Abstract
Visual signals are crucial for parent–offspring communication, although their functioning has been neglected for nocturnal birds. Here, we investigated parental preference for nestling coloration in nocturnal conditions—a question hitherto unexplored—in a nocturnal raptor, the scops owl (Otus scops). We assessed how parents allocated food during the night in relation to a manipulation of ultraviolet (UV) reflectance of the cere (skin above the beak) of their offspring. Reflectance of the cere shows a marked peak in the UV part of the spectrum, and location of the UV peak is related to nestling body mass (i.e. heavier nestlings have a UV peak at lower wavelengths). We found evidence of parental bias in favour of lighter offspring: UV-reduced nestlings gained more weight during the night than their control siblings. This study provides the first experimental evidence of the use of visual cues for parent–offspring communication in a nocturnal bird.
Keywords: parent–offspring communication, nocturnal vision, cere, owls
1. Introduction
Parent–offspring communication in altricial birds often depends upon perception of coloured visual signals. For instance, there is widespread evidence that nestling gape coloration influences parental feeding decisions (e.g. Kilner 2006). Gape coloration can provide parents with information on nestling level of satiation or health (e.g. Kilner 1997; Saino et al. 2003), or with a conspicuous target towards which parents direct their feeds (Heeb et al. 2003; Avilés et al. 2008). Parents may also respond to changes in chromatic characteristics of the body skin (Jourdie et al. 2004; Bize et al. 2006) and ultraviolet (UV) reflectance of the feathers of nestlings (Galván et al. 2008; Tanner & Richner 2008).
So far, all evidence of parental favouritism based on visual cues have come from studies with diurnal bird species. However, recent studies have provided support for a possible role of visual communication in nocturnal birds. Indeed, Penteriani et al. (2007a) showed that male eagle owls Bubo bubo, a nocturnal predator, displayed more frequently towards intruder male mounts displaying low-brightness white badges than towards male mounts with normal white badge brightness. Also, control owlets were in better condition than owlets with brightness-reduced mouths during the post-fledging dependence period (Penteriani et al. 2007b), which could constitute evidence for parental preference based on visual cues.
Here, for the first time, we explored under nocturnal conditions the influence of nestling coloration on parental preferences in a nocturnal predator raptor, the scops owl (Otus scops). Previous studies have found that different components of the cere (skin above the beak, figure 1a) coloration signal adult quality in diurnal raptors (e.g. Mougeot & Arroyo 2006). In this paper, we report on a marked peak in the UV part of spectrum of the cere of scops owl nestlings early in their development (figure 1). We then studied whether UV light reflected by the cere of offspring correlates with nestling weight while they are in the nests. Finally, we experimentally assessed how parents allocated food in relation to a manipulation of UV reflectance of the cere of their offspring.
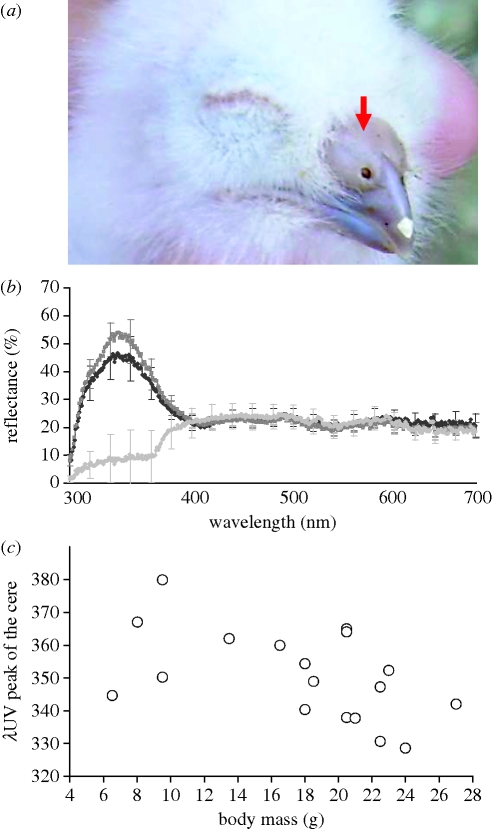
Figure 1.
(a) Photograph of a nestling scops owl showing the cere (red arrow). (b) Average (±s.d.) reflectance spectra of scops owl nestling cere before and after treatment with a petroleum jelly containing or not containing UV-light blocker (black, before; dark grey, control; light grey, UV block). (c) Relationship between nestling body mass and λUV peak of the cere (n = 18 nestlings).
