Abstract
Purpose:
Ventilator-associated pneumonia (VAP) is a nosocomial infection resulting in significant morbidity and mortality. Pseudomonas aeruginosa (P. aeruginosa) and Staphylococcus aureus (S. aureus) are pathogens associated with VAP. Silver (Ag) coating of endotracheal tubes (ETTs) reduces bacterial colonization, however titanium dioxide (TiO2) coating has not been studied.
Methods:
Five types of ETT coatings were applied over silica layer: Ag, solgel TiO2, solgel TiO2 with Ag, Degussa P25 TiO2 (Degussa TiO2), and Degussa TiO2 with Ag. After ETTs were incubated with P. aeruginosa or S. aureus; colonization was determined quantitatively.
Results:
Pseudomonas aeruginosa and S. aureus grew for 5 days on standard ETTs. Compared to standard ETTs, P. aeruginosa growth was significantly inhibited by solgel TiO2 with Ag at 24 hours, and by Degussa TiO2 with Ag at 24 and 48 hours after inoculation. No significant difference in S. aureus growth was observed between the control and any of the five coatings for 5 days.
Conclusion:
In vitro, solgel TiO2 with Ag and Degussa TiO2 with Ag both attenuated P. aeruginosa growth, but demonstrated no effect on S. aureus colonization. Further studies using alternative coating and incorporating UV light exposure are needed to identify their potential utility in reducing VAP.
Keywords: ventilator-associated pneumonia, Degussa titanium dioxide, solgel titanium dioxide, quantitative culture
Introduction
Ventilator-associated pneumonia (VAP) is one of the most common nosocomial infections among intensive care unit patients, which prolongs ventilation and hospitalization, resulting in significant morbidity, mortality and medical costs.1–3 Researchers have developed strategies to prevent VAP, including controlling gastric pH, intratracheal intubation with a high-pressure endotracheal tube (ETT) cuff, continuous subglottic suction to prevent microaspiration, promoting oral hygiene with chlorhexidine, and semi-recumbent positioning.4–9
One strategy to reduce VAP is to treat the ETT with an antiseptic agent to control ETT biofilm formation.10,11 A biofilm is an aggregate of a bacterial community attached to a solid surface and encased in an exopolysaccharide matrix, making it resistant to antibiotic penetration.12 Biofilm formation is most prominent in the distal third of the ETT and can occur as early as sixty hours following endotracheal intubation.13
Due to silver’s antimicrobial properties, Pacheco-Fowler and colleagues studied chlorhexidine and Ag carbonate impregnated ETTs in an in vitro model and observed that bacterial growth of S. aureus, P. aeruginosa, and other organisms was attenuated.14 Similarly, Rello et al found that bacterial colonization was delayed for seven days in adults intubated with Ag-coated ETTs compared with standard ETTs,15 and Kollef and colleagues concluded a statistically significant reduction in the VAP incidence in a randomized multicenter trial.16
Titanium dioxide is widely used as an antimicrobial agent, but it is not commonly employed in the medical setting. In a process known as photocatalysis, TiO2 is activated into potent oxidative species when exposed to UV light, with antiviral, antibacterial as well as fungicidal actions.17 Recent, investigations have suggested that the photocatalytic activity is important in destroying a range of bacteria including S. aureus and P. aeruginosa in aqueous solutions.18 Photocatalytic activity also is implicated in the inhibition of biofilm formation on TiO2 based dental implants.19,20 In view of these antimicrobial actions it is plausible that TiO2-coating of ETTs, potentially in combination with UV light, may sterilize ETTs in patients’ airways, thereby reducing the risk of VAP.
We conducted a pilot study of bacterial growth behavior on TiO2-coated ETTs without UV exposure to evaluate the antimicrobial effect of TiO2 on polyvinyl chloride (PVC) which is used to manufacture ETTs. Initial experiments examined TiO2 without UV light exposure for two reasons. First, the Ag and TiO2 combination has not been studied on PVC material. Second, if the Ag and TiO2 coating has antimicrobial activity without photocatalysis, this would reduce potential toxicity from UV exposure. The objective of this study was to determine if TiO2-coated ETTs with or without Ag would reduce bacterial colonization by two common VAP pathogens (ie, P. aeruginosa or S. aureus) in an in vitro model compared with standard ETTs.
