Abstract
Vocal imitation—the ability to learn a previously unknown acoustic signal from a tutor—is considered to be a key innovation in the evolution of speech. This faculty is very rare and patchily distributed within the animal kingdom, suggesting multiple instances of convergent evolution. It has long been predicted that bats should be capable of vocal imitation and our results provide evidence for this phenomenon. We report that pups of the bat Saccopteryx bilineata learn a complex vocalization through vocal imitation. During ontogeny, pups of both sexes imitate territorial song from adult males, starting with simple precursor songs that develop into genuine renditions. The resemblance of pup renditions to their acoustic model is not caused by physical maturation effects, is independent of pups' gender and relatedness towards adult males and becomes more pronounced during ontogeny, showing that auditory experience is essential for vocal development. Our findings indicate that the faculty of vocal imitation is more widespread than previously thought and emphasize the importance of research on audiovocal communication in bats for a better understanding of the evolutionary origin of vocal imitation.
Keywords: Chiroptera, vocal production learning, mimicry, tutor, auditory input
1. Introduction
The ability to imitate new sounds is prevalent in humans but very rare in other animals and its evolutionary origin is poorly understood (Fitch 2000). Vocal imitation is considered to be a key innovation in the evolution of speech (Fitch 2000), which, together with syntax and semantics, is an important component of language (Hauser et al. 2002). Current evidence for non-human vocal imitation is limited to birds, cetaceans, seals and elephants, which suggests a multiple convergent evolution of this faculty (Janik & Slater 1997; Doupe & Kuhl 1999; Boughman & Moss 2003). Bats are known to modify the structure of simple, innate vocalizations based on social experience (Jones & Ransome 1993; Boughman 1998), but the acquisition of new vocalizations through vocal imitation has never been demonstrated (Boughman & Moss 2003), even though it has long been predicted (Janik & Slater 1997).
The greater sac-winged bat Saccopteryx bilineata is a Neotropical insectivore with a complex social life involving resource-defence polygyny (reviewed in Voigt et al. 2008). Core social groups are termed harems and consist of one harem male and up to eight females, which can have one pup per year. Harem males defend small territories in day-roosts year-round and use various behavioural displays to retain females there. Since males cannot reproductively monopolize females, some pups grow up with a harem male that is not their genetic father. Saccopteryx bilineata exhibits a rich vocal repertoire with context-specific vocalization types, emphasizing the great vocal flexibility of this species (Behr & von Helversen 2004; Voigt et al. 2008). Pup isolation calls mainly elicit maternal care (Knörnschild & von Helversen 2008) but are also important in the development of babbling behaviour. During babbling, pups mix renditions of all adult vocalization types into long bouts, a behaviour probably supporting the vocal repertoire acquisition (Knörnschild et al. 2006). Territorial songs are complex multi-syllabic vocalizations used by adult males for the demarcation of harem territories (Behr & von Helversen 2004).
We studied the vocalizations of pups throughout ontogeny and found that pups of both sexes first produce precursor songs and later true renditions of territorial songs. We compared these pup vocalizations with territorial songs of their harem males and tested whether vocal development was influenced by maturation effects, relatedness, gender, or vocal imitation.
2. Material and methods
We monitored day-roosts with one social group (one male and one to four females with their pups) each at the Biological Station La Selva in Costa Rica from 2005 to 2007. Bats were habituated to humans and identified by plastic bands on their forearms. We used high-quality ultrasonic recording equipment (details in the electronic supplementary material) to record 337 territorial songs/precursors of 17 pups belonging to seven different social groups and 57 territorial songs of the six respective harem males (one male was present and recorded during two seasons). We analysed five to 50 territorial songs/precursors from each pup during two ontogenetic stages (two to six and seven to 10 weeks old; age determined through observations of birth or indirectly through high parturition synchrony within groups), and five to 13 territorial songs from each harem male. Territorial songs/precursors are multi-syllabic; since all syllables other than the buzz syllables are variable and also occur in other vocalization types, we focused only on buzz syllables. All stereotypic, composite syllables consisting of a noisy, buzz-like part preceding a tonal part were considered as buzz syllables (Behr et al. 2006; figure 1). Noisy parts had no discernible fundamental frequency and harmonic structure. Acoustic parameters (details in the electronic supplementary material) belonging to the same syllable part (noisy/tonal) were averaged for every precursor/territorial song and combined into principal components, which we used to conduct discriminant function analyses. The distance between centroids (i.e. the canonical mean of all vocalizations per individual) in a signal space defined by the discriminant functions is a good indicator of acoustic similarity, with similarly sounding individuals clustering together (Boughman 1998; Knörnschild et al. 2007). We used the Euclidean distance between centroids to compare territorial songs of harem males with pup precursor/territorial songs uttered during the two ontogenetic stages. We calculated discriminant functions with the data from the first ontogenetic stage and then mapped the remaining data into the same signal space in order to compare all centroids distances simultaneously. All statistical tests were performed using SPSS v. 11.5. Details on relatedness (i.e. paternity analysis) are shown in the electronic supplementary material.
