Abstract
Acoustic communication involves both the generation and the detection of a signal. In the coqui frog (Eleutherodactylus coqui), it is known that the spectral contents of its calls systematically change with altitude above sea level. Here, distortion product otoacoustic emissions are used to assess the frequency range over which the inner ear is sensitive. It is found that both the spectral contents of the calls and the inner-ear sensitivity change in a similar fashion along an altitudinal gradient. As a result, the call frequencies and the auditory tuning are closely matched at all altitudes. We suggest that the animal's body size determines the frequency particulars of the call apparatus and the inner ear.
Keywords: coqui frog, Eleutherodactylus, otoacoustic emission, vocalization
1. Introduction
The Puerto Rican treefrog, Eleutherodactylus coqui (Anura: Leptodactylidae) is abundant in Puerto Rico and can be found on this island at altitudes that range from sea level to over 1000 m (Narins & Smith 1986). Male coqui frogs produce a characteristic two-note call (‘Co-Qui’) for which it has been shown that each note has different significance for each sex. Males respond to the ‘Co’-note within the call, while females are attracted to the ‘Qui’-note (Narins & Capranica 1976, 1978). The frequency content of these calls varies systematically with the altitude above sea level at which the animal is calling (Narins & Smith 1986).
Successful animal communication not only requires the generation of a signal, but also the presence of an ‘appropriate’ sensor (Ryan 1986). For acoustic communication, this means that the ear has to be sensitive to those spectral (and temporal) cues present in the calls; a correlation that is well established in frogs (Gerhardt & Schwartz 2001). In frogs, as in most tetrapods, the inner ear produces so-called distortion product otoacoustic emissions (DPOAEs). These are weak sounds that arise in response to a two-tone stimulus, and can be recorded by placing a sensitive microphone near the tympanic membrane. In frogs, those frequencies that result in maximum DPOAE amplitudes closely correspond to the frequencies of highest inner-ear sensitivity (Van Dijk et al. 2002; Meenderink et al. 2005b).
Here, we focus on communication between male coqui frogs. We show that the dominant frequencies in the relevant part of the calls (Co-note) and the frequencies of the highest inner-ear sensitivity are closely matched along an altitudinal gradient on a tropical mountain. The similarities in these frequencies seem to be determined by the animal's size, and not under the direct control of external factors (e.g. ambient temperature).
2. Material and methods
(a). Study site
This study was conducted during July 2006 in the Caribbean National Forest in eastern Puerto Rico. Data were obtained along a 13 km stretch of Puerto Rico Highway 191 that transects the northeast face of the Luquillo Mountains up to El Yunque Peak. Data are presented for 43 male E. coqui for which both calls and DPOAEs were recorded. These are a subset of a larger group of animals for which only the calls were recorded (Narins et al. in preparation). The calling sites of these animals ranged from 30 to 1000 m above sea level, spanning the entire altitudinal range at the study site.
(b). Data collection
For each individual, between five and 20 calls were recorded (TC D5M; Sony) by holding a directional microphone (CE8; AKG) with windscreen approximately 1 m from the animal. Following these recordings, the animal was captured and brought to the El Verde Field Station (altitude 350 m). The following day, the animal was anaesthetized (Nembutal 50 mg ml−1; approx. 1.1 µl g−1 body weight; intramuscular injection), and its body size was measured from the tip of the nose to the cloaca (snout-vent length; SVL). A probe, holding two drivers (E-A-RTONE 3A; Aearo) and one microphone (ER-10A; Etymotic) was sealed against the skin surrounding the tympanic membrane for DPOAE measurements. Two equal-level (L1 = L2 > 80 dB SPL) stimulus tones with appropriate absolute (f1, f2) and relative (f2/f1 ≤ 1.1) frequencies were introduced into the ear, each one from a single driver. Simultaneously with the stimulus, ear-canal pressure was recorded, and stored on a computer hard disk for offline analysis. During the recordings, the animal was wrapped in wet gauze to prevent dehydration.
Details of the DPOAE recordings are described elsewhere (Meenderink & Van Dijk 2004). Briefly, for each stimulus presentation, the frequencies f1 and f2 were selected such that they were periodic over the same number of samples. With this paradigm, stimuli can be presented continuously, while allowing the separation of the recorded signal into repetitive periodic ‘blocks’. Artefact-free blocks were subaveraged in two buffers. From these, the amplitude of DPOAEs at 2f1 − f2 and 2f2 − f1, as well as the noise floor, was determined using Fourier analysis. As a control against distortion in the setup, experiments were repeated with the animal replaced by an inanimate object. These did not result in any observable distortion. Therefore, DPOAEs were deemed of biological origin when they exceeded the noise floor by at least 6 dB. A mobile processor (RM2; Tucker-Davis Technologies; Fsample = 12 kHz) that was controlled by custom Matlab (Mathworks) software was used for D/A and A/D conversion. After recovery from anaesthesia, animals were returned to their calling site within 24 h of their capture.
