Abstract
Background and aim:
The general practitioner (GP) is the first contact with the health care system for most patients with COPD in Denmark. We studied, if participating in an educational program could improve adherence to guidelines, not least for diagnosis, staging, and treatment of the disease.
Design and setting:
Two cross-sectional surveys were performed precisely one year apart before and after an educational program for the participating GPs. A total of 124 GPs completed the study; 1716 and 1342 patients with GP-diagnosed COPD and no concomitant asthma, respectively, were included in the two surveys.
Results:
The proportion of patients having FEV1 registered in the GPs files increased from 45% to 69% (P < 0.001); and, furthermore, FEV1 % of predicted was recorded in 30% and 56%, respectively, of the cases (P < 0.001). In line with this, significant improvements were also observed for registration of smoking status (69% to 85%), BMI (8% to 40%), severity of dyspnea (Medical Research Council) (7% to 38%), and FEV1/FVC ratio (28% to 58%) (P < 0.001). Concerning the management options, improvements were also observed with regard to antismoking counseling, inhalator technique, physical activity, and referral for rehabilitation; use of inhaled corticosteroids in patients with mild COPD (FEV1 > 80%pred) declined from 76% to 45%.
Conclusion:
Diagnosis and management of COPD in general practice in Denmark is not according to guidelines, but substantial improvements can be achieved by focused education of GPs and their staff.
Keywords: COPD, guidelines, adherence, education, diagnosis, management
Introduction
Chronic obstructive pulmonary disease (COPD) is a major health problem, not least in industrialized countries, and it has recently been estimated that Denmark, with approximately 5.5 million inhabitants, has more than 300,000 patients suffering from COPD,1 and, like in many countries, a substantial proportion of patients are diagnosed in advanced stages of the disease.2,3 Furthermore, approximately 20% of acute admissions to hospitals in Denmark are due to exacerbations of COPD.2
According to the GOLD-guidelines,3 COPD is both a preventable and treatable disease; and, therefore, more focus on diagnosis, staging and management, including smoking cessation programs and pharmacological treatment, may reduce the overall burden of COPD.3,4 Furthermore, over the recent decades, several sets of national5 and international3,4 guidelines have recommended spirometry and adherence to documented management algorithms with the aim of improving the overall care for patients suffering from COPD. However, previous studies have documented that GPs, in spite of these available resources, do not find it easy to diagnose and treat COPD.6
In Denmark, as well as in many other countries, the general practitioner (GP) often represents the first contact of a COPD patient with the health care system; and, therefore, in order to improve the care of patients with COPD, it seems of outmost importance to improve the knowledge and skills related to diagnosing and managing this common disease among GPs.
In the present study, we therefore studied if participating in an educational program may improve adherence to guidelines, not least for diagnosis, staging, and subsequently, pharmacological treatment of COPD.
Methods
The present study, KVASIMODO II, consisted of two descriptive cross-sectional investigations focusing on the quality of care for patients suffering from COPD in general practice. The initial audit survey of the participating GPs’ patient files (Survey 1) was conducted on recorded patient data obtained before the GP had knowledge of the study, and, after consenting, the GPs and their staff followed an educational program, where after a second audit survey (Survey 2) was conducted exactly 12 months after the first survey.
The primary efficacy parameter was the proportion of patients with spirometric data, and by that correct diagnosis and staging; and the secondary parameters included changes in monitoring of dyspnea score (Medical Research Council [MRC],7 referral for rehabilitation, instruction in use of inhalator-devices, body mass index (BMI [kg/m2]), and anti-smoking counseling.
Denmark has approximately 3600 GPs, covering a population of 5.5 million, and we aimed to include, on a voluntary basis, up to 200 GPs in the study from all over Denmark. Written information about the project and the invitation to participate was distributed by the sponsoring companies’ representatives. Each of the participating GPs had to identify 20 COPD consecutive patients who attended his or her practice from March 1st to June 30th, 2006 (Survey 1) and 20 consecutive patients during a similar period in 2007 (Survey 2).
The case definition of COPD was based on expert opinion as follows: Age ≥ 35 years, and at least two prescriptions for an inhaled bronchodilator (short- or long-acting β2 agonist, short- or long-acting anti-cholinergic drug, or, a combination of the two) within the previous year. Patients who were, by the GP, considered to have asthma were excluded from the study.