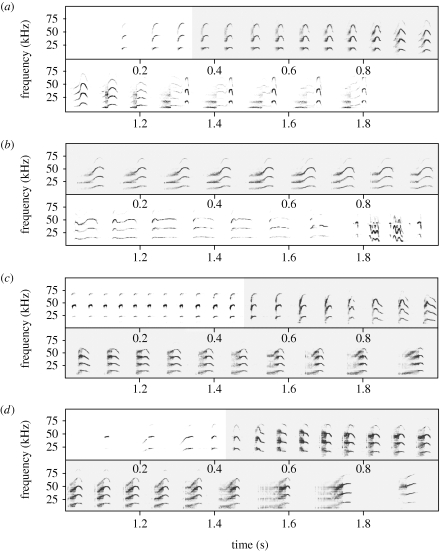
Figure 1.
Sonograms of (a–b) two pup precursor songs and (c) one complete territorial song produced at different ages, and of (d) a territorial song produced by the respective harem male. During ontogeny, territorial song buzz syllables were first produced (a) within isolation calls and later (b) during babbling bouts. Shortly before weaning, pups produced (c) complete territorial songs, which closely resembled (d) adult territorial song. Buzz syllables used for measurements are highlighted in grey. (a) Approximately four week old pup; (b) approximately six week old pup; (c) approximately eight week old pup; and (d) harem male.
3. Results
During ontogeny, pup precursor songs gradually developed into true territorial songs (figure 1). Precursor songs were typically composed of territorial song buzz syllables embedded in isolation calls and later, in babbling bouts. The percentage of buzz syllables per vocalization increased significantly as pups matured (t-test for matched pairs: t16 = −3.290, p = 0.005). Percentage differences between pups and adult males were significant early in ontogeny (t-test: t22 = −3.219, p = 0.004; α = 0.025 after sequential Bonferroni correction) but not later (t-test: t22 = −0.932, p = 0.362), illustrating that precursor songs developed into territorial songs.
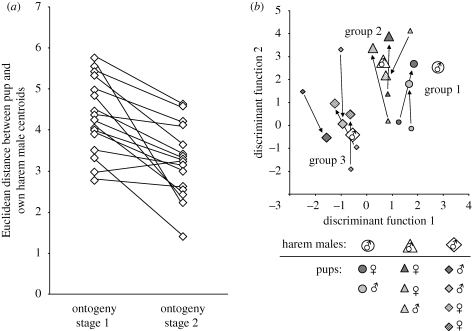
Three different factors can potentially contribute to the vocal similarity between adult males and pups: physical maturation effects, relatedness, and imitation. As an indicator of acoustic similarity between individuals, we used the Euclidean distance between centroids in a signal space based on the acoustic parameters of buzz syllables. Centroids are the canonical mean of all vocalizations per bat and mark each individual's position in signal space. We compared the Euclidean distances between each pup and its harem male, the other males and a ‘species mean’ (i.e. the centroid of all adult males). In order to test whether physical maturation plays the predominant role, we compared the movement of pups in signal space (i.e. the difference in Euclidean distance between ontogeny stages 1 and 2) towards their own harem males and towards the species mean. Pups' vocal movement towards their own harem males was significantly greater than towards the species mean (t-test for matched pairs: t15 = 2.295, p = 0.037), indicating that physical maturation alone is not causing the vocal similarity of pups and adult males. Pups always clustered significantly closer to their own harem male than to the other males in the analysis (t-test for matched pairs; ontogeny stage 1: t16 = −2.963, p = 0.009; ontogeny stage 2: t16 = −4.438, p < 0.0001; α = 0.0167 after sequential Bonferroni correction). Both initial and late similarity between pups and their harem males occurred independently of pups' gender or relatedness towards the harem male (MANOVA with gender and relatedness as fixed factors; gender (nine females, seven males): ontogeny stage 1, F1,13 = 0.516, p = 0.485; ontogeny stage 2, F1,13 = 0.405, p = 0.536; relatedness (eight pups, respectively, sired or not sired by the harem male): ontogeny stage 1, F1,13 = 0.150, p = 0.705; ontogeny stage 2, F1,13 = 1.897, p = 0.192), which indicates that harem males function as tutors for the pups. The distance between pups and their tutors decreased significantly during ontogeny (t-test for matched pairs: t16 = 4.304, p = 0.001; α = 0.0167 after sequential Bonferroni correction), showing that pup renditions converged towards the territorial songs of their respective tutors (figure 2). This convergence over time occurred independently of pups' gender or relatedness towards the tutor (ANOVA with gender and relatedness as fixed factors: gender, F1,13 = 0.070, p = 0.796; relatedness, F1,13 = 2.007, p = 0.180), demonstrating the important role of auditory input in the acquisition of territorial song.