Material and methods
Solgel TiO2 synthesis
Sols of TiO2 were made using a method adapted from Mills and colleagues.21 A solution of 10 mL titanium isopropoxide (AC19470 98+%; Acros Organics, Geel, Belgium) in 2.32 g glacial acetic acid (A465-250; Fisher Chemical, Pittsburgh, PA), was stirred into 60 mL deionized water acidified with 0.59 g concentrated nitric acid (S719721; Fisher Chemical). The reaction solution was then heated rapidly and held at 80°C for 8 hours. The solution was concentrated by heating at 150°C to 11 wt% TiO2 followed by dilution with ethanol (AC61510; Acros Organics) to obtain a translucent white sol containing 6 wt% TiO2 sol.
Endotracheal tubes
The inner lumens of the ETTs were first coated with silica (SiO2) which served as a passivating layer to prevent the TiO2 from degrading the polyvinyl chloride (PVC) and to improve surface wettability by the subsequently applied TiO2 coatings. The SiO2 layer was applied using a nebulizer spray of 15 wt% colloidal SiO2 in deionized water through the ETT, through a custom-designed nozzle, for 1 minute with an air flow rate of 10 liters per minute. The ETT was then dried with 85°C air for 1 minute.
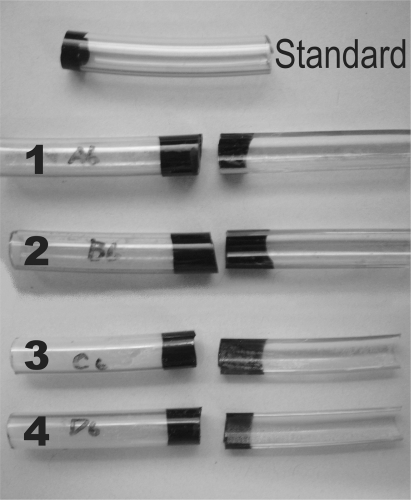
The respective TiO2 and Ag treatments are summarized in Table 1. Five types of coatings were applied over the SiO2 layer (Figure 1): 1) solgel TiO2, 2) solgel TiO2 with Ag, 3) Degussa TiO2 (Evonik Degussa, Parsippany, NJ, USA), 4) Degussa TiO2 with Ag, and 5) Ag (not shown in Figure 1). Each of the coating solutions A–D had 0.19 wt% TiO2 on a dry weight basis. Water and ethanol were used as solvents in variable ratios for each solution. Solgel TiO2 was used for solutions A and B, while C and D solutions consisted of solgel and Degussa TiO2 in the ratio 20:80 wt/wt. Silver nitrate (AgNO3) was added to solutions B and D. For solution E, AgNO3 0.1 wt% was mixed in ethanol-water (50:50 wt/wt). Two types of TiO2 coatings were applied; solgel to increase adherence to PVC for substrate depositition and Degussa TiO2 for potentially high photocatalytic performance. Since Degussa TiO2 suspensions had very poor adhesion to the ETTs, they were mixed with a small amount of sols of solgel TiO2. After TiO2 coating, the ETTs were dried with 85°C air for 1 minute, followed by further drying in an air-circulating oven at 100°C for 15 minutes, then packed while warm.
Table 1.
Coating solutions with their respective titanium dioxide and silver treatments
| Tube | Solution | TiO2 source | Solvent | AgNO3: TiO2 (mol%) |
|---|---|---|---|---|
| 1 | A | Solgel | Water: Ethanol (60:40) | 0 |
| 2 | B | Solgel | Water: Ethanol (60:40) | 5.6 |
| 3 | C | Solgel: Degussa (20:80) | Water: Ethanol (3:97) | 0 |
| 4 | D | Solgel: Degussa (20:80) | Water: Ethanol (5:95) | 5.6 |
| 5 | E | No TiO2 | Water: Ethanol (50:50) | 0.1wt% AgNO3 |
Abbreviations: Ag, silver; AgNO3, silver nitrate; Degussa TiO2, Degussa P25 titanium dioxide; TiO2, titanium dioxide.
Figure 1.
