Abstract
Parasitic Maculinea alcon butterflies can only develop in nests of a subset of available Myrmica ant species, so female butterflies have been hypothesized to preferentially lay eggs on plants close to colonies of the correct host ants. Previous correlational investigations of host-ant-dependent oviposition in this and other Maculinea species have, however, shown equivocal results, leading to a long-term controversy over support for this hypothesis. We therefore conducted a controlled field experiment to study the egg-laying behaviour of M. alcon. Matched potted Gentiana plants were set out close to host-ant nests and non-host-ant nests, and the number and position of eggs attached were assessed. Our results show no evidence for host-ant-based oviposition in M. alcon, but support an oviposition strategy based on plant characteristics. This suggests that careful management of host-ant distribution is necessary for conservation of this endangered butterfly.
Keywords: ant-dependent oviposition, egg-laying, Myrmica, controlled experiment
1. Introduction
The decision where to oviposit is crucial for butterflies (Renwick 1989), especially when relatively immobile caterpillars are dependent on a certain food plant or the protection of another species. At least 50 per cent of the Lycaenidae are associated with ants to some degree (Pierce et al. 2002), ranging from loose commensalism, through caterpillars being frequently tended and protected by ants in exchange for food rewards, to associations where the caterpillars are completely dependent on their ant hosts (Pierce et al. 2002). It has long been observed that lycaenids can adjust their egg-laying behaviour in the presence of tending ants and that the more dependent a lycaenid species is upon tending ants, the more likely that egg-laying behaviour is strongly correlated with the presence of suitable host ants (Pierce & Elgar 1985). Several studies have shown ant-biased oviposition for facultatively ant-associated lycaenids (e.g. Wagner & Kurina 1997), and particularly for obligate mutualists (e.g. Atsatt 1981; Fiedler & Maschwitz 1989; Fraser et al. 2002), but it has proven difficult to demonstrate for the most well-studied obligate ant-dependent lycaenids, the parasitic genus Maculinea. The high degree of host ant specialization of Maculinea (Thomas et al. 1989; Als et al. 2002, 2004) together with its dependence on specific, and often rare, food plants in the early instars (Als et al. 2004), argues for strong selection for an efficient oviposition strategy in this genus.
Previous studies of the correlation between the presence of host ants and the distribution of eggs of Maculinea butterflies have been contradictory, showing either no relationship (Thomas et al. 1997; Nowicki et al. 2005) or a positive correlation (van Dyck et al. 2000; Wynhoff et al. 2008). This latter effect has been postulated to result from differences in development of host plants between habitat patches, rather than direct ant-dependent oviposition (Thomas & Elmes 2001). The oviposition preference of Maculinea butterflies for particular phenological stages of their host-plants has been well documented (Figurny & Woyciechowski 1998; Nowicki et al. 2005).
Most previous studies on Maculinea oviposition have been entirely correlational, so that the effects of plant phenology and host-ant distribution cannot be disentangled. The one exception is the study by Musche et al. (2006), which demonstrated that cues derived from the soil of Myrmica nests are not used in host-plant selection, but this study did not consider any of the other myriad cues that may be provided by the presence of a host-ant nest. In this study, we therefore used potted food plants, matched for size and phenology, to experimentally examine oviposition in areas dominated by natural nests of host and non-host Myrmica ants.
2. Material and methods
In Denmark, Maculinea alcon lays its eggs on the marsh gentian, Gentiana pneumonanthe. At the study site used, Myrmica rubra is the only host of M. alcon (D. R. Nash 2003–2009, unpublished data). Myrmica scabrinodis is also abundant on the study plot, but has never been observed to serve as a host (Als et al. 2002).
Our experiment was conducted during two flight seasons: 2007 (a pilot experiment with potted Gentiana scabra (provided by Højbo Blomster APS), owing to the unavailability of G. pneumonanthe), and 2008 (when a larger number of potted G. pneumonanthe plants (from Staudengärtnerei ‘Alpine Raritäten’, Jürgen Peters) were used).
Myrmica forager densities were assessed by setting out separate honey and tuna-paste baits every 1.5 m along three parallel 50 m transects, 10 m apart. Thirty minutes later, worker ants at the baits were identified with a 10× hand-lens and collected into 96 per cent ethanol for ID verification in the laboratory. This was repeated in 2008 with transects shifted by 5 m. Baiting took place at around 10.00, when the foraging activity of Myrmica ants was high. Having identified areas dominated by M. rubra (host-ant zone, see electronic supplementary material) or M. scabrinodis (non-host-ant zone), six nests of each species were located in 2007 and 16 in 2008, and a potted gentian plant placed 10 cm away from each nest (see electronic supplementary material, figure S1). Plants were paired according to their height and number of flower buds, and one of each pair was placed close to a M. rubra nest and the other close to a M. scabrinodis nest. All plants had been reared under identical conditions, were set out in their pots at similar exposure and watered daily. At the end of the experiment, the ant species next to each plant was checked, and in all cases found to be the same as the initial species.
