Abstract
If a mother's nutritional status predicts the nutritional environment of the offspring, it would be adaptive for mothers experiencing nutritional stress to prime their offspring for a better tolerance to poor nutrition. We report that in Drosophila melanogaster, parents raised on poor larval food laid 3–6% heavier eggs than parents raised on standard food, despite being 30 per cent smaller. Their offspring developed 14 h (4%) faster on the poor food than offspring of well-fed parents. However, they were slightly smaller as adults. Thus, the effects of parental diet on offspring performance under malnutrition apparently involve both adaptive plasticity and maladaptive effects of parental stress.
Keywords: maternal effects, parental effects, egg size, nutritional stress, plasticity, Drosophila
1. Introduction
Parental genotype and environment often influence offspring fitness through non-genetically transmitted parental effects. Such effects may be maladaptive, e.g. malnourished parents may produce offspring of poorer quality (parental stress hypothesis). However, parents may also respond to environmental cues in ways that enhance offspring fitness. In particular, if the nutritional conditions experienced by the mother and offspring are positively correlated, mothers subject to nutritional stress would be favoured to induce plastic changes in the offspring that make the latter more tolerant to nutritional stress. This adaptive hypothesis thus predicts that fitness of offspring on poor diet would be enhanced if their parents also experienced poor diet (Mousseau & Fox 1998; Badyaev & Uller 2009).
One potential mechanism of such adaptive parental effects involves adjustment of investment per offspring, which in organisms lacking parental care can be approximated by egg or newborn size (Azevedo et al. 1997; Mousseau & Fox 1998). Life history theory predicts that under adverse conditions the optimal trade-off between offspring size and number is expected to shift towards fewer but better provisioned offspring (Smith & Fretwell 1974; Roff 1992). Natural selection should thus favour mothers that invest more in individual offspring in response to cues indicative that offspring would experience nutritional stress. One such cue would be the mother's own nutritional environment.
The prevalence of such adaptive parental effects remains unclear. An increase in egg or newborn size in response to poor parental nutrition or high competition has been reported, e.g. in seed beetles (Kawecki 1995), cockroaches (Barrett et al. 2009), Daphnia (McKee & Ebert 1996) and bryozoans (Allen et al. 2008), but more often a decrease or no effect was observed (reviewed by Fox & Czesak 2000). Beneficial effects of poor parental nutrition on offspring themselves facing nutritional stress have been observed, e.g. in Daphnia (Gliwicz & Guisande 1992), butterflies (Rotem et al. 2003) and mosquitoes (Grech et al. 2007), similar plasticity has been suggested in humans (Hales & Barker 2001). However, there are numerous examples of adverse effects of even mild parental nutritional stress (e.g. Diss et al. 1996; Jones & Widemo 2005; Kyneb & Toft 2006; Bonduriansky & Head 2007).
Here, we study the effects of parental larval nutrition (poor versus standard) in Drosophila melanogaster. First, we test the adaptive hypothesis that females raised on a poor diet should produce larger eggs, despite having a smaller body size. Second, we study the effect of parental nutrition on offspring fitness traits (egg-to-adult viability, developmental time and adult body size). A simple stress hypothesis would predict that parents raised on a poor diet would produce offspring of low quality, which survive poorly, take longer to develop and reach a smaller adult size than offspring of well-fed parents. These differences would be particularly manifested if the offspring themselves also developed under nutritional stress. In contrast, according to the adaptive maternal effects hypothesis, mothers raised on poor food would ‘prime’ their offspring for development under nutritional stress such that when the offspring themselves are raised on poor food, they perform better than the offspring of mother raised on a richer diet.
In the only Drosophila study that addressed these questions, mothers maintained on poor food tended to lay larger eggs, but the difference was not significant. Furthermore, poorly fed mothers produced offspring that survived better to adulthood on a rich food, compared with offspring of well-fed mothers, with no difference on the poor food (Prasad et al. 2003). This pattern is not predicted by either the stress hypothesis or the adaptive maternal effects hypothesis. However, the media used in that study differed in the type as well as the concentration of nutrients, with the poor food containing a greater amount of starch than the rich food. In our study, food quality was reduced by diluting the standard food recipe. In contrast to Prasad et al. (2003), we only manipulated the parental larval diet; all parents were maintained as adults on standard food. This may be more ecologically relevant; in nature Drosophila adults tend to use a greater variety of food sources than larvae (Shorrocks 1975).