This practical definition of COPD was chosen, instead of a spirometry-based definition, because evidence from a previous study6 showed that the implementation of spirometry testing was relatively low. Furthermore, this approach gave us the opportunity to investigate how often treatment for COPD is initiated without a verified diagnosis, ie, spirometry, and also potentially made it easier for the participating GPs since prescribing data are always recorded.
After identifying 20 cases of COPD, the GP was asked to perform an internal audit of the information already available in his/her clinical files in order to fill in the clinical record form (CRF) for each of the included patients. No additional patient investigation was allowed in order to complete the CRF, so if the requested information was not available a missing value was recorded.
The CRF consisted of 5 parts: inclusion criteria, exclusion criteria, diagnostic procedures, non-pharmacological treatment, and pharmacological treatment. All data from the individual CRF were entered into a consolidated web-based database by the GP or, in most cases, by a member of their staff. Quality control of the CRFs was performed by consultants from the sponsoring companies.
The education program on COPD, which took place between the two surveys, was based on the GOLD-guidelines and was designed by a group of Danish pulmonologists and GPs with a special interest in COPD (ie, the steering committee of the KVASIMODO II study). It was directed towards the participating GPs and their staff, including nurses, laboratory technicians, and secretaries, and consisted of the following components: 1) An individual meeting with a consultant from one of the sponsoring companies focusing on the GOLD-guidelines, 2) Local meetings with GPs and their staff, where two from the steering committee, a pulmonologist and a GP, discussed several aspects of the guidelines, 3) Regional symposiums for the participating GPs and their staff. The symposium consisted of plenary sessions and workshops, and included practical issues like performing and interpreting spirometry and the teaching of inhaler technique, and, subsequently, 4) An individual meeting with a consultant from one of the sponsoring companies focusing on the participating GPs data, and, not least, changes over the study period, and 5) A final meeting for all participating GPs and their staff, where the results of the study were presented. All participating GPs and their staffs were offered the same educational program, both quantitatively and qualitatively.
Data analysis
Data from individual patient records were analyzed focusing on the overall quality of care according to the previously defined outcome variables; only patient data provided by GPs who completed both surveys were included in the analyses. The FEV1 was expressed as a percentage of the predicted value,8 and the FEV1/FVC ratio as the absolute value. The chi-square test and Mann–Whitney two-sample test were used, as appropriate. The KVASOMODO II project was recommended and approved by the Danish College of General Practitioners.
Results
A total of 194 GPs were interested in participating in the KVASIMODO II study. However, during the two surveys 42 and 28 GPs, respectively, dropped out of the study, primarily due to difficulties in retrieving patient data from their files and excessive daily workload. The final analyses, therefore, comprised data provided by 124 GPs, and included 1716 and 1342 patients, respectively. More females than males were included in the study, but mean age for both genders were 68 years, and mean FEV1 – in patients with available information on age, height, and spirometry – was 59% of the predicted value; further details are given in Table 1. At the start of survey 1 more than 85% of the GPs had direct access to a spirometer.
Table 1.
Characteristics of the patients suffering from COPD enrolled in the first and second survey
| First survey | Second survey | |
|---|---|---|
| Number of GPs | 124 | 124 |
| Number of patients | 1716 | 1342 |
| Mean age (range) | 68 (35–95) | 68 (35–97) |
| Male/Female ratio | 44/56% | 48/52% |
| Smoking, pack-years (range) | 40 (1–110) | 40 (3–120) |
| Body mass index, kg/m2 (range) | 27 (15–44) | 26 (15–48) |
| Dyspnea index, MRC (range) | 3 (1–5) | 3 (1–5) |
Abbreviation: MRC, Medical Research Council.
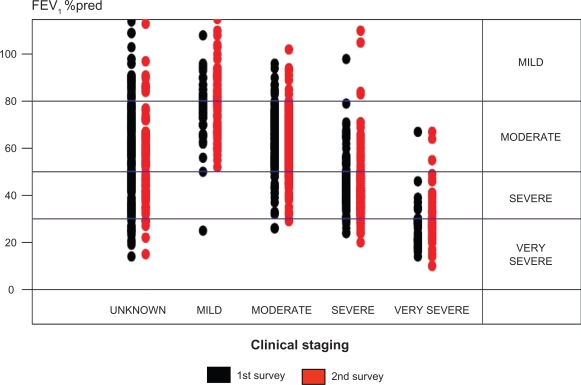
Table 2 shows the results of both audit surveys, ie, the presence of relevant information in the GPs files on indices related to diagnosing and staging of their patients suffering from COPD. At survey 1, only 45 % of the CRFs included relevant spirometric data (FEV1, FVC, and FEV1/FVC ratio), whereas the corresponding figures were 69 % at the 2. survey (P < 0.001). Furthermore, only 21 % of the included patients in survey 1 fulfilled the diagnostic criteria of an FEV1/FVC ratio < 70%, whereas 48 % of the patients included in survey 2 by definition had airway obstruction (P < 0.001). The FEV1 as a percentage of predicted value (requiring data on FEV1, age, and height) was only available for 30% and 56%, respectively, of the included patients at the two surveys (P < 0.001); and the observed correlation between severity of disease as judged by the GP and FEV1 %pred was not optimal (Figure 1).