Standard endotracheal tubes were coated with a passivating layer of colloidal silica and served as controls. Five types of coatings were applied over a silica layer; 1. solgel titanium dioxide without silver, 2. solgel titanium dioxide with silver, 3. Degussa titanium dioxide without silver, 4. Degussa titanium dioxide with silver and 5. silver only (not shown).
Preparation of P. aeruginosa
Freeze-dried P. aeruginosa (ATCC #25668; American Type Culture Collection, Manassas, VA, USA) was rehydrated with nutrient broth in aliquots to be stored at −80°C. After a loopful of frozen bacteria was plated and incubated, a single colony was inoculated into 4 mL tryptic soy broth (TSB) and incubated overnight at 37°C. The following day, the turbid bacterial broth was vortexed and inoculated. To determine the exact inoculums, 100μL of bacterial broth was serially diluted with phosphate-buffered saline (PBS), then optical density was measured to obtain 108 colony forming units/milliliter (CFU/mL).
Preparation of S. aureus
Frozen S. aureus (ATCC #25923) was rehydrated with TSB in the same fashion as P. aeruginosa, and stored at −80°C. Frozen S. aureus was inoculated into 50 mL TSB in a flask, and incubated overnight at 37°C. Turbid S. aureus bacterial broth was transferred to a 50 mL sterile culture tube, centrifuged for 15 minutes, and the supernatant was discarded. A concentrated broth was made by the addition of 1 mL of TSB to the pellet of S. aureus. Optical density was measured to obtain 109 CFU/mL for ETT inoculation.
Bacterial inoculation of ETTs
Each ETT was marked at 4 cm intervals for later division into six equal pieces, then immersed in 75% alcohol for 15 minutes to sterilize, rinsed with 20 mL 0.9% sterile saline, and air dried. After sterilization, each ETT was cut into six pieces, sealed on one end with Tegaderm® (3M, St. Paul, MN), filled with either 8 μl of P. aeruginosa broth plus 800 μl TSB or 100 μl S. aureus broth plus 700 μl TSB, then sealed with Tegaderm. All ETT pieces were placed in a 2 liter dry flask and agitated at 37°C overnight. On the following day, the bacterial fluid was drained from each ETT piece.
Peroxidase assay after coating ETTs
Colorimetric measurement was used to detect whether TiO2 on ETTs generated peroxidase as part its antimicrobial property. EM Quant Peroxide Test Strips (EMD Chemicals Inc. Gibbstown, NJ, USA) which measure peroxidase between 1–100 parts-per million were used. Trypic soy broth 800 μL was placed on the TiO2-coated ETT for 24 hours at 37°C in the dark. Following incubation, the colorimetric strip was dipped into the TSB and the value of peroxidase production was determined.
Observation of bacterial growth
Bacterial inoculated ETT pieces were placed in an incubator to simulate the in vitro human trachea with no ambient light, a relative humidity of >90%, temperature of 37°C and at standard atmospheric pressure. Bacterial growth was observed for 24, 48, 72, 96, 120, and 144 hours after inoculation on the standard and five test ETTs. The inner surface of bacteria was collected by washing with 0.5 mL of sterile PBS and swabbing every 24 hours after inoculation. The collected fluid was serially diluted and plated on tryptic soy agar for incubation overnight at 37°C. Colony counts were expressed as CFU/mL, and each experiment was repeated four times.
Statistical analysis
Data were reported as mean ± standard error of mean (SEM). Bacterial colony counts were analyzed using Mann–Whitney U test comparing coated ETTs with standard ETTs. A P-value of < 0.05 was considered statistically significant.
Results
The coating was slightly opaque using solgel TiO2 compared with Degussa TiO2. Samples with Ag-containing coatings appeared dark due to the reduction of AgNO3 to Ag. The coating was particulate in appearance to the naked eye and peeled off while collecting the bacteria by mechanical force. Peroxidase production was not detected on TiO2-coated ETTs.