2. Material and methods
We used an inbred laboratory strain Canton-S (additional data on two strains recently derived from nature are reported in the electronic supplementary material). The stock had been maintained for several years in our laboratory on a cornmeal medium (30 g sucrose, 60 g glucose, 12.5 g dry yeast, 50 g cornmeal, 0.5 g MgSO4, 0.5 g CaCl2, 30 ml ethanol, 6 ml propionic acid and 1 g nipagin l−1; henceforth referred as standard food).
Flies for the parental generation were raised at a density of 100 eggs per vial, at 25°C with 30 ml of either the standard food or poor food containing one quarter of the amounts of sugars, yeast and cornmeal relative to the standard food. The assays were carried in two separate experiments. In experiment 1, vials with parents raised on poor food were initiated 4 days earlier than those on standard food, to compensate for the development on poor food taking 4 days longer. The eggs for the measurement of eggs size and offspring traits were collected when parents on both food types were 4–6 days old counting from eclosion. In experiment 2, the parental generation on both food types was established simultaneously and eggs were collected 20 days later, when the parents raised on the poor food were 4–6 days old and those raised on standard food were 8–10 days old. In both experiments, the parents were transferred to new vials with standard food and live yeast 2 days before egg collection, which occurred in mass oviposition.
For egg weight, five batches of 30 eggs (from parents raised in five different vials) per experiment × maternal diet combination were rinsed with water, dried on filter paper and weighed to the nearest microgram. To assay offspring traits, four vials with standard food and four with poor food (arranged in two blocks obtained from parents raised in two different vials and offset by several days) were set up for each experiment × parental diet combination, each seeded with 100 eggs. The number of larvae pupating in each vial was scored every 24 h to estimate the time to pupation and pupation success. Adults eclosing daily in each vial were counted and collected (to obtain egg-to-adult viability and developmental time). Twelve females were randomly chosen from the day of peak emergence in each vial, dried at 70°C for 3 days and weighed individually.
To simplify the analysis, we first calculated the mean egg weight per batch and the means of offspring traits per vial. Viability (the proportion of eggs that developed into adults) was angularly transformed. The pupal period was estimated as the difference between the egg-to-adult developmental time and the time to pupation. These values were analysed with ANOVA using JMP statistical software. Parental diet, offspring diet and experiment were fixed factors; block was included as a random factor nested within experiment. For offspring traits, we also carried out separate analysis for the two levels of offspring diet. Interactions and block effect with p > 0.2 were excluded from the final models.
3. Results
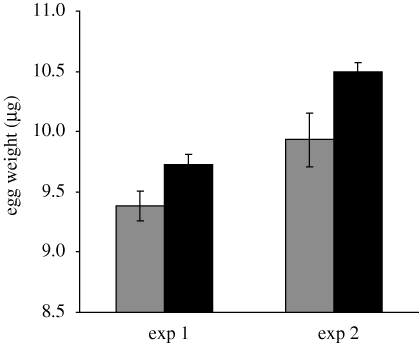
Females raised on poor food laid heavier eggs than mothers raised on standard food (F1,16 = 10.58, p = 0.005; figure 1). This effect was consistent between experiments (interaction F1,16 = 0.66, p = 0.43); the overall greater weight of eggs in experiment 2 (F1,16 = 22.4, p = 0.0002) is likely due to some difference in egg handling (the order of weighing was randomized within experiments).
Figure 1.
Egg weight (mean ± s.e.) as a function of maternal larval diet. Parental diet: grey bar, standard and black bar, poor.