Table 2.
Presence of information in the patient files related to diagnosis and staging of COPD for the patients included in the study by the 124 GPs who participated in both surveys
| Survey 1 | Survey 2 | |
|---|---|---|
| Smoking status | 69% | 85%* |
| Tobacco exposure (pack-years) | 14% | 40%** |
| FEV1 (L) | 45% | 69%** |
| FEV1 (%pred) | 30% | 56%** |
| FEV1/FVC < 70% | 21% | 48%** |
For comparison between survey 1 and 2:
P < 0.01, and
P < 0.001
Figure 1.
Level of FEV1 %pred versus clinical staging of COPD (as judged by the individual patient’s GP based on symptom severity) in the first and the second survey.
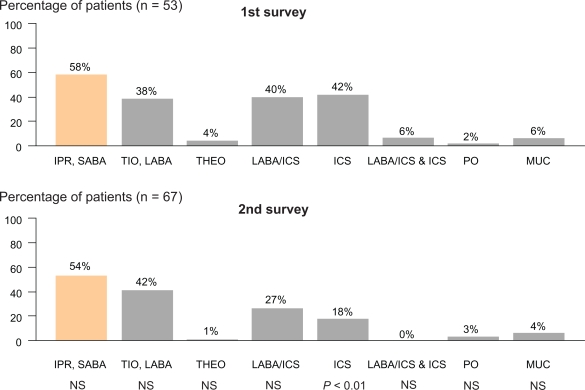
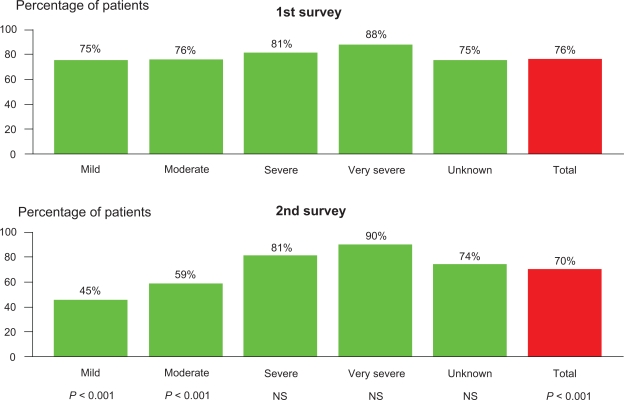
Improvements in several indices of adherence to guidelines for pharmacological and non-pharmacological management were observed from survey 1 to survey 2 (Table 3), including a significant fall in the proportion of patients with mild COPD treated with inhaled corticosteroids (P < 0.01) (Figure 2), especially in patients with available information in the GPs file on FEV1 %pred (76% and 45%, respectively; P < 0.001) (Figure 3).
Table 3.
Presence of information in the patient files related to adherence to COPD guidelines for the patients included in the study by the 124 GPs who participated in both surveys
| Survey 1 | Survey 2 | |
|---|---|---|
| BMI (kg/m2) | 8% | 40%** |
| Dietary instruction given (BMI < 20) | 38% | 59%* |
| Smoking cessation advice given (smokers only) | 40% | 57%* |
| Instruction in inhalation technique documented | 25% | 41%* |
| Dyspnea score recorded (MRC) | 7% | 38%** |
| Referred for COPD rehabilitation | 12% | 16%a |
| Inhaled corticosteroids in mild COPD | 76% | 45%** |
| Inhaled corticosteroids in severe COPD | 86% | 85%a |
For comparison between survey 1 and 2:
NS,
P < 0.01, and
P < 0.001.
Abbreviations: BMI, body mass index; MRC, Medical Research Council.
Figure 2.
Prescribed pharmacological treatment for patients with mild COPD in the first and the second survey.
Abbreviations: IPR, ipratropium bromide; Tio, tiotropium; LABA, long-acting β2 agonist; Theo, theophylline; ICS, inhaled corticosteroids; PO, oral prednisolone; MUC, mucolytics.