Figure 2.
Centroids of territorial songs from pups at two different ontogenetic stages and from their respective harem males plotted in the same two-dimensional signal space. The Euclidean distance between pups and their respective harem males became smaller during ontogeny (a), illustrating that the precursor/territorial songs of pups converged towards the territorial songs of their tutors (b). This convergence occurred independently of pups' gender or relatedness towards the tutor. For clarity, only three of seven social groups are shown. Symbol shape represents social group affiliation. Small symbols denote ontogeny stage 1; large symbols denote ontogeny stage 2.
4. Discussion
Pups of both sexes learned adult territorial songs through vocal imitation of their respective harem males. The acoustic similarity between pups and their tutors was independent of gender or relatedness, showing that auditory input is crucial for the acquisition of territorial song. This similarity is already rather pronounced early in ontogeny, suggesting that pups develop an auditory template (Marler 1976) by listening to their tutors prior to producing any vocalizations. The vocal convergence during ontogeny might partly be influenced by maturation effects. However, the convergence towards a species mean (i.e. a stable vocalization form) was less pronounced than towards the respective tutors. Also, the direction of this convergence was not uniform (figure 2), as could be expected in case of maturation effects (Moss et al. 1997; Vater et al. 2003; but see Hammerschmidt et al. 2000). Therefore, vocal imitation can best explain the observed convergence of pup precursor songs to the territorial songs of their respective tutors.
Vocal imitative abilities in animals seem to be more pronounced in males (Janik & Slater 1997; Boughman & Moss 2003). In S. bilineata, female pups imitate adult territorial songs as readily as male pups, even though only adult males utter territorial songs (Behr & von Helversen 2004). As in some bird species (Doupe & Kuhl 1999), female S. bilineata might have to create or reinforce an acoustic template of male vocalizations as a basis for future mate choice decisions. The faculty of vocal imitation might be more widespread in female mammals than previously thought, especially when considering signals that are important for female mate choice.
A comparative approach that includes extant animal models is important to better understand how vocal imitation evolved. However, mammalian model organisms that are well suited to study vocal imitative abilities in comparison with those of humans are still lacking. Vocal learning in bats may provide an opportunity to fill this gap. Our study represents a first step towards achieving this goal and hopefully inspires investigations into the evolutionary origin of vocal imitation and its functional significance in both a phylogenetic and a neurobiological or learning context. This prospect is especially intriguing because the FOXp2 gene, which is implicated in vocal learning in humans and birds, has been under intense selection in bats (Li et al. 2007).
Acknowledgements
All fieldwork was approved by the Costa Rican authorities (MINAE, SINAC; resolución no. 067-2007, 049-2006, 022-2005).
We thank E. Kalko, S. Yanoviak, O. Behr and three anonymous reviewers for helpful suggestions. Funding was provided by the Deutsche Forschungsgemeinschaft, the German Merit Foundation, and the National Geographic Society.
