Abstract
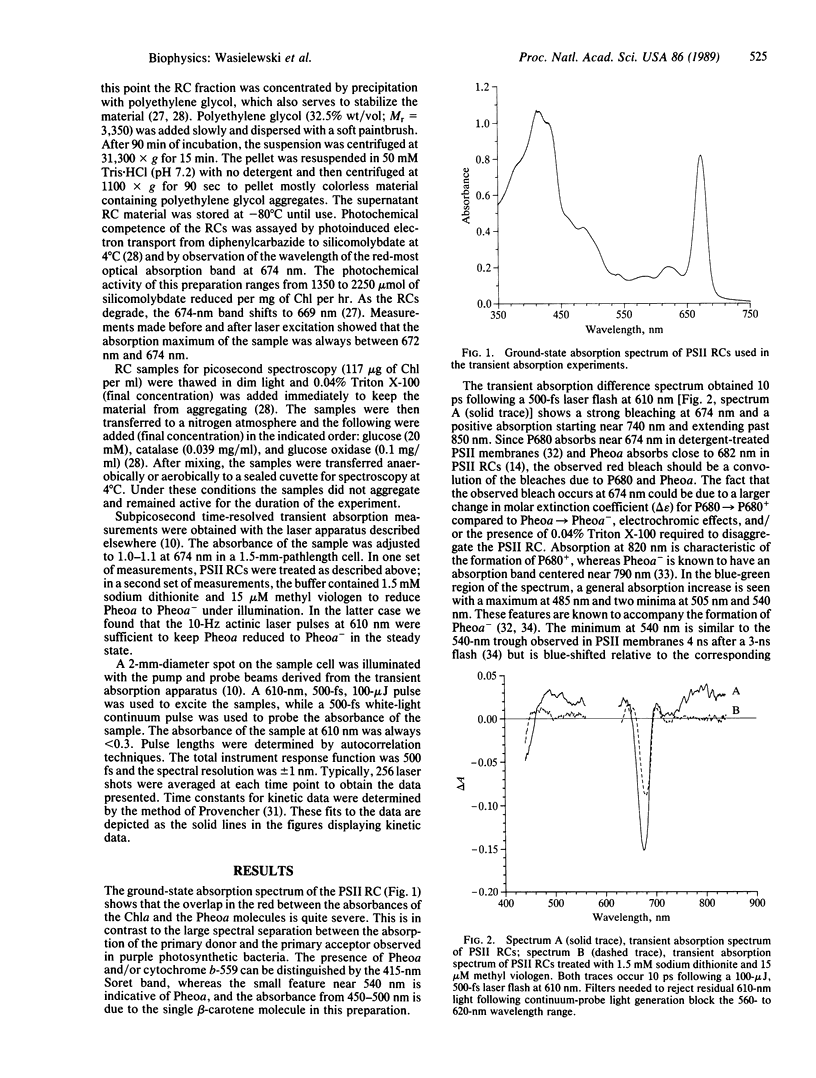
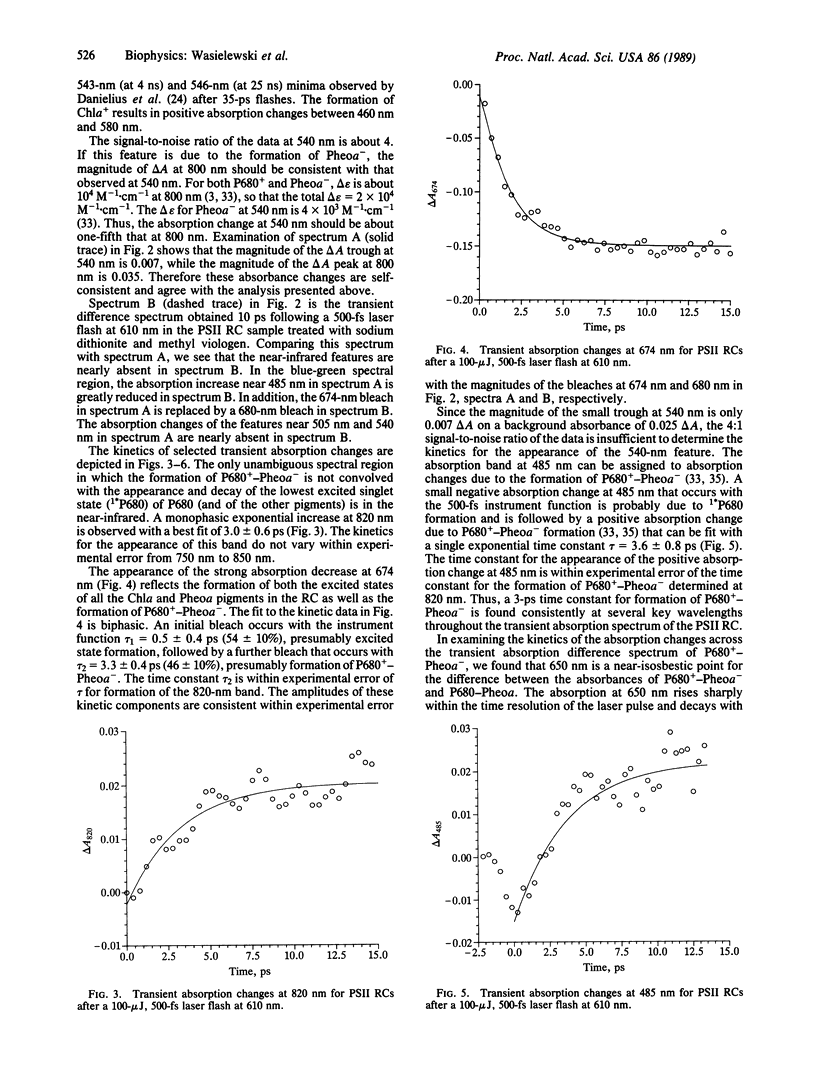
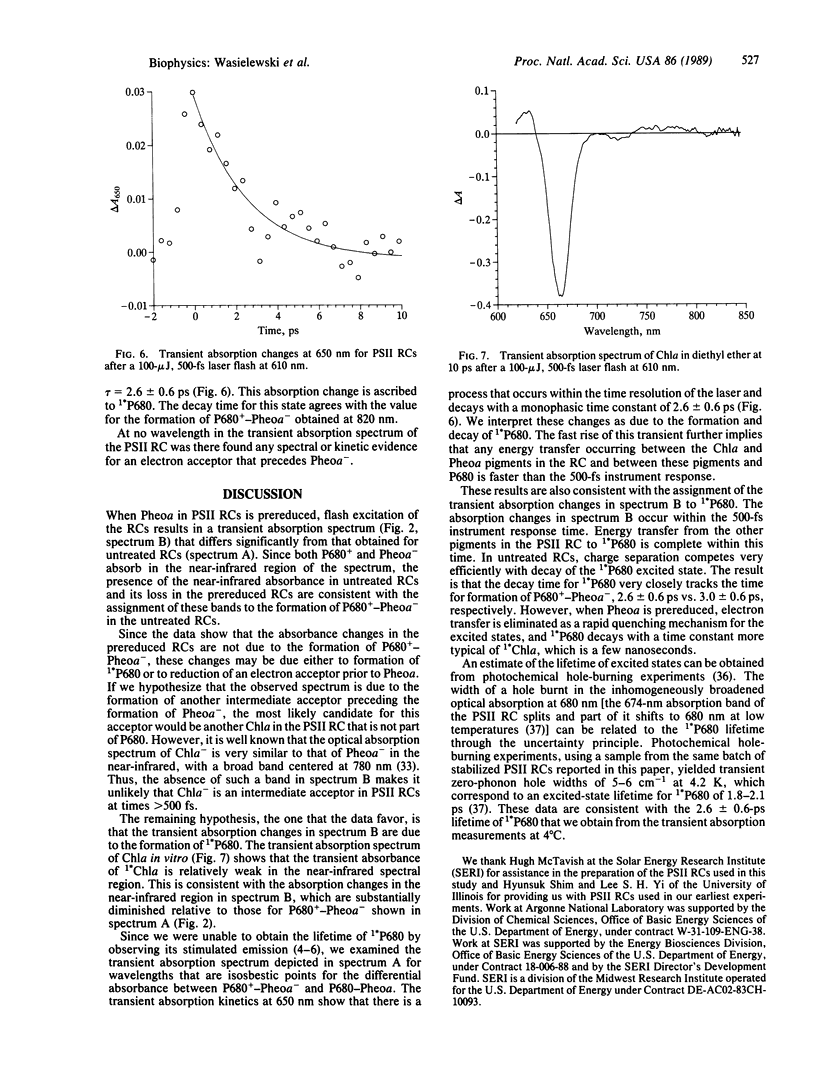
We have measured directly the rate of formation of the oxidized chlorophyll a electron donor (P680+) and the reduced electron acceptor pheophytin a- (Pheoa-) following excitation of isolated spinach photosystem II reaction centers at 4°C. The reaction-center complex consists of D1, D2, and cytochrome b-559 proteins and was prepared by a procedure that stabilizes the protein complex. Transient absorption difference spectra were measured from 440 to 850 nm as a function of time with 500-fs resolution following 610-nm laser excitation. The formation of P680+-Pheoa- is indicated by the appearance of a band due to P680+ at 820 nm and corresponding absorbance changes at 505 and 540 nm due to formation of Pheoa-. The appearance of the 820-nm band is monoexponential with τ = 3.0 ± 0.6 ps. The time constant for decay of 1*P680, the lowest excited singlet state of P680, monitored at 650 nm, is τ = 2.6 ± 0.6 ps and agrees with that of the appearance of P680+ within experimental error. Treatment of the photosystem II reaction centers with sodium dithionite and methyl viologen followed by exposure to laser excitation, conditions known to result in accumulation of Pheoa-, results in formation of a transient absorption spectrum due to 1*P680. We find no evidence for an electron acceptor that precedes the formation of Pheoa-.