Pseudomonas aeruginosa
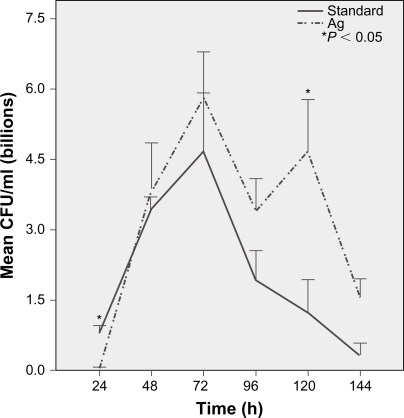
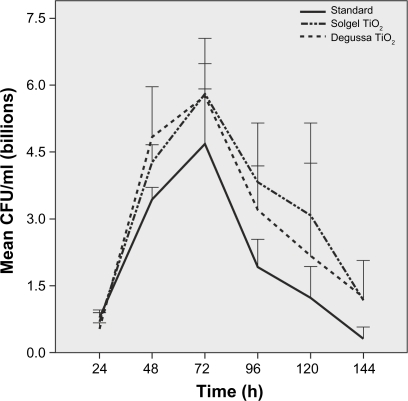
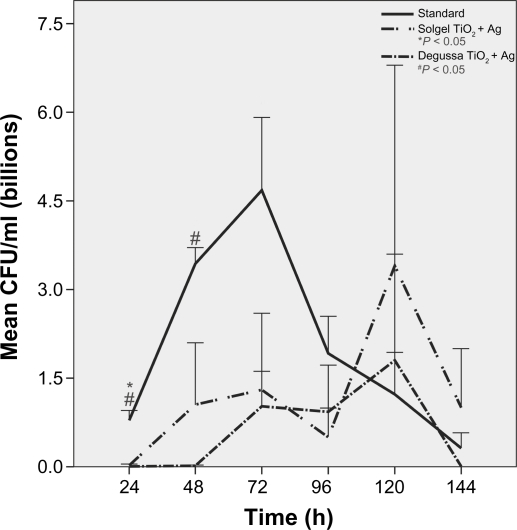
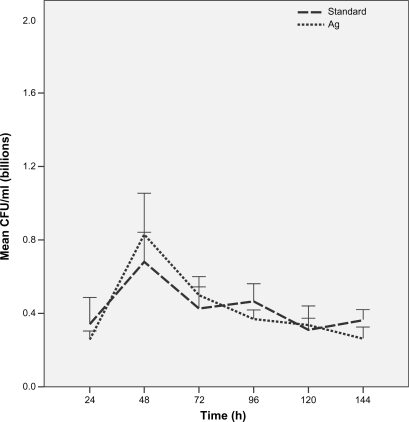
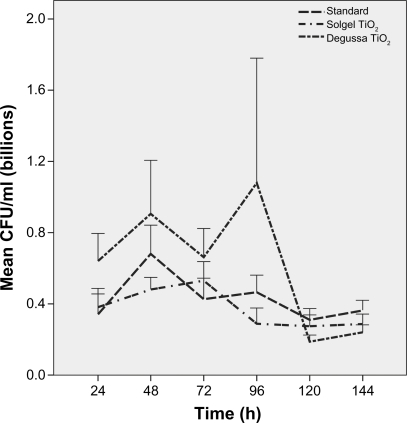
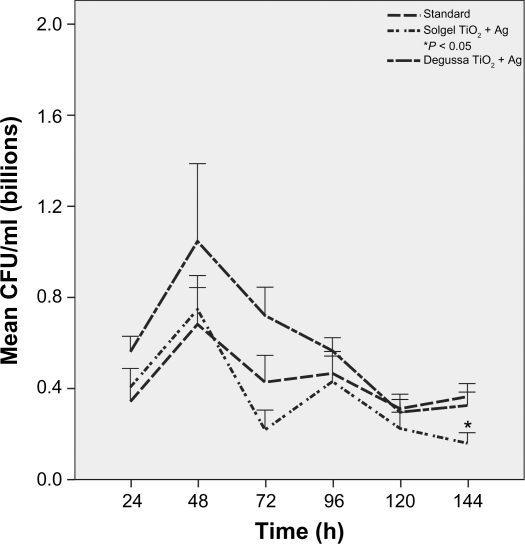
Growth peaked at 72 hours and continued for 144 hours on standard ETTs. Pseudomonas aeruginosa growth was more robust on Ag-coated ETTs compared to standard ETTs at 120 hours (P = 0.04) and 144 hours (P = 0.06) despite initial slow growth in the first 24 hours (Figure 2). There was no difference in P. aeruginosa growth over 144 hours among standard, solgel TiO2 and Degussa TiO2-coated ETTs (Figure 3). Compared to standard ETTs, solgel TiO2 with Ag inhibited P. aeruginosa growth at 24 hours (P = 0.02), and Degussa TiO2 with Ag inhibited P. aeruginosa growth at 24 and 48 hours after inoculation (P = 0.02 and P = 0.02, respectively) (Figure 4).
