Abstract
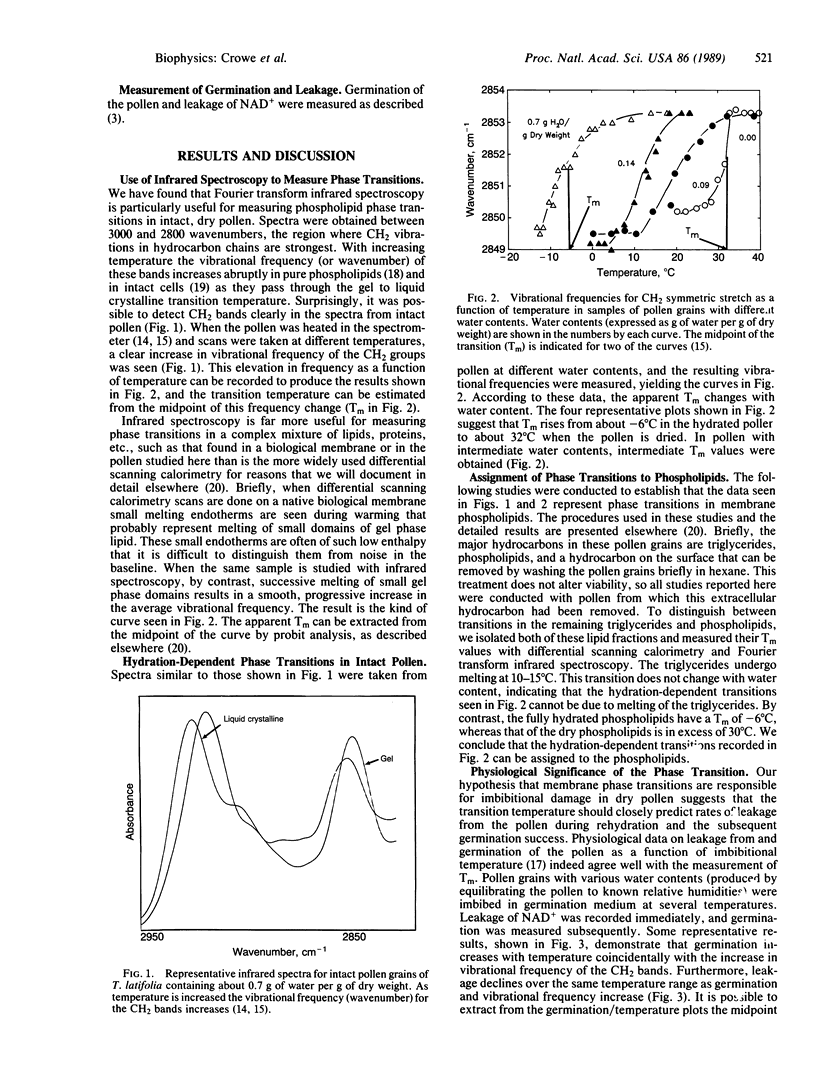
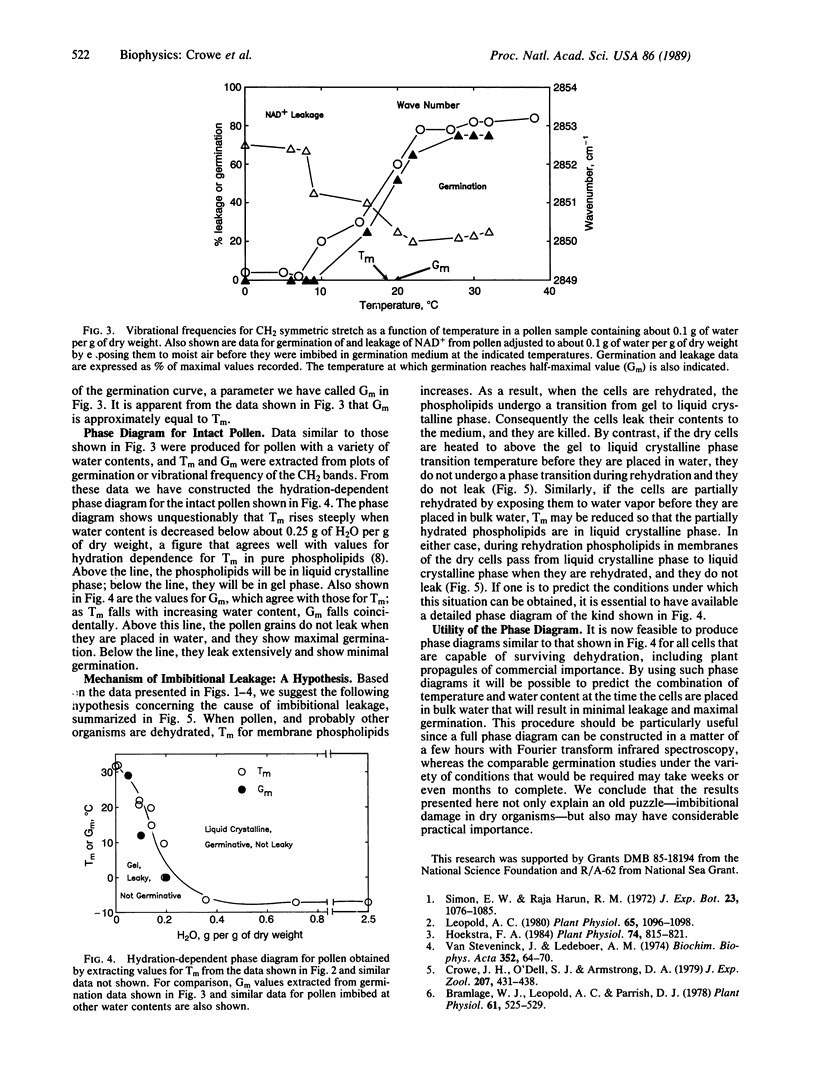
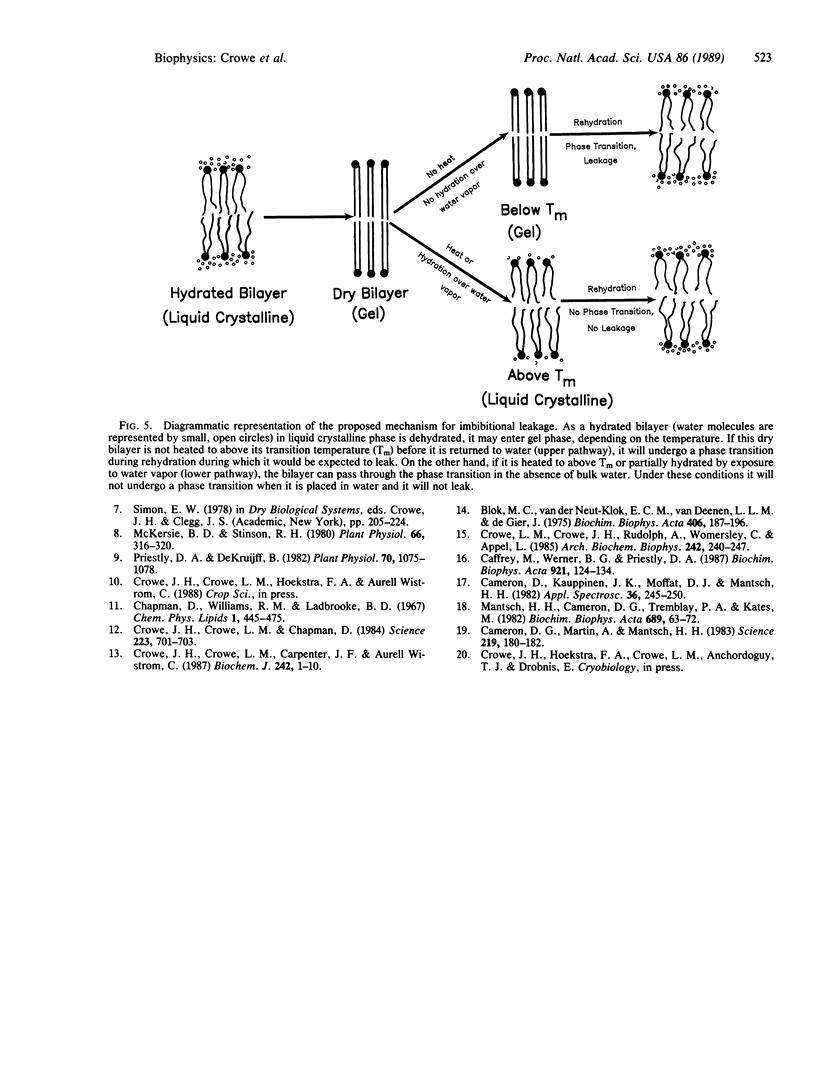
We have found that the most probable cause of the leakage seen when dry cells or organisms such as seeds, pollen, or yeast cells are plunged into water is a gel to liquid crystalline phase transition in membrane phospholipids accompanying rehydration. By using Fourier transform infrared spectroscopy we have recorded infrared spectra of CH2 stretching vibrations in dry and partially hydrated intact pollen grains of Typha latifolia. The vibrational frequency changes abruptly as phospholipids pass through the gel to liquid crystalline phase transition. Below the apparent transition, viable pollen shows low germination and high leakage when placed in water, but above the transition germination increases and leakage decreases. The apparent transition temperature falls with increasing water content, much as in pure phospholipids. By using this phenomenon, it was possible to construct a hydration-dependent phase diagram for the intact pollen. This phase diagram has immediate applications since it has high predictive value for the viability of the pollen when it is placed in water.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blok M. C., van der Neut-Kok E. C., van Deenen L. L., de Gier J. The effect of chain length and lipid phase transitions on the selective permeability properties of liposomes. Biochim Biophys Acta. 1975 Oct 6;406(2):187–196. doi: 10.1016/0005-2736(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Bramlage W. J., Leopold A. C., Parrish D. J. Chilling Stress to Soybeans during Imhibition. Plant Physiol. 