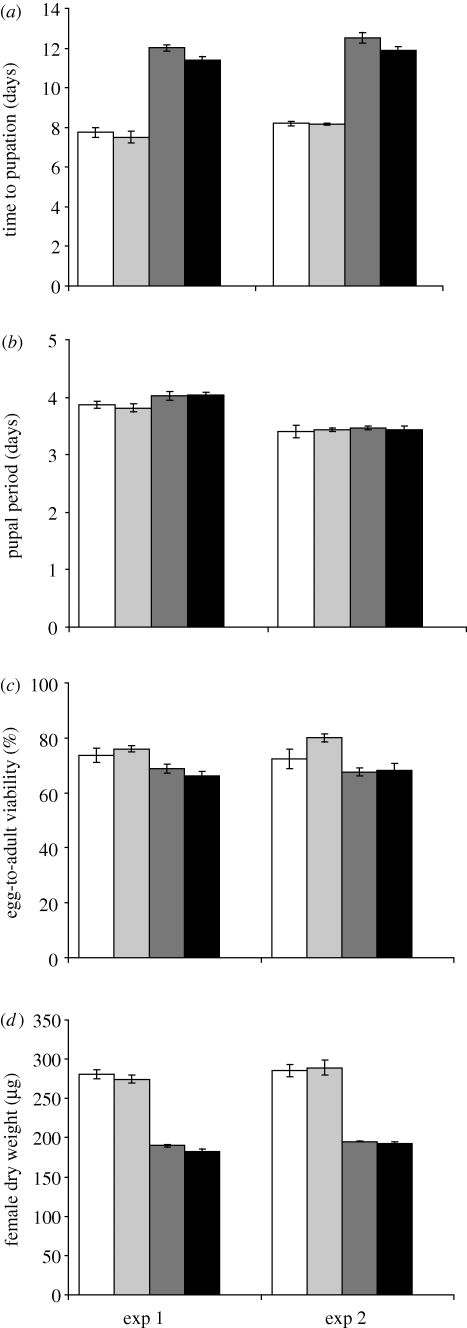
In both experiments offspring raised on poor food pupated earlier and showed a slightly smaller adult weight if their parents also developed on poor food (figure 2, table 1). No effect of parental diet was detected if the offspring were raised on the standard food, although the parental × offspring diet interaction was only marginally significant for time to pupation. In contrast, poor parental diet improved viability on standard but not on poor food, particularly in experiment 2 (figure 2, table 1). The length of the pupal period was unaffected by parental diet (figure 2, table 1).
Figure 2.
Offspring traits (means ± s.e.) as a function of parental and offspring diets: (a) time from oviposition to pupation, (b) the duration of the pupal stage, (c) egg-to-adult viability and (d) female dry weight. Unfilled bar, standard parental and standard offspring diet; light grey bar, poor parental and standard offspring diet; dark grey bar, standard parental and poor offspring diet and black bar, poor parental and poor offspring diet.
Table 1.
Summary of the results of analyses of variance (F-statistics and their significance) on the offspring traits, analysed jointly on both offspring diets and separately for each level of offspring diet. Interactions indicated with ‘—’ had p > 0.2 and were excluded from the model.
| both offspring diets |
||||||||
|---|---|---|---|---|---|---|---|---|
| offspring diet | parental diet | exp | block | offspring × parental diet |
offspring diet × exp | parental diet × exp | ||
| time to pupation | F1,25=1020.6*** | F1.25=8.8*** | F1,25=7.0 | F2,25=2.5 | F1,25=3.5† | — | — | |
| pupal period | F1,25=7.0† | F1,25=0.1 | F1,25=39.7* | F2,25=3.6* | — | F1,25=3.8† | — | |
| egg–adult viability | F1,24=33.4*** | F1.24=2.5 | F1,24=0.2 | F2,24=3.0† | F1,24=4.7* | — | F1,24=2.7 | |
| female dry weight | F1,28=665.5*** | F1,28=0.6 | F1,28=5.8* | — | — | — | — | |
| standard offspring food |
poor offspring food |
|||||||
| parental diet |
exp |
block |
parental diet × exp |
parental diet |
exp |
block |
parental diet × exp |
|
| time to pupation | F1,13=0.5 | F1,13=8.5* | — | — | F1,11=33.2*** | F1,11=1.4 | F2,11=15.7*** | — |
| pupal period | F1,11=0.04 | F1,11=21.8* | F2,11=1.6 | — | F1,11=0.01 | F1,11=31.6* | F2,11=5.7* | — |
| egg–adult viability | F1,10=8.0* | F1,10=0.1 | F2,10=6.6* | F1,10=2.6 | F1,13=0.2 | F1,13=0.1 | — | — |
| female dry weight | F1,11=0.01 | F1,11=1.6 | F2,11=1.2 | — | F1,13=6.5* | F1,13=15.9** | — | — |
Exp: experiment. All remaining p > 0.1. †p < 0.08; *p < 0.05; **p < 0.01; **p < 0.001.