2. Material and methods
The field study was carried out in the surroundings of Guadix (37°18′ N, 3°11′ W), southeastern Spain, in May–June 2009. Scops owls were breeding in nest-boxes recently (2003–2005) installed (more details in Avilés et al. 2008).
We experimentally assessed how parents allocated food in relation to UV reflectance of the cere of their offspring, by applying a petroleum jelly with UV-light blockers to the cere of randomly chosen nestlings and compared their body mass gain with siblings treated with a control petroleum jelly. The experiment was performed on 14 scops owl nests (i.e. 29 and 29 nestlings treated with the UV-light blocker and the control treatment, respectively). The UV-light blocker was composed of an UV absorbing chemical (50/50 w/w blend of Parsol 1789 and MCX; Roche, Dubendorf, Switzerland). When nestlings were 4–6 days old, they were ranked within each brood by weight, and the heaviest nestling was assigned randomly to one of the two treatments, control petroleum jelly or UV-blocking petroleum jelly. The other nestlings in the nest were assigned alternately to each treatment following the ranking order. In order to exclude jostling by nestlings and to increase parental control over food allocation, nestlings were separated by treatment with a cork septum (e.g. Bize et al. 2006). Nestlings were weighed with Pesola spring balances before and after a trial to an accuracy of 0.25 g. Treatments were applied at dusk (i.e. 21–22), and after 10 h, we weighed nestlings again. Nestling body mass gain is positively correlated with the amount of food provided by parents in birds (e.g. Heeb et al. 2003), which suggests that mass gain is a biologically reliable measure of parental provisioning. A linear mixed model in which we controlled for the random effect of nest revealed that body mass of nestlings did not differ between control and UV-reduced nestlings before the manipulation (mean ± s.e.: 21.90 ± 4 g of control versus 20.87 ± 3.51 g of UV-reduced nestlings; treatment effect: F1,28 = 0.57, p = 0.45; nest effect: F12,28 = 31.32, p < 0.0001).
Spectral reflectance (300–700 nm) of the cere was recorded on 18 scops owl nestlings from four different nests following Avilés et al. 2008 (see electronic supplementary material). We summarized cere reflectance data by calculating: (i) total brightness, (ii) UV chroma and (iii) λUV peak (see electronic supplementary material; see Montgomerie 2006). In some nestlings, the measurements were taken before and after providing the UV blocker and the control treatments that allowed us to verify the effect of our experiment on cere reflectance. The UV blocker significantly reduced UV chroma and displaced to longer wavelengths the λUV peak in experimental nestlings (see electronic supplementary material) (figure1b). Mass gain of nestlings in relation to the experiment was analysed with a linear mixed model for repeated measures (see electronic supplementary material).
3. Results
(a). Ultraviolet reflectance of cere
Reflectance of the cere showed a marked peak in the UV part of the spectrum (300–400 nm, figure 1b). A greater UV chroma in the cere was associated with a lower λUV peak (Rp = −0.59, p > 0.01, n = 18), but no significant relationship was found between total brightness and the other colour variables (all p > 0.96).
Variation in body mass among nestlings was not significantly associated with total brightness or UV chroma (Rp = 0.04 and 0.29, respectively; p > 0.23 in the two cases), but it was negatively related with λUV peak (Rp = − 0.51, p > 0.029, n = 18). Thus, heavier nestlings had a λUV peak at lower wavelengths (figure 1c).
(b). Parental preferences in relation to ultraviolet reflectance of nestling cere
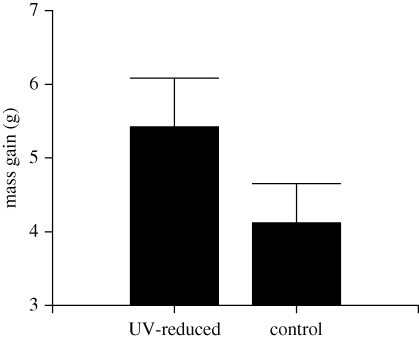
Fifty-five out of 58 scops owl nestlings gained weight during the experiment. Nestlings had an average mass gain (mean ± s.e.) of 4.59 ± 0.39 g, which suggests that parents were actively feeding nestlings during the night. UV-reduced nestlings gained more weight than control siblings during the night (interaction time × treatment: F1,52 = 6.75, p = 0.012, figure 2, electronic supplementary material).