References
- Behr O., von Helversen O.2004Bat serenades: complex courtship songs of the sac-winged bat (Saccopteryx bilineata). Behav. Ecol. Sociobiol. 56, 106–115 (doi:10.1007/s00265-004-0768-7) [Google Scholar]
- Behr O., von Helversen O., Heckel G., Nagy M., Voigt C. C., Mayer F.2006Territorial songs indicate male quality in the sac-winged bat Saccopteryx bilineata. Behav. Ecol. 17, 810–817 (doi:10.1093/beheco/arl013) [Google Scholar]
- Boughman J. W.1998Vocal learning by greater spear-nosed bats. Proc. R. Soc. Lond. B 265, 227–233 (doi:10.1098/rspb.1998.0286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughman J., Moss C. F.2003Vocal learning and development of mammal and bird calls. In Acoustic communication. Springer handbook of auditory research (eds Simmons A. M., Popper A. N., Fay R. R.), pp. 138–213 Berlin, Germany: Springer Press [Google Scholar]
- Doupe A. J., Kuhl P. K.1999Birdsong and human speech: common themes and mechanisms. Annu. Rev. Neurosci. 22, 567–631 (doi:10.1146/annurev.neuro.22.1.567) [DOI] [PubMed] [Google Scholar]
- Fitch W. T.2000The evolution of speech: a comparative review. Trends Cogn. Sci. 4, 258–267 (doi:10.1016/S1364-6613(00)01494-7) [DOI] [PubMed] [Google Scholar]
- Hammerschmidt K., Newman J. D., Champoux M., Suomi S. J.2000Changes in rhesus macaque ‘coo’ vocalizations during early development. Ethology 106, 873–886 (doi:10.1046/j.1439-0310.2000.00611.x) [Google Scholar]
- Hauser M. D., Chomsky N., Fitch W. T.2002The faculty of language: what is it, who has it, and how did it evolve? Science 298, 1569–1579 (doi:10.1126/science.298.5598.1569) [DOI] [PubMed] [Google Scholar]
- Janik V. M., Slater P. J. B.1997Vocal learning in mammals. Adv. Study Behav. 26, 59–99 (doi:10.1016/S0065-3454(08)60377-0) [Google Scholar]
- Jones G., Ransome R. D.1993Echolocation calls of bats are influenced by maternal effects and change over a lifetime. Proc. R. Soc. Lond. B 252, 125–128 (doi:10.1098/rspb.1993.0055) [DOI] [PubMed] [Google Scholar]
- Knörnschild M., von Helversen O.2008Nonmutual vocal mother-pup recognition in the greater sac-winged bat. Anim. Behav. 76, 1001–1009 (doi:10.1016/j.anbehav.2008.05.018) [Google Scholar]
- Knörnschild M., Behr O., von Helversen O.2006Babbling behaviour in the sac-winged bat (Saccopteryx bilineata). Naturwissenschaften 93, 451–454 (doi:10.1007/s00114-006-0127-9) [DOI] [PubMed] [Google Scholar]
- Knörnschild M., von Helversen O., Mayer F.2007Twin siblings sound alike: isolation call variation in the noctule bat, Nyctalus noctula. Anim. Behav. 74, 1055–1063 (doi:10.1016/j.anbehav.2006.12.024) [Google Scholar]
- Li G., Wang J., Rossiter S. J., Jones G., Zhang S.2007Accelerated FoxP2 evolution in echolocating bats. PLoS ONE 2, e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler P.1976Sensory templates in species-specific behavior. In Simpler networks and behavior (ed. Fentrees J.), pp. 314–329 Sunderland, MA: Sinauer Associates [Google Scholar]
- Moss C. F., Redish D., Gounden C., Kunz T.1997Ontogeny of vocal signals in the little brown bat, Myotis lucifugus. Anim. Behav. 54, 131–141 (doi:10.1006/anbe.1996.0410) [DOI] [PubMed] [Google Scholar]
- Vater M., Kössl M., Foeller E., Coro F., Mora E., Russell I. J.2003Development of echolocation calls in the mustached bat, Pteronotus parnellii. J. Neurophysiol. 90, 2274–2290 (doi:10.1152/jn.00101.2003) [DOI] [PubMed] [Google Scholar]
- Voigt C. C., Behr O., Caspers B., von Helversen O., Knörnschild M., Mayer F., Nagy M.2008Songs, scents, and senses: sexual selection in the greater sac-winged bat, Saccopteryx bilineata. J. Mammal. 89, 1401–1410 (doi:10.1644/08-MAMM-S-060.1) [Google Scholar]