Figure 3.
Prescribed treatment inhaled corticosteroids according to severity of COPD based on measurement of FEV1 in the first and the second survey.
Discussion
This study shows that participating in an educational program can substantially improve adherence to COPD guidelines by general practitioners, even though there is still room for improvement with regard to care for patients suffering from COPD in general practice in Denmark.
Our study confirms findings from a previously published similar study.6 However, in contrast to the study by Lange et al,6 the present study included younger subjects (>35 years) and excluded patients with concomitant asthma. These differences have most likely improved our possibility to evaluate real life care of COPD patients in general practice in Denmark, not least for pharmacological treatment due to the exclusion of patients with doctor-diagnosed concomitant asthma. In line with this, it also offered us the opportunity to investigate the quality of care for younger patients with doctor-diagnosed COPD, where the clinical picture will possibly more likely lead to a diagnosis of asthma.
GPs were recruited for the study from all over Denmark, but because participation in the study was voluntary, the enrolled GPs might not be representative of the GPs in Denmark, especially with regard to motivation for improvement in their personal management of patients with COPD. However, due to the fact that a very similar study had previously been conducted in Denmark,6 it seems likely that the GPs recruited for the present study, on average, had less interest in COPD and lung diseases, as GPs were excluded from the present study if they had participated in the previous study.6
Patients were included on the basis of prescription of inhaled bronchodilators, age, and absence of asthma. This practical definition, instead of a spirometry-based definition, was chosen, because one of the major problems in implementation of COPD guidelines in general practice is difficulties in using spirometry, most likely primarily due to the mandatory active patient participation, calibration of spirometer, and interpretation of results.9 However, although this improved our ability to evaluate the main outcome of the study, it might have excluded some patients with milder COPD, as they may not have been prescribed bronchodilators.9,10
Our primary aim was to promote spirometry, and by that correct diagnosis and staging, which is mandatory in the management of COPD.3,4 Performing spirometry is easy, cheap, and takes only a few minutes,11 and it should, therefore, be possible to offer spirometry to all individuals suspected to suffer from COPD. Furthermore, in Denmark, spirometry is reimbursed and the majority of Danish GPs (>85%) have direct access to a spirometer. Although all patients enrolled in the present study had doctor-diagnosed COPD, and were treated with inhaled bronchodilators, spirometry data from survey 1 documented the presence of airway obstruction (FEV1/FVC < 70%) in only 21% of the cases. In line with this, a severity assessment, based on FEV1 %pred, was only available in 30% of the cases. Our study therefore confirm previous findings that spirometry is often underused in general practice;12–14 and this might be one of the main barriers for implementation of GOLD guidelines and/or national COPD guidelines. Fortunately, a significant improvement in these figures were observed at survey 2 (48% and 56%, respectively), but remained substantially lower than found in other comparable studies9,10,15 although the majority of the GPs who participated in the study actually had a spirometer in their practice.
It has previously been shown that inaccurate diagnosis and staging of COPD leads to inadequacy of administered treatments, including non-pharmacological management.9,10,15 Bourbeau et al10 identified, looking at data reported by a large number of GPs, that the pharmacological treatment matched guideline recommendations in only 35% of the patients. This obvious discrepancy between prescribed therapy and guideline recommendations of therapy tailored to disease severity was also observed in the present study (Figure 1).
In contrast to bronchodilators, inhaled corticosteroids were, at the time of this study, only recommended to patients with severe and very severe COPD experiencing repeated exacerbations.3,4 With regard to pharmacological treatment, we therefore decided to focus on the use of inhaled corticosteroids. Survey 1 showed that inhaled corticosteroids were prescribed for 76% of the patients with mild COPD, which is comparable to figures reported by Bourbeau et al10 Furthermore, Jones et al9 has reported that inhaled corticosteroids are overprescribed for COPD in general practice (recommended for 17%, taken by 60%). The education program in the present study included information on the evidence-based recommended pharmacological and non-pharmacological treatment, and in accordance with this we observed an improvement in most relevant indices, including the use of inhaled corticosteroids (Table 3, Figure 2). In keeping with previous findings from Denmark by Schaefer et al,16 it is therefore possible, through education, to change GPs’ prescription patterns without interfering with patient access to relevant treatment or with GPs’ clinical freedom.