Figure 2.
Characterization of P. aeruginosa growth on polyvinyl chloride endotracheal tubes over a 144-hour period. Comparison of standard and silver only coated endotracheal tubes. There was less P. aeruginosa growth on silver-coated endotracheal tubes at 24 hours (P < 0.01), but more growth at 120 hours (P = 0.04) and 144 hours (P = 0.06) compared to standard endotracheal tubes.
Notes: Mean ± standard error of mean, *P < 0.05 compared to standard endotracheal tube. N = 4.
Abbreviation: CFU, colony-forming units.
Figure 3.
Characterization of P. aeruginosa growth on polyvinyl chloride endotracheal tubes coated with titanium dioxide over 144 hour period. Comparison of standard, solgel titanium dioxide and Degussa titanium dioxide endotracheal tubes. P. aeruginosa growth was not statistically significant at all time points.
Notes: Mean ± standard error of mean, N = 4.
Abbreviation: CFU, colony-forming units.
Figure 4.
Characterization of P. aeruginosa growth on polyvinyl chloride endotracheal tubes coated with titanium dioxide with silver over a 144-hour period. Comparison of standard, solgel titanium dioxide with silver, and Degussa titanium dioxide with silver endotracheal tubes. P. aeruginosa growth was inhibited significantly by solgel titanium dioxide with silver at 24 hours and by Degussa titanium dioxide with silver at 24 and 48 hours, respectively.
Notes: Mean ± standard error of mean; *P < 0.05 compared to standard endotracheal tube, # P < 0.05 compared to standard endotracheal tube. N = 4.
Abbreviation: CFU, colony-forming units.
Staphylococcus aureus
Growth was observed up to 144 hours. Staphylococcus aureus growth peaked at 48 hours after inoculation on both standard and Ag-coated ETTs without a difference over 144 hours (Figure 5). Furthermore, there was also no difference in S. aureus growth over 144 hours between standard, solgel TiO2 and Degussa TiO2-coated ETTs (Figure 6). Compared to standard ETTs, neither solgel TiO2 with Ag nor Degussa TiO2 with Ag showed a difference in S. aureus growth over a 144 hour period except solgel TiO2 with Ag at the 144 hour point after inoculation in which there was a significant attenuation of S. aureus growth compared to the standard ETT (Figure 7).
Figure 5.
Characterization of S. aureus growth on polyvinyl chloride endotracheal tubes coated with silver only over a 144-hour period. Comparison of standard and silver only coated endotracheal tubes. There was no difference in S. aureus growth between standard and silver coated endotracheal tubes at any time point over 144 hours compared with standard endotracheal tubes.
Note: N = 4.
Abbreviation: CFU, colony-forming units.
Figure 6.
Characterization of S. aureus growth on polyvinyl chloride endotracheal tubes coated with titanium dioxide only over a 144-hour period. Comparison of standard, solgel titanium dioxide and Degussa titanium dioxide endotracheal tubes. There was no difference in S. aureus growth in the titanium dioxide-coated groups compared to standard endotracheal tubes at any time point.
Note: N = 4.
Abbreviation: CFU, colony-forming units.
Figure 7.
Characterization of S. aureus growth on polyvinyl chloride endotracheal tubes coated with titanium dioxide with silver over a 144-hour period. Comparison of standard, solgel titanium dioxide with silver and Degussa titanium dioxide with silver endotracheal tubes. There was no difference in S. aureus growth in the titanium dioxide with silver-coated groups compared to standard endotracheal tubes except at 144 hours with solgel titanium dioxide with silver endotracheal tubes.
Note: N = 4.
Abbreviation: CFU, colony-forming units.