1978 Apr;61(4):525–529. doi: 10.1104/pp.61.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey M., Werner B. G., Priestley D. A. A crystalline lipid phase in a dry biological system: evidence from X-ray diffraction analysis of Typha latifolia pollen. Biochim Biophys Acta. 1987 Sep 4;921(1):124–134. doi: 10.1016/0005-2760(87)90178-0. [DOI] [PubMed] [Google Scholar]
- Cameron D. G., Martin A., Mantsch H. H. Membrane isolation alters the gel to liquid crystal transition of Acholeplasma laidlawii B. Science. 1983 Jan 14;219(4581):180–182. doi: 10.1126/science.6849129. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Carpenter J. F., Aurell Wistrom C. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J. 1987 Feb 15;242(1):1–10. doi: 10.1042/bj2420001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984 Feb 17;223(4637):701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- Crowe L. M., Crowe J. H., Rudolph A., Womersley C., Appel L. Preservation of freeze-dried liposomes by trehalose. Arch Biochem Biophys. 1985 Oct;242(1):240–247. doi: 10.1016/0003-9861(85)90498-9. [DOI] [PubMed] [Google Scholar]
- Hoekstra F. A. Imbibitional chilling injury in pollen: involvement of the respiratory chain. Plant Physiol. 1984 Apr;74(4):815–821. doi: 10.1104/pp.74.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold A. C. Temperature effects on soybean imbibition and leakage. Plant Physiol. 1980 Jun;65(6):1096–1098. doi: 10.1104/pp.65.6.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch H. H., Cameron D. G., Tremblay P. A., Kates M. Phosphatidylsulfocholine bilayers. An infrared spectroscopic characterization of the polymorphic phase behavior. Biochim Biophys Acta. 1982 Jul 14;689(1):63–72. doi: 10.1016/0005-2736(82)90189-4. [DOI] [PubMed] [Google Scholar]
- McKersie B. D., Stinson R. H. Effect of Dehydration on Leakage and Membrane Structure in Lotus corniculatus L. Seeds. Plant Physiol. 1980 Aug;66(2):316–320. doi: 10.1104/pp.66.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell N. L., Sharawy M. H., Schuster G. S. Effects of in vivo single and multiple isoproterenol injections on subsequently explanted submandibular glands. Acta Anat (Basel) 1979;105(4):431–438. doi: 10.1159/000145150. [DOI] [PubMed] [Google Scholar]
- Priestley D. A., de Kruijff B. Phospholipid Motional Characteristics in a Dry Biological System : A P-Nuclear Magnetic Resonance Study of Hydrating Typha latifolia Pollen. Plant Physiol. 1982 Oct;70(4):1075–1078. doi: 10.1104/pp.70.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steveninck J., Ledeboer A. M. Phase transitions in the yeast cell membrane. The influence of temperature on the reconstitution of active dry yeast. Biochim Biophys Acta. 1974 May 30;352(1):64–70. doi: 10.1016/0005-2736(74)90179-5. [DOI] [PubMed] [Google Scholar]