(c). Call analysis
Recorded calls were digitized (Audigy SE; Creative Labs; Fsample = 44 kHz), and for each call the dominant Co-note frequency was determined (resolution = 43 Hz) using the software package SoundRuler (Gridi-Papp 2003). For each frog, all recorded calls were analysed. The obtained values are given as their median and interquartile (25–75%) range.
(d). DPOAE analysis
DPOAEs were recorded by varying stimulus frequency f1 between 0.3 and 3.2 kHz, while keeping the ratio f2/f1, and the stimulus levels (L1, L2) constant. This yields so-called DPOAE audiograms—plots of DPOAE amplitude versus frequency. From both ears in each animal, several DPOAE audiograms were obtained for different combinations of the fixed-stimulus parameters. From each DPOAE audiogram, the frequency that evoked the maximum DPOAE amplitude (FmaxDP) was extracted. As these frequencies showed no systematic variation within individuals, the obtained values are given as their median ± interquartile range.
3. Results
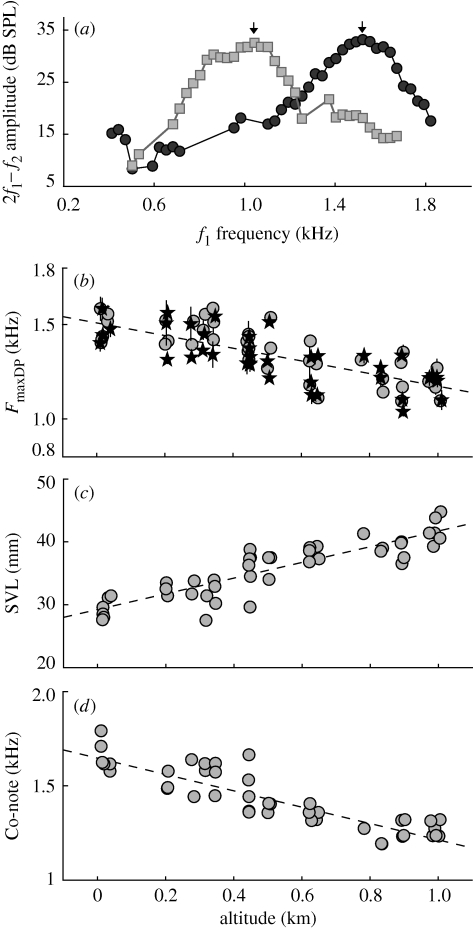
Two typical DPOAE audiograms, obtained from two animals caught at different altitudes, are shown in figure 1a. The stimulus frequencies that resulted in maximum DPOAE amplitudes (FmaxDP) clearly differ between the two audiograms. This is confirmed in figure 1b, which shows FmaxDP versus altitude for all animals.
Figure 1.
(a) DPOAE audiograms showing 2f1 – f2 amplitudes for two animals caught at the altitude extremes in this study. Stimulus parameters: f1 = 0.3 … 3.2 kHz in 30 Hz steps; f2/f1 = 1.1; L1 = L2 = 90 dB SPL. Downward arrows indicate maxima in DPOAE audiograms. Filled circles, 30 m (PR57/b); filled squares, 1005 m (PR86/b). Scatter plots showing (b) the frequencies resulting in maximum DPOAE amplitude (FmaxDP), (c) snout-vent length (SVL) and (d) dominant Co-note frequency as a function of altitude. Error bars give interquartile ranges. To avoid overlap, data points were offset by small random numbers along both abscissa and ordinate. (b) shows results for both 2f1 − f2 (grey circles; n = 43) and 2f2 − f1 (black stars; n = 40) DPOAEs. The ordinate gives f1 for 2f1 − f2 and DPOAE frequency for 2f2 − f1 (see text). Data were fitted by straight (dashed) lines: (b) freq = 1.51 − 0.33*Alt, r2 = 0.589; (c) SVL = 29.23 + 12.5*Alt, r2 = 0.777; (d) Co = 1.65 − 0.43*Alt, r2 = 0.773.
For 2f 1 – f2 DPOAEs, FmaxDP is given as the stimulus frequency f1, while for the 2f2 – f1 DPOAEs it is the emission frequency itself. These different choices for FmaxDP are motivated by previous observations in frogs (e.g. Meenderink et al. 2005a), which showed that these particular frequencies are the most stable predictor for maxima in DPOAE audiograms. Given that the difference between f1 and fDPOAE is small (since fDPOAE = 2f2 – f1 = f1(2 * f2/f1 − 1), where f2/f1 ≤ 1.1), this differential choice has little effect, but represents the most accurate outcome.
