Abstract
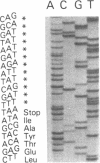
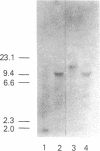
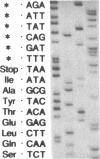
Two B apolipoproteins (apo) are present in human plasma, designated apoB-100 and apoB-48, and represent translational products from mature apoB mRNAs that differ by a single base. Either the glutamine codon encoded by the single-copy apoB gene at nucleotide 6666 is transcribed and translated to produce apoB-100 or an RNA-editing mechanism substitutes a uracil for cytosine, altering this glutamine codon (CAA) to a stop codon (UAA), prematurely terminating translation to produce apoB-48. In the present report, editing of rat apoB transcripts was evaluated by amplification of RNA with the polymerase chain reaction by use of primers based on the apoB cDNA cloned from a rat liver cDNA library. The combined results of this study show that (i) a single copy of the apoB gene exists in the rat; (ii) the rat apoB gene encodes only the glutamine codon for the synthesis of apoB of higher molecular weight (apoBH); (iii) rat apoB transcripts undergo RNA editing; (iv) apoBH and apoB of lower molecular weight (apoBL) in the rat represent structural equivalents of apoB-100 and apoB-48 in humans, respectively; (v) RNA editing occurs in both the liver and intestine of the rat; (vi) rat hepatic apoB RNA is more extensively edited than is human hepatic apoB RNA, which is consistent with the marked increase in apoBL secretion by the rat liver when compared with human; and (vii) the definitive identification of apoBH mRNA as well as apoBL mRNA in the rat intestine provides a mechanism for the biosynthesis of both apoBH and apoBL by the rat intestine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackhart B. D., Ludwig E. M., Pierotti V. R., Caiati L., Onasch M. A., Wallis S. C., Powell L., Pease R., Knott T. J., Chu M. L. Structure of the human apolipoprotein B gene. J Biol Chem. 1986 Nov 25;261(33):15364–15367. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Habib G., Yang C. Y., Gu Z. W., Lee B. R., Weng S. A., Silberman S. R., Cai S. J., Deslypere J. P., Rosseneu M. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987 Oct 16;238(4825):363–366. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Haynes E. B., Sand T. M., Davis R. A. Developmental coordinate expression of triacylglycerol and small molecular weight apoB synthesis and secretion by rat hepatocytes. J Lipid Res. 1988 Jan;29(1):33–42. [PubMed] [Google Scholar]
- Demmer L. A., Levin M. S., Elovson J., Reuben M. A., Lusis A. J., Gordon J. I. Tissue-specific expression and developmental regulation of the rat apolipoprotein B gene. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8102–8106. doi: 10.1073/pnas.83.21.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Higuchi K., Hospattankar A. V., Law S. W., Meglin N., Cortright J., Brewer H. B., Jr Human apolipoprotein B (apoB) mRNA: identification of two distinct apoB mRNAs, an mRNA with the apoB-100 sequence and an apoB mRNA containing a premature in-frame translational stop codon, in both liver and intestine. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1772–1776. doi: 10.1073/pnas.85.6.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K., Monge J. C., Lee N., Law S. W., Brewer H. B., Jr, Sakaguchi A. Y., Naylor S. L. The human apoB-100 gene: apoB-100 is encoded by a single copy gene in the human genome. Biochem Biophys Res Commun. 1987 May 14;144(3):1332–1339. doi: 10.1016/0006-291x(87)91456-2. [DOI] [PubMed] [Google Scholar]
- Hospattankar A. V., Higuchi K., Law S. W., Meglin N., Brewer H. B., Jr Identification of a novel in-frame translational stop codon in human intestine apoB mRNA. Biochem Biophys Res Commun. 1987 Oct 14;148(1):279–285. doi: 10.1016/0006-291x(87)91107-7. [DOI] [PubMed] [Google Scholar]
- Kane J. P. Apolipoprotein B: structural and metabolic heterogeneity. Annu Rev Physiol. 1983;45:637–650. doi: 10.1146/annurev.ph.45.030183.003225. [DOI] [PubMed] [Google Scholar]
- Lee D. M., Koren E., Singh S., Mok T. Presence of B-100 in rat mesenteric chyle. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1149–1156. doi: 10.1016/s0006-291x(84)80253-3. [DOI] [PubMed] [Google Scholar]
- Lusis A. J., West R., Mehrabian M., Reuben M. A., LeBoeuf R. C., Kaptein J. S., Johnson D. F., Schumaker V. N., Yuhasz M. P., Schotz M. C. Cloning and expression of apolipoprotein B, the major protein of low and very low density lipoproteins. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4597–4601. doi: 10.1073/pnas.82.14.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel Y. L., Innerarity T. L., Spilman C., Mahley R. W., Protter A. A., Milne R. W. Mapping of human apolipoprotein B antigenic determinants. Arteriosclerosis. 1987 Mar-Apr;7(2):166–175. doi: 10.1161/01.atv.7.2.166. [DOI] [PubMed] [Google Scholar]
- Powell L. M., Wallis S. C., Pease R. J., Edwards Y. H., Knott T. J., Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987 Sep 11;50(6):831–840. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks C. E., Marsh J. B. Metabolic heterogeneity of apolipoprotein B in the rat. J Lipid Res. 1981 Mar;22(3):519–527. [PubMed] [Google Scholar]
- Tsao S. G., Brunk C. F., Pearlman R. E. Hybridization of nucleic acids directly in agarose gels. Anal Biochem. 1983 Jun;131(2):365–372. doi: 10.1016/0003-2697(83)90185-9. [DOI] [PubMed] [Google Scholar]
- Van't Hooft F. M., Hardman D. A., Kane J. P., Havel R. J. Apolipoprotein B (B-48) of rat chylomicrons is not a precursor of the apolipoprotein of low density lipoproteins. Proc Natl Acad Sci U S A. 1982 Jan;79(1):179–182. doi: 10.1073/pnas.79.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]