In addition to describing the overall quality of care for patients suffering from COPD, we also wanted to evaluate if participation in an educational program can improve adherence to guidelines, and by that improve the quality of care. Similar to other studies,9,10 the method used was an internal audit. Since the GPs themselves completed the CRFs, we cannot exclude an element of ‘observer bias’, especially during survey 2, although some cross-checking was done on a random sample of the cases included.
In conclusion, this study showed that diagnosis, staging, and management, including pharmacological and non-pharmacological treatment, is not according to guidelines in general practice in Denmark, but substantial improvements can be achieved in most relevant indices of quality of care of this very common chronic disease through educating the GPs and their staff. Further studies are needed in order to ascertain how the achieved improvements can be maintained and expanded.
Footnotes
Disclosure
This study was sponsored by Pfizer Denmark and Boehringer-Ingelheim Denmark.
References
- 1.Løkke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow-up study of the general population. Thorax. 2006;6:935–939. doi: 10.1136/thx.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juel K, Døssing M. København: Statens Institut for Folkesundhed; 2003. KOL i Danmark [COPD in Denmark]. Sygdommen der hver dag koster 10 danskere livet [The disease that kills 10 Danes every day] [Google Scholar]
- 3.Global Initiative for Chronic Obstructive Lung Disease Global Strategy for Diagnosis, Management and Prevention of COPD. Guidelines available from the GOLD website www.goldcopd.com Accessed December, 2009).
- 4.National Institute for Clinical Excellence (NICE) Chronic obstructive pulmonary disease: national clinical guideline for management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax. 2004;59(Suppl 1):1–232. [PMC free article] [PubMed] [Google Scholar]
- 5.Lange P, Hansen JG, Iversen M, et al. Diagnostik og behandling af kronisk obstruktiv lungesygdom: oversigt og vejledende retningslinier. Ugeskr Laeg. 1998;160(Suppl 1):1–38. [Google Scholar]
- 6.Lange P, Rasmussen FV, Borgeskov H, Dollerup J, Jensen MS, Roslind K, Nielsen LM, on behalf of the KVASIMODO study group The quality of COPD care in general practice in Denmark: the KVASIMOLDO study. Prim Care Respir J. 2007;16:174–181. doi: 10.3132/pcrj.2007.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bestall JC, Paul EA, Garrod R, Granham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quanjer PH, Tammeling GJ. Summary of recommendations. Standardized lung function testing. Report Working Party, European Community for Coal and Steel. Bull Eur Physiopathol Respir. 1983;19(Suppl 5):7–20. [PubMed] [Google Scholar]
- 9.Jones RC, Dickson-Spillmann M, Mather MJ, Marks D, Schackell BS. Accuracy of diagnostic registers and management of chronic obstructive pulmonary disease: The Devon primary care audit. Respir Res. 2008;9:62. doi: 10.1186/1465-9921-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourbeau J, Sebaldt RJ, Day A, et al. Practice patterns in the management of chronic obstructive pulmonary disease in primary practice: the CAGE study. Can Respir J. 2008;15:13–19. doi: 10.1155/2008/173904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson GJ. Clinical Tests of Respiratory Function. Chapman and Hall; London, UK: [Google Scholar]
- 12.Bolton CE, Ionescu AA, Edwards PH, Faulner TA, Edwards SM, Shale DJ. Attaining correct diagnosis of COPD in general practice. Respir Med. 2005;99:493–500. doi: 10.1016/j.rmed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Caramori G, Bettoncelli G, Tosatto R, et al. Underuse of spirometry by general practitioners for the diagnosis of COPD in Italy. Monaldi Arch Chest Dis. 2005;63:6–1. doi: 10.4081/monaldi.2005.651. [DOI] [PubMed] [Google Scholar]
- 14.Weidinger P, Nilsson JLG, Lindblad U. Adherence to diagnostic guidelines and quality indicators in asthma and COPD in Swedish primary care. Pharmacoepidemiol Drug Saf. 2009;18:393–400. doi: 10.1002/pds.1734. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths C, Feder G, Wedzicha J, Foster G, Livingstone A, Marlowe GS. Feasibility of spirometry and reversibility testing for the identification of patients with chronic obstructive pulmonary disease on asthma registers in general practice. Respir Med. 1999;93:903–8. doi: 10.1016/s0954-6111(99)90057-4. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer K, Hansen AO, Maerkedahl H, Rehfeld C, Birk HO, Henriksen LO. Changing GPs’ prescription patterns through guidelines and feed-back. Intervention study. Pharmacoepidemiol Drug Saf. 2007;16:694–704. doi: 10.1002/pds.1404. [DOI] [PubMed] [Google Scholar]