Near the base of the mountain, one encounters small coqui frogs that produce high-pitched calls at high repetition rates. As one gains altitude while progressing up Luquillo Mountain the frogs systematically increase in length. At the same time, their calls exhibit a systematic drop in pitch and a concomitant reduction in call rate (Narins & Smith 1986). These observations are formalized in figure 1c,d, which show the animal's SVL and the dominant Co-note frequency in relation to the altitude above sea level at which the animal was calling.
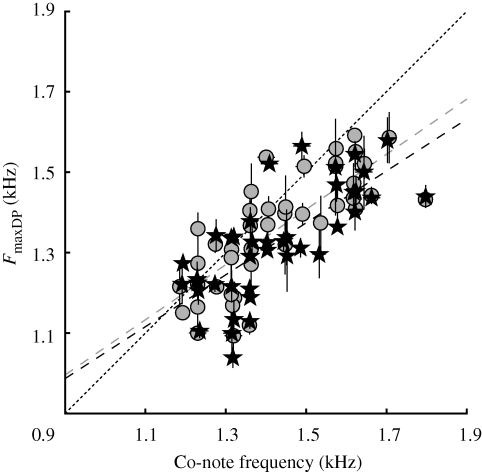
Data from figure 1b,d are plotted again in figure 2. Dominant Co-note and FmaxDP show a clear correlation. This indicates that the frequency sensitivity of the ears and the frequency contents of the calls covary, and are matched for any given altitude.
Figure 2.
Scatter plot combining data from figures 1b,d showing the relationship between the frequency at maximum DPOAE amplitude (FmaxDP) and the dominant Co-note frequency in the call. Points give median values ± interquartile ranges. Grey circles, 2f1 − f2; black stars, 2f2 − f1. To avoid overlap, data points were offset by small random numbers along both abscissa and ordinate. The diagonal (dashed) line indicates Co-note frequency FmaxDP. Regression lines to the 2f1 − f2 and 2f2 − f1 data are given; grey dashed line, 2f1 − f2 = 0.37+0.69*Co, r2 = 0.599; black dashed line, 2f2 − f1 = 0.40+0.65*Co, r2 = 0.547.
4. Discussion
DPOAE audiograms exhibited band-pass characteristics; they have a single maximum, with frequencies other than this maximum invariably resulting in lower DPOAE amplitudes (figure 1a). This differs from DPOAE audiograms recorded in several other anurans, which are more or less ‘M-shaped’ with two relative maxima (Van Dijk & Manley 2001; Vassilakis et al. 2004; Meenderink et al. 2005a). This bimodal shape results from DPOAE generation in both auditory end organs in the frog (the amphibian papilla and the basilar papilla; Lewis & Narins 1999), which are most sensitive to different frequency ranges that are not necessarily continuous. This results in an intermediate frequency region with diminished DPOAEs. The DPOAEs reported here most probably originated from the amphibian papilla, which is sensitive up to ca 1.5 kHz (Narins & Capranica 1976; Stiebler & Narins 1990).
Along the altitudinal gradient, the Co-note frequency varies considerably (figure 1d; Narins & Smith 1986). With this, two alternatives may be entertained: (i) each animal's ear is sensitive over a limited frequency range, requiring a shift in frequency sensitivity with altitude that parallels the shift in call frequency; and (ii) the frog ear has a broad sensitivity range that allows detection of all call frequencies it may encounter (irrespective of altitude). Our results (figure 2) point towards the former strategy, a finding that is supported by behavioural experiments (Narins 1983; Narins & Smith 1986).
Factors that caused the observed systematic gradients are likely to show a systematic dependence on altitude as well. One candidate is ambient temperature, which changes approximately 6°C over the 1000 m altitudinal gradient. In several ectothermic animals, both the spectral content of calls and the tuning of the ear vary with ambient temperature. However, temperature-dependent shifts in call frequency (Gerhardt & Mudry 1986) and auditory tuning (Stiebler & Narins 1990) are too small to explain the observed gradients. Moreover, FmaxDP showed a clear dependence on altitude (figure 1b), while DPOAE recordings were all made at the same ambient temperature (range: 23.9–25.8°C), and only after animals were allowed to acclimate for several hours. Rather than this external factor, we suggest that the observed gradients result from a morphological factor (SVL), which also varies systematically with altitude (figure 1c). The correlation between body size and dominant call frequency is well established in frogs (Ryan 1988), and from our work it appears that hearing sensitivity and body size are correlated as well. We are unaware of previous reports on a similar dependence in auditory sensitivity. Presumably, this dependence on body size is linked to corresponding size changes in the inner ear. It is unknown whether and what morphological variation occurs with varying body size (e.g. hair cell morphology) and whether such variation can explain the relationship between body size and hearing sensitivity we observed.
Acknowledgements
The experimental protocol adhered to the ABS guidelines for the use of animals in research and was approved by the UCLA Animal Research Committee (protocol no. 094-086-51).
