Abstract
Despite decades of interest, adaptive explanations for biased offspring sex ratios in mammals remain contentious, largely because direct tests of the underlying fitness assumptions of adaptive hypotheses are rarely conducted. These tests are complicated by the difficulty of manipulating offspring sex prior to significant maternal investment owing to the biological constraints of viviparity. We test the adaptive advantage of sex allocation through cross-fostering offspring by sex in tammar wallabies. We examine whether offspring sex is correlated with maternal investment ability (i.e. Trivers–Willard hypothesis, TWH). In addition, we test the assumption that maternal investment has a greater influence on the fitness of sons than of daughters. We failed to find statistical support for maternal investment ability influencing a son's weaning success or body size more than a daughter's, although this result was probably owing to small sample sizes. In support of the TWH, females that gave birth to a son had higher investment ability (likelihood of weaning an offspring) regardless of the sex of offspring fostered.
Keywords: sex allocation, sex ratio, marsupial, maternal condition, Trivers–Willard hypothesis, cross-fostering
1. Introduction
Despite a large amount of empirical research, consensus support for biases in offspring sex ratio in mammals representing adaptive sex allocation has not emerged (Cockburn et al. 2002; Sheldon & West 2004; West 2009). The lack of clear support is owing, in part, to the infrequency of tests of the underlying assumptions of sex allocation hypotheses (Hewison & Gaillard 1999). The Trivers–Willard hypothesis (TWH) predicts that, where variation in reproductive success is greater in males than in females, the probability that an individual mother produces a son, rather than a daughter, increases with her capacity to invest (Trivers & Willard 1973). The hypothesis assumes that sons gain more in reproductive success from being produced by a mother of relatively good condition than daughters do, and has subsequently been broadened to assume that sex-differential maternal effects exist for at least one component of offspring lifetime fitness (juvenile survival, adult lifespan or reproductive success; e.g. Schwanz et al. 2006). However, this assumption has rarely been tested (Hewison & Gaillard 1999).
The ideal test of TWH is to manipulate offspring sex and determine whether offspring fitness depends on maternal condition in a sex-specific fashion, and whether mothers invest in offspring sex according to these fitness differences. Cross-fostering by sex provides several advantages over correlative studies. First, it decouples maternal investment ability from sex-specific response to investment by offspring (e.g. male-specific vulnerability to resource limitation; Clutton-Brock et al. 1985). Maternal ‘condition’ is measured functionally as a female's ability to rear relatively fit offspring, thus removing the necessity to rely upon potentially erroneous morphological measures of the condition (Cameron 2004; Sheldon & West 2004). Second, cross-fostering provides data on offspring fitness across a range of maternal condition, which may not be available in natural systems.
Manipulation of offspring sex ratio has, to our knowledge, only been performed once in a mammal after non-negligible amounts of maternal investment had occurred (Koskela et al. 2009). Cross-fostering is ideally performed prior to any resource investment by mothers, for which marsupials provide an inherent advantage given the early stages of development at which birth occurs (e.g. prior to 90% of maternal investment; Hayssen et al. 1985). We present results from an experiment in tammar wallabies (Macropus eugenii derbianus) where offspring were cross-fostered by sex to test (i) the main TWH prediction that more sons are produced by mothers with relatively greater investment ability and (ii) the TWH assumption that sons gain more in fitness than daughters from being reared by mothers of greater investment ability. We monitored weaning success and offspring growth over a year to test whether the production of a given sex represents an adaptive strategy based on maternal investment ability (Sunnucks & Taylor 1997). Weaning success provides one component of offspring fitness, along with phenotypic quality, and persistent maternal effects on the size of sons provides an indicator of potential adult reproductive success (Rudd 1994).
2. Material and methods
(a). Experimental protocol
Thirty-two adult female tammar wallabies (M. eugenii derbianus) were captured in Tutanning Nature Reserve, Western Australia (32°33′ S, 117°20′ E) in April 2008. At the time of capture, all females were carrying a single pouch young (13 carrying daughters, 19 carrying sons) of less than 100 days of age (based on growth tables; Poole et al. 1991). Females were weighed (g), measured (pes (foot) length, mm) and ear-tagged. Cross-fostering was performed using the established protocol (Taggart 2002). Young were weighed (g), sexed (via visual inspection of genital region), micro-chipped, assigned to one of the five treatment groups and reattached to teats. Treatments were based on the sex birthed and the sex fostered by the mother (birthed/fostered): (i) female/female, n = 5, (ii) female/male, n = 6, (iii) male/female, n = 6, (iv) male/male, n = 9, and (v) mothers that reared their own offspring following brief removal from the teat (sham manipulation, n = 6, four males and two females). All cross-fostering was performed with offspring of equivalent developmental stages to avoid problems owing to changes in milk composition over the course of lactation that impacts growth rate (Findlay & Renfree 1984).