Discussion
Clinically, VAP is diagnosed after patients have been mechanically ventilated for more than 48 hours similar to the duration of bacterial growth in our simulated model. The growth of P. aeruginosa was consistent with the findings of other investigators under comparable conditions when monitoring growth in the first 24 hours.22,23 An initial suppression of P. aeruginosa in Ag-coated ETT was also observed in sections of intraventricular tubing impregnated with Ag.23 Moreover, our experience was similar to results of others who observed an undulating pattern of bacterial growth over a 50 hour period in Ag-coated ETTs. 24 The second P. aeruginosa growth peak observed in Figure 2 was thought to be an experimental variation due to the small sample size.
A similar growth pattern on Ag-coated ETT was also observed in a study evaluating the growth of P. aeruginosa and S. aureus on PVC even in the presence of antimicrobials. Both P. aeruginosa and S. aureus produce a biofilm; its robustness is strain dependent.25–27 Biofilm generating species of S. aureus exhibit accelerating growth kinetics in the first 6 days in animal models. Furthermore, these strains form microcolonies on catheter substrates that serve as sites of biofilm formation.28 Thus, it is likely that S. aureus growth was probably blunted in our model until a glycocalyx developed, then growth proceeded in the presence of nutrients and favorable conditions.
The antimicrobial properties of Ag result from Ag ions binding to bacterial sulphydryl- or histidyl-containing proteins, which disrupt transmembranous energy metabolism and electrolyte transport systems.29 Because the cell wall composition and thickness vary between gram positive and gram negative bacteria, silver’s antimicrobial effect may be greater in P. aerugionsa than in S. aureus during the initial phase of bacterial multiplication.
The characteristics of discolored Ag-coated particles were not analyzed in this experiment. However, AgNO3 is known to undergo photochemical reduction to metallic Ag upon exposure to light as described by the reaction:
This leads to the tiny particles precipitation of Ag that appears black or brown. Future studies are needed to define the specific mechanism involved in the antimicrobial properties of Ag as well as the physiological impact of discolored Ag. Silver nitrate was not expected to react with SiO2 under ambient conditions, since SiO2 is quite nonreactive.
Application and treatment of ETTs with these chemicals may also change the surface charge and hydrophobicity, as in this study, thus changing the PVC characteristics from hydrophobic to hydrophilic.
This study demonstrated that the addition of TiO2 significantly augmented the antibacterial effects of Ag on P. aeruginosa during the first 72 hours, but had no effect on S. aureus. In our limited study, TiO2 photocatalysis was not observed as determined by the release of hydrogen peroxide as a part of reactive oxygen species. Silver and TiO2 might have reacted with each other releasing Ag ion, and this reactant might have a synergistic antimicrobial effect even in a dark environment without photocatalysis.
In contrast, Yao showed that application of thin films of Ag and TiO2 for sterilization purposes on silicone catheters promptly eliminates S. aureus within 90 minutes without TiO2-photocatalysis. They also showed inhibition of growth of Escherichia coli (E. coli) and P. aeruginosa, although they only observed growth for 24 hours.30 Li and colleagues demonstrated that AgNO3 and TiO2 pulverized to surgical masks reduced E. coli and S. aureus by 100%. In Li’s study, Ag plus TiO2 pulverized masks were exposed to UV-C irradiation prior to bacterial inoculation.31 Pseudomonas aeruginosa behaves very differently in the initial phase of bacterial attachment to the surface compared to S. aureus. Rogers and colleagues demonstrated that S. aureus multiplies and produces a biofilm on the surface that thickens monotonically, becoming hard during growth and softening during starvation. In comparison, P. aeruginosa showed much less reproducible behavior; the organism was loosely adhered and detached from the surface after several hours in vitro.32 Until P. aerugionosa attachment is established, this initial motile motion phase may be inhibited or delayed by synergistic bactericidal activity of Ag and either form of TiO2 as we demonstrated.