4. Discussion
Females raised on poor food weigh 30 per cent less than those raised on the standard food (see also Kolss et al. 2009). Given that intraspecific correlations between maternal size and egg size in arthropods are typically positive (Azevedo et al. 1997), one would expect their eggs also to be smaller. Yet, in both experiments females raised on the poor food laid 3–6% heavier eggs, confirming the trend observed by Prasad et al. (2003) and indicating a specific, evolved plastic response. The same response was also observed in two outbred strains recently derived from natural populations (electronic supplementary material). Presumably, the larger egg size reflects enhanced egg provisioning, consistent with the adaptive response predicted by life history theory (Smith & Fretwell 1974).
The prediction of the adaptive parental effects hypothesis was only upheld for one offspring trait, time from oviposition to pupation on poor food: parents raised on poor food produced offspring that pupated on average about 14 h (4%) earlier than offspring of parents raised on standard food. No such effect was detected when the offspring developed on standard food; this (marginally significant) interaction between maternal and offspring diet is also consistent with the adaptive parental effects hypothesis. Development on poor food is generally slow, and under these conditions being able to develop faster may be particularly advantageous. First, the already initially poor nutritional environment will deteriorate as the meagre resources are used up by competing larvae and waste products accumulate. Second, even in the absence of competition the larval food sources (decomposing fruit) are likely to become increasingly unsuitable due to rotting or desiccation.
Other offspring traits did not conform to the adaptive parental effects hypothesis. The slightly smaller body weight of daughters whose parents were raised on poor food possibly reflects a trade-off with the faster development of these offspring, but is also consistent with the maternal stress hypothesis. Poor parental food enhanced viability of Canton-S flies on standard rather than poor food, similar to a result reported by Prasad et al. (2003). However, additional data from two other strains indicate that this viability effect may be strain specific, in contrast to the effect on time to pupation and body weight, which were consistent among strains (electronic supplementary material).
While in principle a non-genetic paternal effect on developmental time or body weight cannot be excluded, such paternal effects have been rarely observed in Drosophila (Pitnick & Karr 1998). Thus, the effects of parental diet on developmental time and weight of offspring raised on poor food are presumably mediated by maternal effects. We cannot say to what extent the faster development of offspring of parents raised on poor food is due to the larger egg size rather than to maternal effects mediated otherwise. On the one hand, larger egg size in insects, including Drosophila, is typically associated with shorter development (Azevedo et al. 1997). On the other hand, egg size also usually correlates positively with larval viability and adult size (Azevedo et al. 1997); if anything, offspring of mothers raised on the poor food showed opposite trends.
One can speculate that without the plastic increase in eggs size, the reduction in offspring viability and body size would be even greater. If so, both the parental stress hypothesis and the adaptive parental effects hypothesis may be true. A parental history of malnourishment may have an adverse effect on some aspect of offspring performance, but adaptive plastic responses may act to alleviate these adverse effects. For some traits (here time to pupation) the plastic response would be strong enough for the offspring of the malnourished parents to perform better under nutritional stress than the offspring of well-fed parents. This would require that the effects of parental diet on offspring performance are mediated by multiple underlying variables rather than just egg size, which is also what the results of this study suggest.
Acknowledgements
We thank F. Rosengren and A. Von Ungern-Sternberg for help with the experiments, and two reviewers for comments. This work was supported by the Swiss National Science Foundation.