Following cross-fostering, females were transported to the University of Western Australia's Native Animal Facility where they were housed in six replicate naturally vegetated outdoor enclosures, with treatments spread among enclosures, supplemented with ad libitum Kangaroo cubes (Glen Forrest Stockfeeders, Western Australia), mixed vegetables and water. Prior to release into their new enclosures, females were examined to ensure pouch young had successfully reattached to the teat. We measured offspring survival and body size at weaning (November 2008, approx. 300 days old) and approximately 1 year of age (May 2009). We additionally measured maternal body mass and condition (see below) over the course of lactation.
(b). Statistical analysis
Maternal condition index (MCI) was calculated as the residuals from a regression between maternal mass (MM) and pes length (both variables log-transformed). The influence of offspring sex on MM and MCI at weaning was analysed by ANOVA with the treatment group as the variable. The influence of MM and MCI at cross-fostering on offspring sex was examined with separate logistic regressions. To determine which factors affected weaning success (weaned or failed) we performed logistic model selection using stepwise elimination. The full model contained the predictors birth sex, foster sex, birth sex*foster sex and MM and MCI at cross-fostering. Significance of each variable was assessed by likelihood ratio tests of the difference in deviance of the model with and without a variable. The likelihood ratio statistic (G statistic) was compared with a χ2 distribution. A significant birth sex*foster sex term would indicate a significant treatment effect. Offspring size at weaning and 1 year was analysed by ANOVA with foster sex, birth sex and birth sex*foster sex as variables. A significant treatment effect would be revealed by a significant interaction term.
3. Results
There was no significant difference in MM or MCI among treatment groups at the commencement of the study (MM: F4,27 = 0.533, p = 0.713; MCI: F4,27 = 0.45, p = 0.771) or at weaning (MM: F4,26 = 2.164, p = 0.101; MCI: F4,26 = 1.51, p = 0.228).
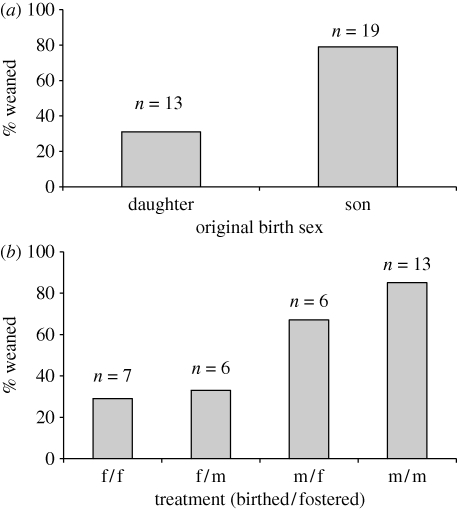
Offspring sex birthed by a mother was not related to MM (model χ2 = 0.71, p = 0.40) or MCI (model χ2 = 0.19, p = 0.67) at the time of cross-fostering. Weaning success was 61.5 per cent for all cross-fostered offspring (n = 26) and 50 per cent for sham offspring (n = 6), indicating that cross-fostering did not reduce weaning success (χ2 = 0.27, p = 0.61). Offspring weaning success for a given mother was predicted by the sex of the offspring originally produced by the mother and the MM and MCI of the mother at the time of cross-fostering (table 1). A mother that originally birthed a son had a significantly greater probability of weaning her pouch young irrespective of its sex than a mother that birthed a daughter (p = 0.004, table 1; figure 1a), indicating that mothers with greater investment ability were more likely to produce sons. Although weaning success of male offspring was more strongly influenced than that of a female offspring by the sex of offspring originally produced by a mother, neither the interaction term (i.e. cross-fostering treatment) nor foster sex itself were significant predictors of weaning success (figure 1b).
Table 1.
Analysis of deviance in logistic regression for the probability that a mother will wean an offspring. (Models predict the log odds ratio of weaned/failed. MM, maternal mass at time of swapping; MCI, maternal condition index at time of swapping; BS, offspring sex birthed by a mother; FS, offspring sex fostered by a mother. Asterisk denotes significant.)
| deviance | models compared | G | Δdf | p | |
|---|---|---|---|---|---|
| model selection | |||||
| 1. null model | 43.22 | ||||
| 2. full model: WS = MM + MCI + BS + FS + BS*FS | 27.82 | 2 versus 1 | 15.4 | 5 | 0.009* |
| 3. WS = (2) − MCI | 34.34 | 3 versus 2 | 6.52 | 1 | 0.01* |
| 4. WS = (2) − MM | 34.50 | 4 versus 2 | 6.68 | 1 | 0.01* |
| 5. WS = (2) − BS*FS | 28.50 | 5 versus 2 | 0.68 | 1 | 0.41 |
| 6. WS = (5) − FS | 28.62 | 6 versus 5 | 0.12 | 1 | 0.73 |
| 7. WS = (6) − BS | 36.76 | 7 versus 6 | 8.14 | 1 | 0.004* |
| model selected: WS = MM + MCI + BS | |||||
| variable | estimate |
s.e. |
|||
| MM | 0.006 | 0.003 | |||
| MCI | −22.53 | 11.68 | |||
| BS (female) | −1.36 | 0.57 | |||
Figure 1.
(a) Percentage of young weaned in relation to the sex birthed by the mother (daughter, n = 13; son, n = 19), and (b) percentage of young weaned successfully in relation to sex birthed/sex fostered by the mother (f/f, n = 5; f/m, n = 6; m/f, n = 6; m/m, n = 13).
Mass of surviving offspring at weaning was not influenced by the sex of offspring originally produced by the mother (F1,17 = 0.06, p = 0.81), foster sex (F1,17 = 0.29, p = 0.60) or their interaction (F1,17 = 0.02, p = 0.90). At 1 year of age, males were significantly larger than females in body size but not body mass, but the sex birthed by the mother and its interaction with foster sex had no influence on these measures (1 year pes: foster sex, F1,17 = 15.16, p = 0.001; birth sex, F1,17 = 2.03, p = 0.17; birth sex*foster sex, F1,17 = 1.37, p = 0.26; 1 year mass: foster sex, F1,17 = 2.70, p = 0.12; birth sex, F1,17 = 0.24, p = 0.63; birth sex*foster sex, F1,17 = 0.12, p = 0.73).
4. Discussion
The present study provides experimental evidence that female tammar wallabies with greater investment ability give birth to more sons. This study is, to our knowledge, the first to employ manipulations of offspring sex in mammals and find support for adaptive condition-dependent sex allocation. Importantly, by cross-fostering offspring according to sex, the relationship between birth sex and weaning success was disassociated. In correlative studies, higher weaning success of sons cannot be attributed definitively to maternal rearing ability or male survivability. Here, the higher weaning success for mothers that birthed sons occurred in both male and female foster young, thus is clearly attributed to maternal investment during lactation.
The role of maternal investment ability was not reflected in relationships between MM or condition and offspring sex. Maternal condition at fertilization may be more closely linked to offspring sex than our measures at cross-fostering (Cameron 2004). Cross-fostering occurred as much as a full year after fertilization (tammar wallabies exhibit embryonic diapause; Rudd 1994). However, maternal investment in our study was not linked to changes in capital resources (i.e. body mass) regardless of the sex birthed or reared. Instead, an unmeasured component of female quality (e.g. foraging efficiency, immunological health) or pre-determined resources for investment appears to exist that is not altered by the sex being reared. Across mammals, maternal food intake or dominance rank appears to relate to offspring sex at least as often as morphological indicators typically employed in empirical studies (Hewison & Gaillard 1999; Sheldon & West 2004). Here, we use cross-fostering methodologies to demonstrate that reproductive females allocate to offspring sex according to a measure of maternal investment ability that is not apparent when examining maternal morphological condition during lactation.
In kangaroos and wallabies, pouch young survival is often linked to maternal condition and health, which is predominantly dictated by environmental conditions (Frith & Sharman 1964; Bolton et al. 1985). This relationship has been attributed to inadequate milk supply as environmental conditions deteriorate (Frith & Sharman 1964) or to strong intraspecific competition (Bolton et al. 1985). Examining milk components in females birthing daughters and sons may provide some insight into the mechanisms of maternal investment ability.
We did not find convincing evidence for the main assumption of the TWH—that the fitness of sons depends more than the fitness of daughters on maternal investment ability. Survival to weaning in sons appeared to be more strongly influenced than in daughters by maternal investment ability; however, this effect was not significant. We suspect that this was owing to insufficient sample sizes and plan to extend the study with a large field experiment in tammar wallabies. Alternatively, a sex-specific effect of maternal investment ability on offspring lifetime fitness may have been masked by our benign captive conditions, or may exist in components not measured in this study (e.g. offspring reproductive success).
