Abstract
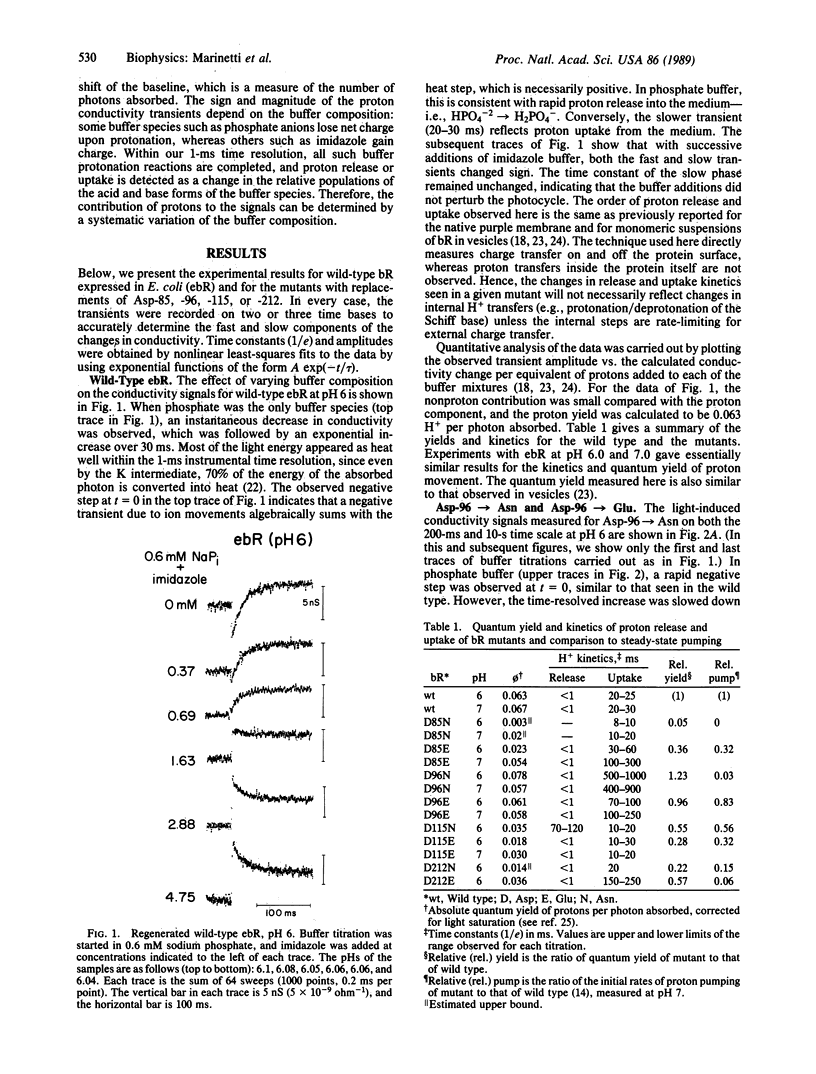
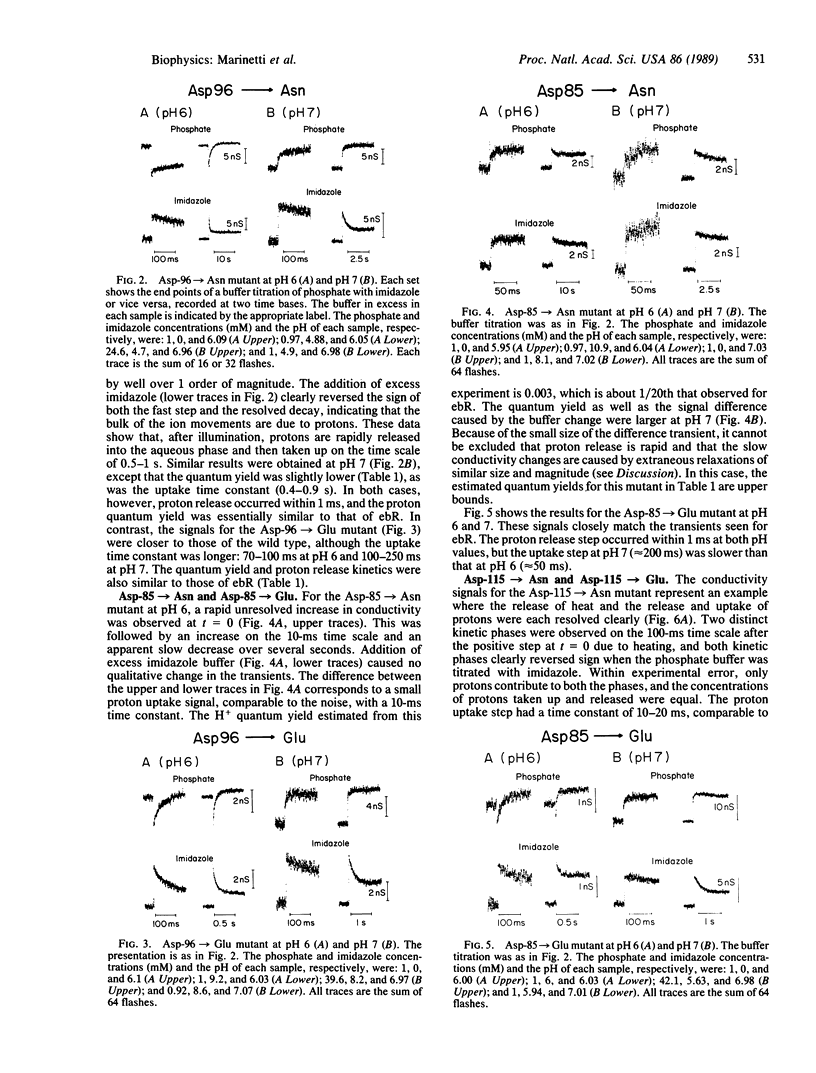
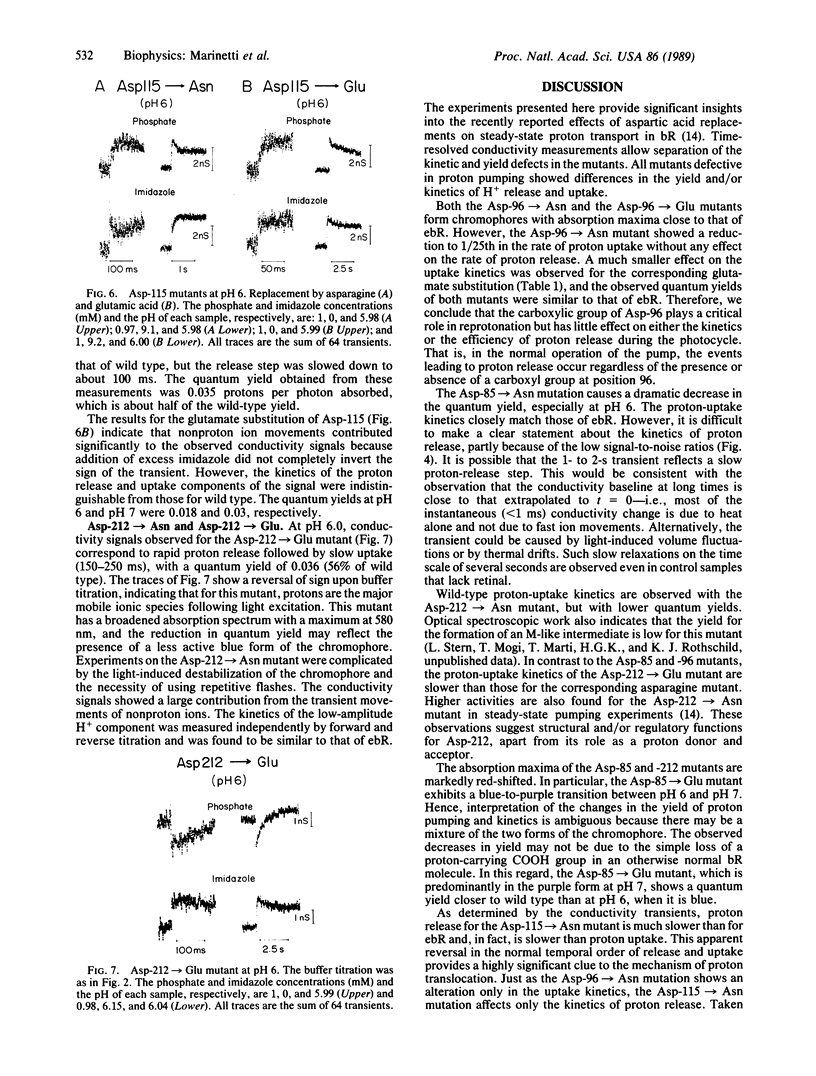
Recently, a number of aspartic acid mutants of bacteriorhodopsin have been shown to be defective in steady-state proton transport. Here we report time-resolved measurements of light-induced proton release and uptake for these mutants. Proton transfers between the protein and the aqueous phase were directly monitored by measuring changes in the bulk conductivity of a micellar solution of bacteriorhodopsin. For the Asp-96----Asn mutant, proton uptake was slowed by greater than 1 order of magnitude with no observable effect on the release step. For Asp-85----Asn, H+ uptake occurred with normal kinetics, but the yield was significantly lower compared with either the Asp-96----Asn mutant or wild type, especially at pH 6. Substitution of glutamate for Asp-85 or Asp-96 had smaller but detectable effects on the kinetics and quantum yield of proton movements. Both asparagine and glutamate substitutions of aspartates at positions 115 and 212 lowered the proton quantum yields. Of these, only the Asp-115----Asn mutant showed an effect on the proton release step, and only the Asp-212----Glu mutation decreased the proton uptake rate. These experiments imply an obligatory role for Asp-96 in H+ uptake in the normal operation of the bacteriorhodopsin proton pump. The results also indicate that the amino acid substitutions affect the kinetics of either H+ release or H+ uptake, but not both. This implies that the two steps occur independently of each other after initiation of the photocycle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahl P. L., Stern L. J., Düring D., Mogi T., Khorana H. G., Rothschild K. J. Effects of amino acid substitutions in the F helix of bacteriorhodopsin. Low temperature ultraviolet/visible difference spectroscopy. J Biol Chem. 1988 Sep 25;263(27):13594–13601. [PubMed] [Google Scholar]
- Birge R. R., Cooper T. M. Energy storage in the primary step of the photocycle of bacteriorhodopsin. Biophys J. 1983 Apr;42(1):61–69. doi: 10.1016/S0006-3495(83)84369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Stern L. J., Hackett N. R., Chao B. H., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: I. Tyrosine-185 protonates and deprotonates during the photocycle. Proteins. 1988;3(4):219–229. doi: 10.1002/prot.340030403. [DOI] [PubMed] [Google Scholar]
- