Abstract
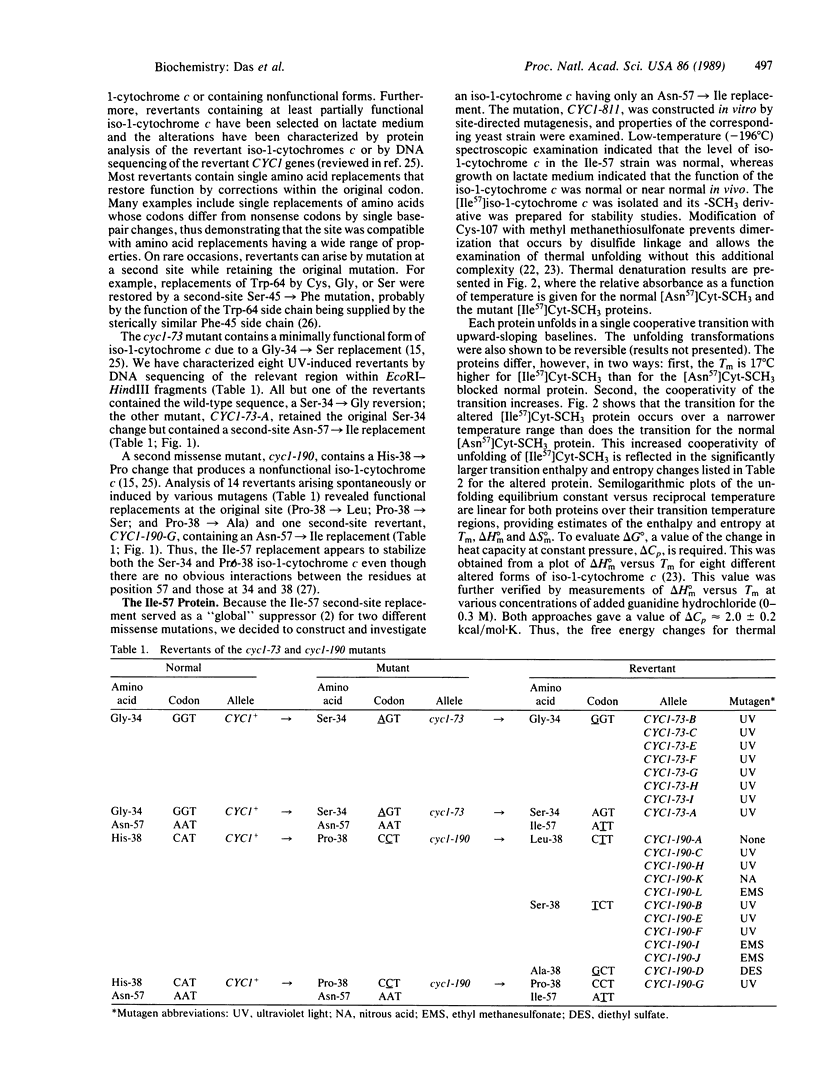
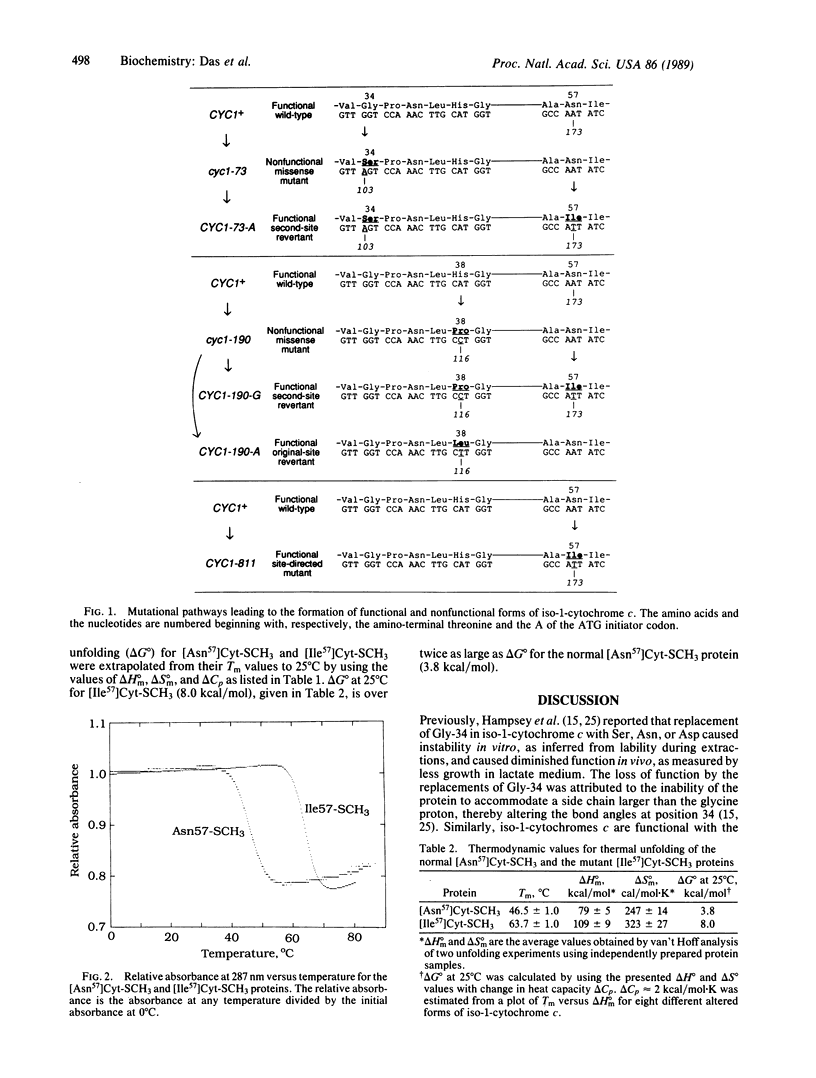
Two Saccharomyces cerevisiae yeast mutants, cyc1-73 and cyc1-190, contain nonfunctional and presumably unstable forms of iso-1-cytochrome c due to Gly-34----Ser and His-38----Pro replacements, respectively. Second-site reversions that produced Asn-57----Ile replacements at least partially restored function, presumably by alleviating the instability of these two altered iso-1-cytochromes c. Introduction of the Ile-57 replacement by site-directed mutagenesis in an otherwise normal protein resulted in a 17 degrees C increase in the transition temperature (Tm), corresponding to over a 2-fold increase in the free energy change (delta G degrees) for thermal unfolding.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T., Bell J. A., Sun D. P., Nicholson H., Wozniak J. A., Cook S., Matthews B. W. Replacements of Pro86 in phage T4 lysozyme extend an alpha-helix but do not alter protein stability. Science. 1988 Feb 5;239(4840):631–635. doi: 10.1126/science.3277275. [DOI] [PubMed] [Google Scholar]
- Alber T., Sun D. P., Wilson K., Wozniak J. A., Cook S. P., Matthews B. W. Contributions of hydrogen bonds of Thr 157 to the thermodynamic stability of phage T4 lysozyme. Nature. 1987 Nov 5;330(6143):41–46. doi: 10.1038/330041a0. [DOI] [PubMed] [Google Scholar]
- Alber T., Wozniak J. A. A genetic screen for mutations that increase the thermal stability of phage T4 lysozyme. Proc Natl Acad Sci U S A. 1985 Feb;82(3):747–750. doi: 10.1073/pnas.82.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan P. N., Rollence M. L., Pantoliano M. W., Wood J., Finzel B. C., Gilliland G. L., Howard A. J., Poulos T. L. Proteases of enhanced stability: characterization of a thermostable variant of subtilisin. Proteins. 1986 Dec;1(4):326–334. doi: 10.1002/prot.340010406. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Hampsey D. M., Das G., Sherman F. Amino acid replacements in yeast iso-1-cytochrome c. Comparison with the phylogenetic series and the tertiary structure of related cytochromes c. J Biol Chem. 1986 Mar 5;261(7):3259–3271. [PubMed] [Google Scholar]
- Hampsey D. M., Das G., Sherman F. Yeast iso-1-cytochrome c: genetic analysis of structural requirements. FEBS Lett. 1988 Apr 25;231(2):275–283. doi: 10.1016/0014-5793(88)80834-2. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Grutter M. G., Schellman J. Thermodynamic stability and point mutations of bacteriophage T4 lysozyme. J Mol Biol. 1984 May 15;175(2):195–212. doi: 10.1016/0022-2836(84)90474-1. [DOI] [PubMed] [Google Scholar]
- Hecht M. H., Sturtevant J. M., Sauer R. T. Effect of single amino acid replacements on the thermal stability of the NH2-terminal domain of phage lambda repressor. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5685–5689. doi: 10.1073/pnas.81.18.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht M. H., Sturtevant J. M., Sauer R. T. Stabilization of lambda repressor against thermal denaturation by site-directed Gly----Ala changes in alpha-helix 3. Proteins. 1986 Sep;1(1):43–46. doi: 10.1002/prot.340010108. [DOI] [PubMed] [Google Scholar]
- Hickey D. R., McLendon G., Sherman F. Thermodynamic stabilities of yeast iso-1-cytochromes c having amino acid substitutions for lysine 32. J Biol Chem. 1988 Dec 5;263(34):18298–18305. [PubMed] [Google Scholar]
- Holzschu D., Principio L., Conklin K. T., Hickey D. R., Short J., Rao R., McLendon G., Sherman F. Replacement of the invariant lysine 77 by arginine in yeast iso-1-cytochrome c results in enhanced and normal activities in vitro and in vivo. J Biol Chem. 1987 May 25;262(15):7125–7131. [PubMed] [Google Scholar]
- Imanaka T., Shibazaki M., Takagi M. A new way of enhancing the thermostability of proteases. Nature. 1986 Dec 18;324(6098):695–697. doi: 10.1038/324695a0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Louie G. V., Hutcheon W. L., Brayer G. D. Yeast iso-1-cytochrome c. A 2.8 A resolution three-dimensional structure determination. J Mol Biol. 1988 Jan 20;199(2):295–314. doi: 10.1016/0022-2836(88)90315-4. [DOI] [PubMed] [Google Scholar]
- Matthews B. W., Nicholson H., Becktel W. J. Enhanced protein thermostability from site-directed mutations that decrease the entropy of unfolding. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6663–6667. doi: 10.1073/pnas.84.19.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews C. R., Crisanti M. M., Gepner G. L., Velicelebi G., Sturtevant J. M. Effect of single amino acid substitutions on the thermal stability of the alpha subunit of tryptophan synthase. Biochemistry. 1980 Apr 1;19(7):1290–1293. doi: 10.1021/bi00548a004. [DOI] [PubMed] [Google Scholar]
- Mitchinson C., Baldwin R. L. The design and production of semisynthetic ribonucleases with increased thermostability by incorporation of S-peptide analogues with enhanced helical stability. Proteins. 1986 Sep;1(1):23–33. doi: 10.1002/prot.340010106. [DOI] [PubMed] [Google Scholar]
- Pantoliano M. W., Ladner R. C., Bryan P. N., Rollence M. L., Wood J. F., Poulos T. L. Protein engineering of subtilisin BPN': enhanced stabilization through the introduction of two cysteines to form a disulfide bond. Biochemistry. 1987 Apr 21;26(8):2077–2082. doi: 10.1021/bi00382a002. [DOI] [PubMed] [Google Scholar]
- Perry L. J., Wetzel R. Disulfide bond engineered into T4 lysozyme: stabilization of the protein toward thermal inactivation. Science. 1984 Nov 2;226(4674):555–557. doi: 10.1126/science.6387910. [DOI] [PubMed] [Google Scholar]
- Ramdas L., Sherman F., Nall B. T. Guanidine hydrochloride induced equilibrium unfolding of mutant forms of iso-1-cytochrome c with replacement of proline-71. Biochemistry. 1986 Nov 4;25(22):6952–6958. doi: 10.1021/bi00370a032. [DOI] [PubMed] [Google Scholar]
- Russel M., Kidd S., Kelley M. R. An improved filamentous helper phage for generating single-stranded plasmid DNA. Gene. 1986;45(3):333–338. doi: 10.1016/0378-1119(86)90032-6. [DOI] [PubMed] [Google Scholar]
- Sauer R. T., Hehir K., Stearman R. S., Weiss M. A., Jeitler-Nilsson A., Suchanek E. G., Pabo C. O. An engineered intersubunit disulfide enhances the stability and DNA binding of the N-terminal domain of lambda repressor. Biochemistry. 1986 Oct 7;25(20):5992–5998. doi: 10.1021/bi00368a024. [DOI] [PubMed] [Google Scholar]
- Schellman J. A. The thermodynamic stability of proteins. Annu Rev Biophys Biophys Chem. 1987;16:115–137. doi: 10.1146/annurev.bb.16.060187.000555. [DOI] [PubMed] [Google Scholar]
- Schweingruber M. E., Stewart J. W., Sherman F. Primary site and second site revertants of missense mutants of the evolutionarily invariant tryptophan 64 in iso-1-cytochrome c from yeast. J Biol Chem. 1979 May 25;254(10):4132–4143. [PubMed] [Google Scholar]
- Sherman F., Jackson M., Liebman S. W., Schweingruber A. M., Stewart J. W. A deletion map of cyc1 mutants and its correspondence to mutationally altered iso-1-cytochromes c of yeast. Genetics. 1975 Sep;81(1):51–73. doi: 10.1093/genetics/81.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F., Stewart J. W., Jackson M., Gilmore R. A., Parker J. H. Mutants of yeast defective in iso-1-cytochrome c. Genetics. 1974 Jun;77(2):255–284. doi: 10.1093/genetics/77.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Lin B. Genetic analysis of staphylococcal nuclease: identification of three intragenic "global" suppressors of nuclease-minus mutations. Genetics. 1985 Aug;110(4):539–555. doi: 10.1093/genetics/110.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Maggio E. T., Kenyon G. L. Simple alkanethiol groups for temporary blocking of sulfhydryl groups of enzymes. Biochemistry. 1975 Feb 25;14(4):766–771. doi: 10.1021/bi00675a019. [DOI] [PubMed] [Google Scholar]
- Wells J. A., Powers D. B. In vivo formation and stability of engineered disulfide bonds in subtilisin. J Biol Chem. 1986 May 15;261(14):6564–6570. [PubMed] [Google Scholar]