Eggs laid on flower buds were counted every day after 19.00, when most of the butterflies were inactive, and their position on the plants noted. The number of eggs associated with host and non-host ants, and with buds of different characteristics, was tested using general linear mixed models taking into account overdispersion in the data (see electronic supplementary material for details).
3. Results
Myrmica rubra dominated our study site, with foragers at 63 per cent of our baits in 2007 and 51 per cent in 2008. M. scabrinodis was collected at 19 and 37 per cent of our baits, respectively, and Myrmica ruginodis at a single bait in 2008. At the remaining baits, we found either Formica spp. or no ants.
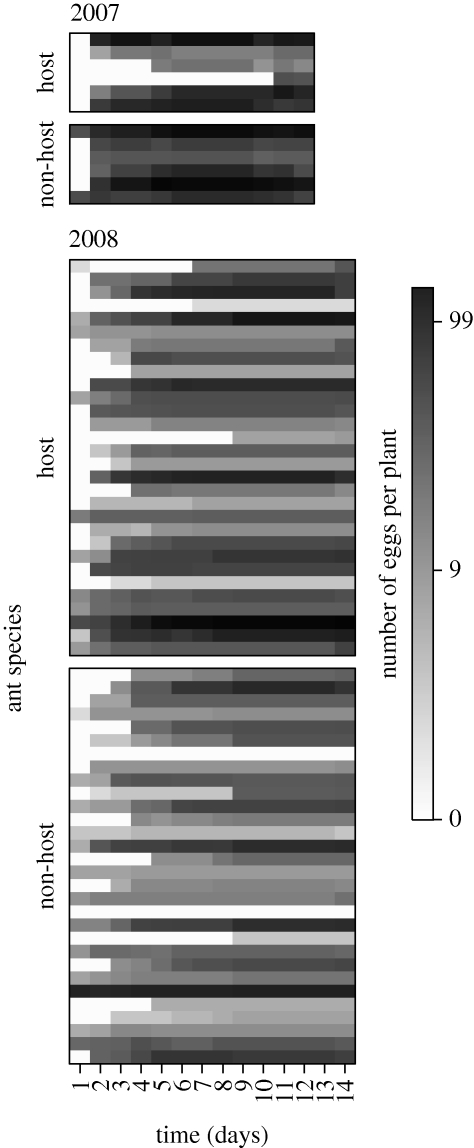
Neither in 2007 nor in 2008 could we find a significant difference for the number of eggs laid on plants close to host ants and non-host ants (2007: Δd = 2.76, d.f. = 1, p = 0.10; 2008: Δd = 1.25, d.f. = 1, p = 0.26) (figure 1). Power analysis revealed that the increased sample size used in 2008 would give a 69 per cent chance of detecting a difference as large as that observed in 2007, giving us a high degree of confidence in rejecting the hypothesis of host-ant-dependent oviposition. In both years, there was, unsurprisingly, a significant increase in the number of eggs over time (2007: Δd = 4.8, d.f. = 1, p = 0.03; 2008: Δd = 32.96, d.f. = 1, p < 0.001), but the interaction between ant species and time was not significant (2007: Δd = 0.03, d.f. = 1, p = 0.85; 2008: Δd = 1.76, d.f. = 1, p = 0.18). The number of eggs laid in both years was similar to the number laid on natural G. pneumonanthe plants of similar height and number of buds at this site (likelihood ratio χ2 = 0.726, d.f. = 1, p = 0.394; see electronic supplementary material for details).
Figure 1.
Cumulative number of eggs per plant over the observation period, shown as a heat map (plotted on a log scale).
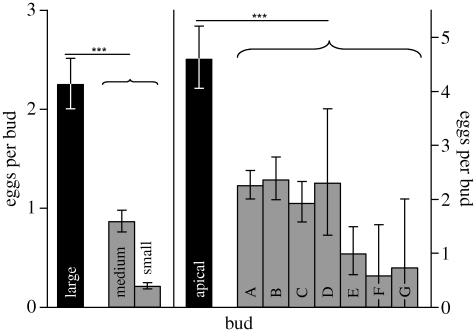
Large buds of G. scabra and apical buds of G. pneumonanthe had received significantly more eggs than smaller and lower buds by the last day (2007: day 11: Wald χ2 = 38.04; d.f. = 1, p < 0.001; 2008: day 15: Wald χ2 = 28.05; d.f. = 1, p < 0.001; figure 2).
