]
- Melendez K, Wallen ES, Edwards BS, Mobarak CD, Bear DG, Moseley PL. Heat shock protein 70 and glycoprotein 96 are differentially expressed on the surface of malignant and nonmalignant breast cells. Cell Stress Chaperones. 2006;11:334–42. doi: 10.1379/CSC-187.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf P, Fusek M. Two crystal structures for cathepsin D: the lysosomal targeting signal and active site. Embo J. 1993;12:1293–302. doi: 10.1002/j.1460-2075.1993.tb05774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–96. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, et al. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61:272–9. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- Munro S, Pelham HR. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986;46:291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Nylandsted J, Wick W, Hirt UA, Brand K, Rohde M, Leist M, et al. Eradication of glioblastoma, and breast and colon carcinoma xenografts by Hsp70 depletion. Cancer Res. 2002;62:7139–42. [PubMed] [Google Scholar]
- O’Malley K, Mauron A, Barchas JD, Kedes L. Constitutively expressed rat mRNA encoding a 70-kilodalton heat-shock-like protein. Mol Cell Biol. 1985;5:3476–83. doi: 10.1128/mcb.5.12.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort H, Glondu M, Sahla ME, Platet N, Garcia M. How to target estrogen receptor-negative breast cancer? Endocr Relat Cancer. 2003;10:261–6. doi: 10.1677/erc.0.0100261. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Schmid SL. Enzymatic recycling of clathrin from coated vesicles. Cell. 1986;46:5–9. doi: 10.1016/0092-8674(86)90852-4. [DOI] [PubMed] [Google Scholar]
- Rohde M, Daugaard M, Jensen MH, Helin K, Nylandsted J, Jaattela M. Members of the heat-shock protein 70 family promote cancer cell growth by distinct mechanisms. Genes Dev. 2005;19:570–82. doi: 10.1101/gad.305405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE, Schmid SL. Enzymatic recycling of clathrin from coated vesicles. Cell. 1986;46:5–9. doi: 10.1016/0092-8674(86)90852-4. [DOI] [PubMed] [Google Scholar]
- Saito K, Dai Y, Ohtsuka K. Enhanced expression of heat shock proteins in gradually dying cells and their release from necrotically dead cells. Exp Cell Res. 2005;310:229–236. doi: 10.1016/j.yexcr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Sorger PK, Pelham HR. Cloning and expression of a gene encoding hsc73, the major hsp70-like protein in unstressed rat cells. Embo J. 1987;6:993–8. doi: 10.1002/j.1460-2075.1987.tb04850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulier S, Vilotte JL, L’Huillier PJ, Mercier JC. Developmental regulation of murine integrin beta 1 subunit- and Hsc73-encoding genes in mammary gland: sequence of a new mouse Hsc73 cDNA. Gene. 1996;172:285–9. doi: 10.1016/0378-1119(96)00169-2. [DOI] [PubMed] [Google Scholar]
- Srivastava PK. Immunotherapy for human cancer using heat shock protein-peptide complexes. Curr Oncol Rep. 2005;7:104–8. doi: 10.1007/s11912-005-0035-8. [DOI] [PubMed] [Google Scholar]
- Thanner F, Sutterlin MW, Kapp M, Rieger L, Kristen P, Dietl J, et al. Heat-shock protein 70 as a prognostic marker in node-negative breast cancer. Anticancer Res. 2003;23:1057–62. [PubMed] [Google Scholar]
- Terlecky SR, Dice JF. Polypeptide import and degradation by isolated lysosomes. J Biol Chem. 1993;268:23490–5. [PubMed] [Google Scholar]
- Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torronteguy C, Frasson A, Zerwes F, Winnikov E, da Silva VD, Menoret A, et al. Inducible heat shock protein 70 expression as a potential predictive marker of metastasis in breast tumors. Cell Stress Chaperones. 2006;11:34–43. doi: 10.1379/CSC-159R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara F, Yoshioka T, Muraki T. Molecular and functional characterization of HSC54, a novel variant of human heat-shock cognate protein 70. Mol Pharmacol. 2000;58:1257–63. doi: 10.1124/mol.58.6.1257. [DOI] [PubMed] [Google Scholar]
- Tsukahara F, Maru Y. Identification of novel nuclear export and nuclear localization-related signals in human heat shock cognate protein 70. J Biol Chem. 2004;279:8867–72. doi: 10.1074/jbc.M308848200. [DOI] [PubMed] [Google Scholar]
- Tutar Y, Song Y, Masison DC. Primate chaperones Hsc70 (constitutive) and Hsp70 (induced) differ functionally in supporting growth and prion propagation in Saccharomyces cerevisiae. Genetics. 2006;172:851–61. doi: 10.1534/genetics.105.048926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–6. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Roig LM, Fanelli MA, Lopez LA, Gago FE, Tello O, Aznar JC, et al. Heat shock proteins and cell proliferation in human breast cancer biopsy samples. Cancer Detect Prev. 1997;21:441–51. [PubMed] [Google Scholar]
- Vargas-Roig LM, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock protein expression and drug resistance in breast cancer patients treated with induction chemotherapy. Int J Cancer. 1998;79:468–75. doi: 10.1002/(sici)1097-0215(19981023)79:5<468::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Zou N, Ao L, Cleveland JC, Jr, Yang X, Su X, Cai GY, et al. Critical role of extracellular heat shock cognate protein 70 in the myocardial inflammatory response and cardiac dysfunction after global ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2008;294:H2805–2813. doi: 10.1152/ajpheart.00299.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