The study presented here is, to our knowledge, the first to test the adaptive basis of sex allocation in mammals using cross-fostering techniques prior to most maternal investment (Koskela et al. 2009). It illustrates the profound utility of this experimental technique by providing support for condition-dependent sex allocation that could not have been gained with correlative analysis. Moreover, the study highlights the advantage that marsupials provide for the study of sex allocation owing to the ability to cross-foster early in maternal investment.
Acknowledgements
The project was approved by the University of Western Australia's Animal Ethics Committee (approval no.: RA/3/100/695) and Department of Environment and Conservation (permit no.: SF006445).
Financial support provided to K.A.R. from the University of Western Australia Research Grants Scheme (RA/1/485/846).
References
- Bolton B. L., Newsome A. E., Merchant J. C.1985Reproduction in the agile wallaby: opportunistic breeding in a seasonal environment. Proc. Ecol. Soc. Aust. 13, 73–79 [Google Scholar]
- Cameron E. Z.2004Facultative adjustment of mammalian sex ratios in support of the Trivers–Willard hypothesis: evidence for a mechanism. Proc. R. Soc. Lond. B 271, 1723–1728 (doi:10.1098/rspb.2004.2773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T. H., Albon S. D., Guinness F. E.1985Parental investment and sex differences in juvenile mortality in birds and mammals. Nature 313, 131–133 (doi:10.1038/313131a0) [Google Scholar]
- Cockburn A., Legge S., Double M. C.2002Sex ratios in birds and mammals: can the hypotheses be disentangled? In Sex ratios: concepts and research methods (ed. Hardy I. C. W.), pp. 266–286 Cambridge, UK: Cambridge University Press [Google Scholar]
- Findlay L., Renfree M. B.1984Growth, development and secretion of the mammary gland of macropodid marsupials. Symp. Zool. Soc. Lond. 51, 403–432 [Google Scholar]
- Frith H. J., Sharman G. B.1964Breeding in wild populations of the red kangaroo, Megaleia rufa. Wildl. Res. 9, 86–114 [Google Scholar]
- Hayssen V., Lacy R. C., Parker P. J.1985Metatherian reproduction: transitional or transcending. Am. Nat. 126, 617–632 (doi:10.1086/284443) [Google Scholar]
- Hewison A. J. M., Gaillard J. M.1999Successful sons or advantaged daughters? The Trivers–Willard model and sex-biased maternal investment in ungulates. Trends Ecol. Evol. 14, 229–234 (doi:10.1016/S0169-5347(99)01592-X) [DOI] [PubMed] [Google Scholar]
- Koskela E., Mappes T., Niskanen T., Rutkowska J.2009Maternal investment in relation to sex ratio and offspring number in a small mammal: a case for Trivers and Willard theory? J. Anim. Ecol. 78, 1007–1014 (doi:10.1111/j.1365-2656.2009.01574.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole W. E., Simms N. G., Wood J. T., Luboloa M.1991Tables for age determination of the Kangaroo Island Wallaby (Tammar) Macropus eugenii, from body measurements. Technical Memorandum no. 32. CSIRO (Division of Wildlife and Ecology), Canberra, Australia [Google Scholar]
- Rudd C. D.1994Sexual behaviour of the male and female tammar wallabies (Macropus eugenii) at postpartum oestrus. J. Zool. 232, 151–162 (doi:10.1111/j.1469-7998.1994.tb01565.x) [Google Scholar]
- Schwanz L. E., Bragg J. G., Charnov E. L.2006Maternal condition and facultative sex ratios in population with overlapping generations. Am. Nat. 168, 521–530 (doi:10.1086/507993) [DOI] [PubMed] [Google Scholar]
- Sheldon B. C., West S. A.2004Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am. Nat. 163, 40–54 (doi:10.1086/381003) [DOI] [PubMed] [Google Scholar]
- Sunnucks P., Taylor A. C.1997Sex of pouch young related to maternal weight in Macropus eugenii and M. parma (Marsupialia: Macropodidae). Aust. J. Zool. 45, 573–578 (doi:10.1071/ZO97038) [Google Scholar]
- Taggart D.2002Use of pouch young removal and cross-fostering techniques to accelerate breeding and recruitment in the threatened brush-tailed rock wallaby, Petrogale penicillata. ANZCCART News. 15, 1–3 [Google Scholar]
- Trivers R. L., Willard D. E.1973Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92 (doi:10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- West S. A.2009Sex allocation Monographs in population biology series Princeton, NJ: Princeton University Press [Google Scholar]