Figure 2.
Average (±s.e.) body mass gain (g) of scops owl siblings that were treated with a petroleum jelly containing or not containing UV-light blocker (UV-reduced and control nestlings, respectively). Sample size was 14 broods (29 and 29 UV-reduced and control nestlings, respectively).
4. Discussion
We have shown for the first time that the cere of nestlings of a nocturnal raptor strongly reflects in the UV. In addition, our findings show that UV cere reflectance by scops owl nestlings is related to their body mass, and the existence of biases in food allocation toward nestlings presenting signal intensities suggesting small body size (UV-blocked nestlings). To our knowledge, this is the first experimental evidence of a role of visual communication in parent–offspring communication for a nocturnal species.
Parents are expected to decide which young to feed in a way that maximizes their own reproductive success by feeding disproportionately to nestlings with the largest fitness return per amount of food invested (Godfray 1995; Mock & Parker 1997). The optimal feeding strategy should change from feeding young in poorer to better body condition when resources shift from abundance to that limitation (Bize et al. 2006). Risk of complete brood failure is low in our scops owl population (D. Parejo & J. M. Avilés 2009, unpublished data); thus by preferentially feeding lighter offspring parents may maximize the number of offspring at fledging. In agreement, we found that heavier nestlings have a more UV-biased peak of reflectance of their cere and that parents preferentially feed nestlings with a less UV-biased peak of reflectance. The mechanism by which nestling body mass relates to UV cere reflectance is yet unknown. It has been suggested that, in adult birds, variation in UV skin reflectance could result from differences in the organization of parallel collagen fibres in the dermis (Prum & Torres 2003). It follows that subtle changes in UV reflectance can arise by derma shrinkage and derma growth affecting the collagen array size. Thus, perhaps in scops owl nestlings UV reflectance of the cere reflects to what extent collagen fibres were properly disposed with lower reflective UV cere revealing a worse organization. The organization of collagen fibres of the dermis are likely to change throughout nestling development (Prum & Torres 2003), and a slight sexual size dimorphism has been reported for adult scops owls (Blanco et al. 2002). Therefore, parents might show colour preferences revealing age and/or sex when feeding nestlings. Although the exact mechanism promoting the UV cere coloration–body mass link clearly deserves further research, our results confirm the existence of such a relationship which is a prerequisite for the use of cere coloration as a signal revealing nestling quality (i.e. condition, age and/or sex) to provisioning parents.
The fact that parents responded to a manipulation of UV reflectance of their offspring can not be due to UV coloration, because owls probably do not have a VS or UVS cone (Bowmaker & Martin 1978) and, at night, there probably is not enough light for colour vision. It is possible that the measured effect was due to a change in perceived brightness mediated by scotopic (rod) vision. Compared to diurnal species, owls have very few cones in their rod-dominated retina (Hart 2001). Owls, like some bats (e.g. Winter et al. 2003), may have a UV-transmitting lens and cornea and so the secondary (beta) peak of the pigment in their rods would capture UV light contributing to achromatic vision at low light levels. It is also possible that, even at low light levels, photopic discrimination was involved, but again the effect of UV would be via the stimulation of the beta peak of pigments in the cones. It has been reported that owls have cones containing oil droplets that are either colourless or only a pale yellow (Walls 1942), which presumably transmit UV light.
In conclusion, our results provide strong support for an important, though long overlooked, role of nocturnal vision in parent–offspring communication in a nocturnal bird. Coloured patches (i.e. cere and beaks) are, however, frequent in nestlings of several nocturnal bird species and their use as visual signals revealing nestling quality for adjusting parental effort might, therefore, be widespread in nature.
Acknowledgements
This research was funded by the Spanish Ministry of Education and Science/FEDER (CGL2008-00718).