Our study has several limitations. First, it was conducted in an in vitro model that was designed to replicate the human tracheal environment. However, the results may not be applicable to the in vivo setting where other factors may impact the ETT surface such as frequent suctioning, nebulizer therapy or host factors. Second, the concomitant use of Ag and TiO2 would seem to be marginally effective against VAP if it does not inhibit a common cause of VAP (ie, S. aureus). Third, minimizing the particulate surface that facilitates bacterial binding could be effective by reducing the surface area for bacterial adherence and growth. Fourth, the coating, peeled off under mechanical stress, could be dislodged by a suction catheter and produce potentially harmful Ag particles in the human airway and lung parenchyma. Nevertheless, titanium dioxide is known to be safe in humans and is used in such products as cosmetics, toothpaste, and sun screen. Hence, the combination of Ag and TiO2 needs to be evaluated in a clinical setting. Lastly, this study did not directly evaluate the attractive feature of TiO2 photocatalysis. The potency of TiO2 either individually or in combination with Ag needs to be examined in conjunction with photocatalysis. However, exposing tracheal mucosa to UV light involves potential toxicity. This potential risk and, more importantly, its clinical feasibility, need to be ascertained in future studies.
Conclusion
One strategy to reduce the incidence of VAP is to pretreat ETT with an antiseptic agent to reduce or delay bacterial biofilm formation. The use of TiO2 with Ag-coated ETTs effectively inhibited P. aeruginosa growth, but not S. aureus growth, in an in vitro model of a human trachea environment.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose in this work.
References
- 1.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 2.Elward AM, Warren DK, Fraser VJ. Ventilator-associated pneumonia in pediatric intensive care unit patients: risk factors and outcomes. Pediatrics. 2002;109:758–764. doi: 10.1542/peds.109.5.758. [DOI] [PubMed] [Google Scholar]
- 3.Warren DK, Shukla SJ, Olsen MA, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003;31:1312–1317. doi: 10.1097/01.CCM.0000063087.93157.06. [DOI] [PubMed] [Google Scholar]
- 4.Cook D, Reeve B, Guyatt G, et al. Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analyses. JAMA. 1996;275:308–314. [PubMed] [Google Scholar]
- 5.Crnich C, Safdar N, Maki D. The role of the intensive care unit environment in the pathogenesis and prevention of ventilator-associated pneumonia. Respir Care. 2005;50:813–836. discussion, 836–818. [PubMed] [Google Scholar]
- 6.Rello J, Sonora R, Jubert P, et al. Pneumonia in intubated patients: role of respiratory airway care. Am J Respir Crit Care Med. 1996;154:111–115. doi: 10.1164/ajrccm.154.1.8680665. [DOI] [PubMed] [Google Scholar]
- 7.Gujadhur R, Helme BW, Sanni A, et al. Continuous subglottic suction is effective for prevention of ventilator associated pneumonia. Interact Cardiovasc Thorac Surg. 2005;4:110–115. doi: 10.1510/icvts.2004.100149. [DOI] [PubMed] [Google Scholar]
- 8.DeRiso AJ, 2nd, Ladowski JS, Dillon TA, et al. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556–1561. doi: 10.1378/chest.109.6.1556. [DOI] [PubMed] [Google Scholar]
- 9.Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851–1858. doi: 10.1016/S0140-6736(98)12251-1. [DOI] [PubMed] [Google Scholar]
- 10.Adair CG, Gorman SP, Byers LM, et al. Eradication of endotracheal tube biofilm by nebulised gentamicin. Intensive Care Med. 2002;28:426–431. doi: 10.1007/s00134-002-1223-8. [DOI] [PubMed] [Google Scholar]
- 11.Safdar N, Crnich C, Maki D. The pathogenesis of ventilator-associated pneumonia: its relevance to developing effective strategies for prevention. Respir Care. 2005;50:725–739. discussion, 739–741. [PubMed] [Google Scholar]
- 12.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 13.Feldman C, Kassel M, Cantrell J, et al. The presence and sequence of endotracheal tube colonization in patients undergoing mechanical ventilation. Eur Respir J. 1999;13:546–551. doi: 10.1183/09031936.99.13354699. [DOI] [PubMed] [Google Scholar]