We thank Jill Johnson and Hilda Lugo for their logistical help at the El Verde Field Station and Brittany Barker for assistance with obtaining permits. Supported by grants from NIH (R01DC00222), and the UCLA Academic Senate (no. 3501) to P.M.N. and from the Netherlands Organisation for Scientific Research (NWO-VENI 863.08.003) to S.W.F.M.
References
- Gerhardt H. C., Mudry K. M.1986Temperature effects on frequency preferences and mating call frequencies in the green treefrog Hyla cinerea (Anura: Hylidae). J. Comp. Physiol. 137, 1–6 (doi:10.1007/BF00656911) [Google Scholar]
- Gerhardt H. C., Schwartz J. J.2001Auditory tuning and frequency preferences in anurans. In Anuran communication (ed. Ryan M. J.), pp. 73–85 Washington, DC: Smithsonian Institution Press [Google Scholar]
- Gridi-Papp M. SoundRuler: acoustic analysis for research and teaching, v. 0.9.6.0. 2003. Available via SourceForge. See http://soundruler.sourceforge.net . [Google Scholar]
- Lewis E. R., Narins P. M.1999The acoustic periphery of amphibians; anatomy and physiology. In Comparative hearing: fish and amphibians, vol. 11, Springer Handbook of Auditory Research (eds Fay R. R., Popper A. N.), pp. 101–154 New York, NY: Springer [Google Scholar]
- Meenderink S. W. F., Van Dijk P.2004Level dependence of distortion product otoacoustic emissions in the leopard frog Rana pipiens pipiens. Hear. Res. 192, 107–118 (doi:10.1016/j.heares.2004.01.015) [DOI] [PubMed] [Google Scholar]
- Meenderink S. W. F., Narins P. M., Van Dijk P.2005aDetailed f1, f2 area study of distortion product otoacoustic emissions in the frog. J. Assoc. Res. Otolaryngol. 6, 37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meenderink S. W. F., Van Dijk P., Narins P. M.2005bComparison between distortion product otoacoustic emissions and nerve fiber responses from the basilar papilla of the frog. J. Acoust. Soc. Am. 117, 3165–3173 (doi:10.1121/1.1871752) [DOI] [PubMed] [Google Scholar]
- Narins P. M.1983Synchronous vocal response mediated by the amphibian papilla in a neotropical treefrog: behavioural evidence. J. Exp. Biol. 105, 95–105 [Google Scholar]
- Narins P. M., Capranica R. R.1976Sexual differences in the auditory system of the tree frog Eleutherodactylus coqui. Science 192, 378–380 (doi:10.1126/science.1257772) [DOI] [PubMed] [Google Scholar]
- Narins P. M., Capranica R. R.1978Communicative significance of the two-note call of the treefrog Eleutherodactylus coqui. J. Comp. Physiol. 127, 1–9 (doi:10.1007/BF00611921) [Google Scholar]
- Narins P. M., Smith S. L.1986Clinal variation in anuran advertisement calls: basis for acoustic isolation? Behav. Ecol. Sociobiol. 19, 135–141 (doi:10.1007/BF00299948) [Google Scholar]
- Narins P. M., Kits M., Meenderink S. W. F.In preparation Global warming drives shift in tropical anuran acoustic communication signals. [Google Scholar]
- Ryan M. J.1986Factors influencing the evolution of acoustic communication: biological constraints. Brain Behav. Evol. 28, 70–82 (doi:10.1159/000118693) [DOI] [PubMed] [Google Scholar]
- Ryan M. J.1988Constraints and patterns in the evolution of anuran acoustic communication. In The evolution of the amphibian auditory system (ed. Fritzsch B.), pp. 637–677 Hoboken, NJ: Wiley & Sons [Google Scholar]
- Stiebler I. B., Narins P. M.1990Temperature-dependence of auditory nerve response properties in the frog. Hear. Res. 46, 63–82 (doi:10.1016/0378-5955(90)90140-K) [DOI] [PubMed] [Google Scholar]
- Van Dijk P., Manley G. A.2001Distortion product otoacoustic emissions in the tree frog Hyla cinerea. Hear. Res. 153, 14–22 (doi:10.1016/S0378-5955(00)00251-3) [DOI] [PubMed] [Google Scholar]
- Van Dijk P., Mason M. J., Narins P. M.2002Distortion product otoacoustic emissions in frogs: correlation with middle and inner ear properties. Hear. Res. 173, 100–108 [DOI] [PubMed] [Google Scholar]
- Vassilakis P. N., Meenderink S. W. F., Narins P. M.2004Distortion product otoacoustic emissions provide clues to hearing mechanisms in the frog ear. J. Acoust. Soc. Am. 116, 3713–3726 (doi:10.1121/1.1811571) [DOI] [PubMed] [Google Scholar]