Figure 2.
Mean number of eggs (±s.e.) on different buds in 2007 and 2008 on their last day of exposure to the butterflies. Large and apical buds (black bars) were contrasted against other buds (grey bars; buds A–G are successively distant from the plant apex). ***p < 0.001.
4. Discussion
We found no evidence for host-ant-mediated oviposition in M. alcon. The results demonstrate that oviposition choice is not influenced by the presence of host ants, but that developmental stage and position within a plant of a flower bud are good predictors of oviposition choice.
Our results are consistent with the findings of Thomas & Elmes (2001) that plant development rather than host-ant presence influences the butterflies’ oviposition choice, but differ in preferred bud size found for G. scabra. In their study, small buds attracted most eggs, while in ours large buds were favoured. This may reflect differences in the phenology of G. scabra, which has dense growth. Large buds provide a larger surface area and are more exposed than small buds, which are rather inaccessible. In G. pneumonanthe, where bud size was more consistent within plants, the apical buds were most attractive to the butterflies, supporting the finding of Nowicki et al. (2005) that exposed plants are the most attractive for M. alcon oviposition.
Contrary to the study of van Dyck et al. (2000), who found a positive correlation between host-ant presence and egg numbers early in the flight season, which subsequently disappeared, we found no significant change in oviposition choice over time.
The absence of host-ant-dependent oviposition by M. alcon may reflect either an absence of selection for the use of ant cues, or selection against their use by Maculinea. Among the former class of explanations is the possible lack of suitable host ant recognition cues. Even though butterflies are very efficient in detecting odours (Hansson 1995), it is possible that they simply do not encounter suitable cues from their host-ants. For example, ant trail pheromones have been considered the most likely oviposition cues (Henning 1983), but are mainly short-lived volatile compounds (Vander Meer & Alonso 1998), which may not be encountered by ovipositing females since the main foraging periods of Myrmica (morning and evening; Thomas 2002) are temporally separated from those when most oviposition takes place (mid-day and afternoon; M. A. Fürst 2006–2008, personal observations). Another possible constraint is the limited oviposition time available to Maculinea females in the field (Kőrösi et al. 2008), which may make delays in oviposition choice based on ant discrimination costly.
Alternatively, it is possible that ant-dependent oviposition has not been selected because more effective exploitation of the host ants by more effective oviposition strategies may lead to overexploitation of the host ant population and therefore to increased extinction risk.
Our study has clear implications for the conservation of endangered Maculinea species. Lack of ant-dependent oviposition means that active management of sites to increase the overlap of food plant and host ant distributions is required, as the butterflies cannot be relied upon to find their own host ants when rare.
Acknowledgements
We are grateful to Maëlle Durey, Félix Teillard, Elisabeth Stafflinger, Rune Ritz, Birgit Meyer and Nicky Bos for help in the field and the Center for Social Evolution for providing a great working environment. Funding was via a Danish Natural Science Foundation grant to the Center for Social Evolution.