Keywords: electron transfer, ultrafast spectroscopy, photosynthesis, Spinacia oleracea
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G., Yeates T. O., Komiya H., Rees D. C. Structure of the reaction center from Rhodobacter sphaeroides R-26: the protein subunits. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6162–6166. doi: 10.1073/pnas.84.17.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton J., Martin J. L., Migus A., Antonetti A., Orszag A. Femtosecond spectroscopy of excitation energy transfer and initial charge separation in the reaction center of the photosynthetic bacterium Rhodopseudomonas viridis. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5121–5125. doi: 10.1073/pnas.83.14.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Tiede D., Tang J., Smith U., Norris J., Schiffer M. Structure of Rhodopseudomonas sphaeroides R-26 reaction center. FEBS Lett. 1986 Sep 1;205(1):82–86. doi: 10.1016/0014-5793(86)80870-5. [DOI] [PubMed] [Google Scholar]
- Davis M. S., Forman A., Fajer J. Ligated chlorophyll cation radicals: Their function in photosystem II of plant photosynthesis. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4170–4174. doi: 10.1073/pnas.76.9.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Jankowiak R., Small G. J. Hole-burning spectroscopy and relaxation dynamics of amorphous solids at low temperatures. Science. 1987 Aug 7;237(4815):618–625. doi: 10.1126/science.237.4815.618. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Breton J., Hoff A. J., Migus A., Antonetti A. Femtosecond spectroscopy of electron transfer in the reaction center of the photosynthetic bacterium Rhodopseudomonas sphaeroides R-26: Direct electron transfer from the dimeric bacteriochlorophyll primary donor to the bacteriopheophytin acceptor with a time constant of 2.8 +/- 0.2 psec. Proc Natl Acad Sci U S A. 1986 Feb;83(4):957–961. doi: 10.1073/pnas.83.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. H., Brock H., Holzwarth A. R. Picosecond kinetics of fluorescence and absorbance changes in photosystem II particles excited at low photon density. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8414–8418. doi: 10.1073/pnas.84.23.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert M., Picorel R., Rubin A. B., Connolly J. S. Spectral, Photophysical, and Stability Properties of Isolated Photosystem II Reaction Center. Plant Physiol. 1988 Jun;87(2):303–306. doi: 10.1104/pp.87.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gorkom H. J., Pulles M. P., Wessels J. S. Light-induced changes of absorbance and electron spin resonance in small photosystem II particles. Biochim Biophys Acta. 1975 Dec 11;408(3):331–339. doi: 10.1016/0005-2728(75)90134-6. [DOI] [PubMed] [Google Scholar]
- Woodbury N. W., Becker M., Middendorf D., Parson W. W. Picosecond kinetics of the initial photochemical electron-transfer reaction in bacterial photosynthetic reaction centers. Biochemistry. 1985 Dec 17;24(26):7516–7521. doi: 10.1021/bi00347a002. [DOI] [PubMed] [Google Scholar]
- Yeates T. O., Komiya H., Rees D. C., Allen J. P., Feher G. Structure of the reaction center from Rhodobacter sphaeroides R-26: membrane-protein interactions. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6438–6442. doi: 10.1073/pnas.84.18.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]