References
- Avilés J. M., Pérez-Contreras T., Navarro C., Soler J. J.2008Dark nests and conspicuousness in color patterns of nestlings of altricial birds. Am. Nat. 171, 327–338 (doi:10.1086/527493) [DOI] [PubMed] [Google Scholar]
- Blanco G., Dávila J. A., López Septiem J. A., Rodríguez R., Martínez F.2002Sex-biased initial eggs favours sons in the slightly size-dimorphic scops owl (Otus scops). Biol. J. Linn. Soc. 76, 1–7 (doi:10.1111/j.1095-8312.2002.tb01709.x) [Google Scholar]
- Bize P., Piault R., Moureau B., Heeb P.2006A UV signal of offspring condition mediates context-dependent parental favouritism. Proc. R. Soc. B 273, 2063–2068 (doi:10.1098/rspb.2006.3546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J. K., Martin G. R.1978Visual pigments and colour vision in a nocturnal bird, Strix aluco (tawny owl). Vis. Res. 18, 1125–1130 (doi:10.1016/0042-6989(78)90095-0) [DOI] [PubMed] [Google Scholar]
- Galván I., Amo L., Sanz J. J.2008Ultraviolet–blue reflectance of some nestling plumage patches mediates parental favouritism in great tits Parus major. J. Avian Biol 39, 277–282 (doi:10.1111/j.0908-8857.2008.04273.x) [Google Scholar]
- Godfray H. C. J.1995Signalling of need between parents and young: parent–offspring conflict and sibling rivalry. Am. Nat. 146, 1–24 (doi:10.1086/285784) [Google Scholar]
- Hart N. S.2001The visual ecology of avian photoreceptors. Prog. Retin. Eye Res. 20, 675–703 (doi:10.1016/S1350-9462(01)00009-X) [DOI] [PubMed] [Google Scholar]
- Heeb P., Schwander T., Faoro S.2003Nestling detectability affects parental feeding preferences in a cavity-nesting bird. Anim. Behav. 66, 637–642 (doi:10.1006/anbe.2003.2238) [Google Scholar]
- Jourdie V., Moureau B., Bennett A. T. D., Heeb P.2004Ultraviolet reflectance by the skin of nestlings. Nature 431, 262–262 (doi:10.1038/431262a) [DOI] [PubMed] [Google Scholar]
- Kilner R.1997Mouth colour is a reliable signal of need in begging canary nestlings. Proc. R. Soc. Lond. B 264, 963–968 (doi:10.1098/rspb.1997.0133) [Google Scholar]
- Kilner R.2006Function and evolution of color in young birds. In Bird coloration, vol. 2: function and evolution (eds Hill G. E., McGraw K. J.), pp. 201–232 Harvard, MA: Harvard University Press [Google Scholar]
- Mock D. W., Parker G. A.1997. In The evolution of sibling rivalry Oxford, UK: Oxford University Press [Google Scholar]
- Montgomerie R.2006Analyzing colors. In Bird coloration, vol. 1: mechanism and measurements (eds Hill G. E., McGraw K. J.), pp. 90–147 Harvard, MA: Harvard University Press [Google Scholar]
- Mougeot F., Arroyo B. E.2006Ultraviolet reflectance by the cere of raptors. Biol. Lett. 2, 173–176 (doi:10.1098/rsbl.2005.0434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penteriani V., Delgado M. M., Alonso-Alvárez C., Sergio F.2007aThe importance of visual cues for nocturnal species: eagle owl signal by badge brightness. Behav. Ecol. 18, 143–147 (doi:10.1093/beheco/arl060) [Google Scholar]
- Penteriani V., Delgado M. M., Alonso-Alvárez C., Viqueira Pina N., Sergio F., Bartolommei P., Thompson L. J.2007bThe importance of visual cues for nocturnal species: eagle owl fledgings signal with white mouth feathers. Ethology 113, 934–943 [Google Scholar]
- Prum R. O., Torres R.2003Structural colouration of avian skin: convergent evolution of coherently scattering dermal collagen arrays. J. Exp. Biol. 206, 2409–2429 (doi:10.1242/jeb.00431) [DOI] [PubMed] [Google Scholar]
- Saino N., Ambrosini R., Martinelli R., Ninni P., Møller A. P.2003Gape coloration reliably reflects immunocompetence of barn swallow (Hirundo rustica) nestlings. Behav. Ecol. 14, 16–22 (doi:10.1093/beheco/14.1.16) [Google Scholar]
- Tanner M., Richner H.2008Ultraviolet reflectance of plumage for parent–offspring communication in the great tit (Parus major). Behav. Ecol. 19, 369–373 (doi:10.1093/beheco/arm142) [Google Scholar]
- Walls G. L.1942The vertebrate eye vol. 19 Bloomfield Hills, MI: Canbrook Institute of Science Bulletin [Google Scholar]
- Winter Y., López J., von Helversen O.2003Ultraviolet vision in a bat. Nature 425, 612–614 (doi:10.1038/nature01971) [DOI] [PubMed] [Google Scholar]