References
- Als T. D., Nash D. R., Boomsma J. J.2002Geographical variation in host-ant specificity of the parasitic butterfly Maculinea alcon in Denmark. Ecol. Entomol. 27, 403–414 (doi:10.1046/j.1365-2311.2002.00427.x) [Google Scholar]
- Als T., Vila R., Kandul N., Nash D. R., Yen S. H., Hsu Y., Mignault A., Boomsma J. J., Pierce N. E.2004The evolution of alternative parasitic life histories in large blue butterflies. Nature 432, 386–390 (doi:10.1038/nature03020) [DOI] [PubMed] [Google Scholar]
- Atsatt P. R.1981Ant-dependent food plant selection by the mistletoe butterfly Ogyris amaryllis (Lycaenidae). Oecologia 48, 60–63 (doi:10.1007/BF00346988) [DOI] [PubMed] [Google Scholar]
- Fiedler K., Maschwitz U.1989The symbiosis between the weaver ant Oecophylla smaragdina, and Anthene emolus, an obligate myrmecophilous lycaenid butterfly. J. Nat. Hist. 23, 833–846 (doi:10.1080/00222938900770441) [Google Scholar]
- Figurny E., Woyciechowski M.1998Flowerhead selection for oviposition by females of the sympatric butterfly species Maculinea teleius and M. nausithous (Lepidoptera: Lycaenidae). Entomol. Gen. 23, 215–222 [Google Scholar]
- Fraser A. M., Tregenza T., Wedell N., Elgar M. A., Pierce N. E.2002Oviposition tests of ant preference in a myrmecophilous butterfly. J. Evol. Biol. 15, 861–870 (doi:10.1046/j.1420-9101.2002.00434.x) [Google Scholar]
- Hansson B. S.1995Olfaction in Lepidoptera. Experientia 51, 1003–1027 (doi:10.1007/BF01946910) [Google Scholar]
- Henning S. F.1983Biological groups within the Lycaenidae (Lepidoptera). J. Entomol. Soc. S. Afr. 46, 65–85 [Google Scholar]
- Kőrösi A., Örvössy N., Batáry P., Kövér S., Peregovits L.2008Restricted within-habitat movement and time-constrained egg laying of female Maculinea rebeli butterflies. Oecologia 156, 455–464 (doi:10.1007/s00442-008-0986-1) [DOI] [PubMed] [Google Scholar]
- Musche M., Anton C., Worgan A., Settele J.2006No experimental evidence for host ant related oviposition in a parasitic butterfly. J. Insect Behav. 19, 631–643 (doi:10.1007/s10905-006-9053-0) [Google Scholar]
- Nowicki P., Witek M., Skorka P., Woyciechowski M.2005Oviposition patterns in the myrmecophilous butterfly Maculinea alcon Denis & Schiffermüller (Lepidoptera: Lycaenidae) in relation to characteristics of foodplants and presence of ant hosts. Pol. J. Ecol. 53, 409–417 [Google Scholar]
- Pierce N. E., Elgar M. A.1985The influence of ants on host plant selection by Jalmenus evagoras, a myrmecophilous lycaenid butterfly Behav. Ecol. Sociobiol. 16, 209–222 [Google Scholar]
- Pierce N. E., Braby M. F., Heath A., Lohman D. J., Mathew J., Rand D. B., Travassos M. A.2002The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu. Rev. Entomol. 47, 733–771 (doi:10.1146/annurev.ento.47.091201.145257) [DOI] [PubMed] [Google Scholar]
- Renwick J. A. A.1989Chemical ecology of oviposition in phytophagous insects. Experientia 45, 223–228 (doi:10.1007/BF01951807) [Google Scholar]
- Thomas J. A.2002Larval niche selection and evening exposure enhance adoption of a predacious social parasite, Maculinea arion (large blue butterfly), by Myrmica ants. Oecologia 132, 531–537 [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Elmes G. W.2001Food-plant niche selection rather than the presence of ant nests explains oviposition patterns in the myrmecophilous butterfly genus Maculinea. Proc. R. Soc. Lond. B 268, 471–477 (doi:10.1098/rspb.2000.1398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Elmes G. W., Wardlaw J. C., Woyciechowski M.1989Host specificity among Maculinea butterflies in Myrmica ant nests. Oecologia 79, 452–457 (doi:10.1007/BF00378660) [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Elmes G. W., Clarke R. T., Kim K. G., Munguira M. L., Hochberg M. E.1997Field evidence and model predictions of butterfly-mediated apparent competition between gentian plants and red ants. Acta Oecologica 18, 671–684 (doi:10.1016/S1146-609X(97)80050-1) [Google Scholar]
- Vander Meer R. K., Alonso L. E.1998Pheromone directed behavior in ants. In Pheromone communication in social insects: ants, wasps, bees and termites (eds Vander Meer R. K., Breed M. D., Espelie K. E., Winston M. L.), pp. 159–192 Oxford, UK: Westview Press [Google Scholar]
- van Dyck H., Oostermeijer J. G. B., Talloen W., Feenstra V., van der Hidde A., Wynhoff I.2000Does the presence of ant nests matter for oviposition to a specialized myrmecophilous Maculinea butterfly? Proc. R. Soc. Lond. B 267, 861–866 (doi:10.1098/rspb.2000.1082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D., Kurina L.1997The influence of ants and water availability on oviposition behavior and survivorship of a facultatively ant-tended herbivore. Ecol. Entomol. 22, 352–360 (doi:10.1046/j.1365-2311.1997.00077.x) [Google Scholar]
- Wynhoff I., Grutters M., van Langevelde F.2008Looking for the ants: selection of oviposition sites by two myrmecophilous butterfly species. Anim. Biol. 58, 371–388 (doi:10.1163/157075608X383683) [Google Scholar]