- 14.Pacheco-Fowler V, Gaonkar T, Wyer PC, et al. Antiseptic impregnated endotracheal tubes for the prevention of bacterial colonization. J Hosp Infect. 2004;57:170–174. doi: 10.1016/j.jhin.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Rello J, Kollef M, Diaz E, et al. Reduced burden of bacterial airway colonization with a novel silver-coated endotracheal tube in a randomized multiple-center feasibility study. Crit Care Med. 2006;34:2766–2772. doi: 10.1097/01.CCM.0000242154.49632.B0. [DOI] [PubMed] [Google Scholar]
- 16.Kollef MH, Afessa B, Anzueto A, et al. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA. 2008;300:805–813. doi: 10.1001/jama.300.7.805. [DOI] [PubMed] [Google Scholar]
- 17.Fujishima A. TiO2 Photocatalysis: Fundamentals and applications. Tokyo, Japan: BKC, Inc; 1999. [Google Scholar]
- 18.Tsuang YH, Sun JS, Huang YC, et al. Studies of photokilling of bacteria using titanium dioxide nanoparticles. Artif Organs. 2008;32:167–174. doi: 10.1111/j.1525-1594.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 19.Arai T, Ueda T, Sugiyama T, et al. Inhibiting microbial adhesion to denture base acrylic resin by titanium dioxide coating. J Oral Rehabil. 2009;36:902–908. doi: 10.1111/j.1365-2842.2009.02012.x. [DOI] [PubMed] [Google Scholar]
- 20.Veltri M, Ferrari M, Balleri P. Stability values of titanium dioxide-blasted dental implants in edentulous maxillas: a 3-year pilot study. J Oral Rehabil. 2009. Nov 4, [Epub ahead of print] [DOI] [PubMed]
- 21.Mills A, Elliott N, Hill G, et al. Preparation and characterisation of novel thick sol-gel titania film photocatalysts. Photochem Photobiol Sci. 2003;2:591–596. doi: 10.1039/b212865a. [DOI] [PubMed] [Google Scholar]
- 22.Ramstedt M, Houriet R, Mossialos D, et al. Wet chemical silver treatment of endotracheal tubes to produce antibacterial surfaces. J Biomed Mater Res B Appl Biomater. 2007;83:169–180. doi: 10.1002/jbm.b.30781. [DOI] [PubMed] [Google Scholar]
- 23.Secer HI, Kural C, Kaplan M, et al. Comparison of the efficacies of antibiotic-impregnated and silver-impregnated ventricular catheters on the prevention of infections. An in vitro laboratory study. Pediatr Neurosurg. 2008;44:444–447. doi: 10.1159/000172966. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann M, Guttmann J, Muller B, et al. Reduction of the bacterial load by the silver-coated endotracheal tube (SCET), a laboratory investigation. Technol Health Care. 1999;7:359–370. [PubMed] [Google Scholar]
- 25.Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008;322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corrigan RM, Rigby D, Handley P, et al. The role of Staphylococcus aureus surface protein SasG in adherence and biofilm formation. Microbiology. 2007;153:2435–2446. doi: 10.1099/mic.0.2007/006676-0. [DOI] [PubMed] [Google Scholar]
- 27.Gotz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43:1367–1378. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 28.Fluckiger U, Ulrich M, Steinhuber A, et al. Biofilm formation, icaADBC transcription, and polysaccharide intercellular adhesin synthesis by staphylococci in a device-related infection model. Infect Immun. 2005;73:1811–1819. doi: 10.1128/IAI.73.3.1811-1819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guggenbichler JP, Boswald M, Lugauer S, et al. A new technology of microdispersed silver in polyurethane induces antimicrobial activity in central venous catheters. Infection. 1999;27(Suppl 1):S16–23. doi: 10.1007/BF02561612. [DOI] [PubMed] [Google Scholar]
- 30.Yao Y, Ohko Y, Sekiguchi Y, et al. Self-sterilization using silicone catheters coated with Ag and TiO2 nanocomposite thin film. J Biomed Mater Res B Appl Biomater. 2008;85:453–460. doi: 10.1002/jbm.b.30965. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Leung P, Yao L, et al. Antimicrobial effect of surgical masks coated with nanoparticles. J Hosp Infect. 2006;62:58–63. doi: 10.1016/j.jhin.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Rogers SS, van der Walle C, Waigh TA. Microrheology of bacterial biofilms in vitro: Staphylococcus aureus andPseudomonas aeruginosa. Langmuir. 2008;24:13549–13555. doi: 10.1021/la802442d. [DOI] [PubMed] [Google Scholar]