References
- Allen R. M., Buckley Y. M., Marshall D. J.2008Offspring size plasticity in response to intraspecific competition: an adaptive maternal effect across life-history stages. Am. Nat. 171, 225–237 (doi:10.1086/524952) [DOI] [PubMed] [Google Scholar]
- Azevedo R. B. R., French V., Partridge L.1997Life-history consequences of egg size in Drosophila melanogaster. Am. Nat. 150, 250–282 (doi:10.1086/286065) [DOI] [PubMed] [Google Scholar]
- Badyaev A. V., Uller T.2009Parental effects in ecology and evolution: mechanisms, processes and implications. Philos. Trans. R. Soc. B 364, 1169–1177 (doi:10.1098/rstb.2008.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. L. B., Moore A. J., Moore P. J.2009Diet and social conditions during sexual maturation have unpredictable influences on female life history trade-offs. J. Evol. Biol. 22, 571–581 (doi:10.1111/j.1420-9101.2008.01671.x) [DOI] [PubMed] [Google Scholar]
- Bonduriansky R., Head M.2007Maternal and paternal condition effects on offspring phenotype in Telostylinus angusticollis (Diptera: Neriidae). J. Evol. Biol. 20, 2379–2388 (doi:10.1111/j.1420-9101.2007.01419.x) [DOI] [PubMed] [Google Scholar]
- Diss A. L., Kunkel J. G., Montgomery M. E., Leonard D. E.1996Effects of maternal nutrition and egg provisioning on parameters of larval hatch, survival and dispersal in the gypsy moth, Lymantria dispar L. Oecologia 106, 470–477 (doi:10.1007/BF00329704) [DOI] [PubMed] [Google Scholar]
- Fox C. W., Czesak M. E.2000Evolutionary ecology of progeny size in arthropods. Annu. Rev. Entomol. 45, 341–369 (doi:10.1146/annurev.ento.45.1.341) [DOI] [PubMed] [Google Scholar]
- Gliwicz Z. M., Guisande C.1992Family-planning in Daphnia—resistance to starvation in offspring born to mothers grown at different food levels. Oecologia 91, 463–467 (doi:10.1007/BF00650317) [DOI] [PubMed] [Google Scholar]
- Grech K., Maung L. A., Read A. F.2007The effect of parental rearing conditions on offspring life history in Anopheles stephensi. Malaria J. 6, 130 (doi:10.1186/1475-2875-6-130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. N., Barker D. J. P.2001The thrifty phenotype hypothesis. Br. Med. Bull. 60, 5–20 (doi:10.1093/bmb/60.1.5) [DOI] [PubMed] [Google Scholar]
- Jones T. M., Widemo F.2005Survival and reproduction when food is scarce: implications for a lekking Hawaiian Drosophila. Ecol. Entomol. 30, 397–405 (doi:10.1111/j.0307-6946.2005.00705.x) [Google Scholar]
- Kawecki T. J.1995Adaptive plasticity of egg size in response to competition in the cowpea weevil, Callosobruchus maculatus (Coleoptera, Bruchidae). Oecologia 102, 81–85 [DOI] [PubMed] [Google Scholar]
- Kolss M., Vijendravarma R. K., Schwaller G., Kawecki T. J.2009Life-history consequences of adaptation to larval nutritional stress in Drosophila. Evolution 63, 2389–2401 [DOI] [PubMed] [Google Scholar]
- Kyneb A., Toft S.2006Effects of maternal diet quality on offspring performance in the rove beetle Tachyporus hypnorum. Ecol. Entomol. 31, 322–330 (doi:10.1111/j.1365-2311.2006.00775.x) [Google Scholar]
- McKee D., Ebert D.1996The interactive effects of temperature, food level and maternal phenotype on offspring size in Daphnia magna. Oecologia 107, 189–196 (doi:10.1007/BF00327902) [DOI] [PubMed] [Google Scholar]
- Mousseau T. A., Fox C. W.1998The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407 (doi:10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- Pitnick S., Karr T. L.1998Paternal products and by-products in Drosophila development. Proc. R. Soc. Lond. B 265, 821–826 (doi:10.1098/rspb.1998.0366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad N. G., Shakarad M., Rajamani M., Joshi A.2003Interaction between the effects of maternal and larval levels of nutrition on pre-adult survival in Drosophila melanogaster. Evol. Ecol. Res. 5, 903–911 [Google Scholar]
- Roff D. A.1992The evolution of life histories New York, NY: Chapman and Hall [Google Scholar]
- Rotem K., Agrawal A. A., Kott L.2003Parental effects in Pieris rapae in response to variation in food quality: adaptive plasticity across generations? Ecol. Entomol. 28, 211–218 (doi:10.1046/j.1365-2311.2003.00507.x) [Google Scholar]
- Shorrocks B.1975Distribution and abundance of woodland species of British DrosophiIa (Diptera, Drosophilidae). J. Anim. Ecol. 44, 851–864 (doi:10.2307/3723) [Google Scholar]
- Smith C. C., Fretwell S. D.1974Optimal balance between size and number of offspring. Am. Nat. 108, 499–506 (doi:10.1086/282929) [Google Scholar]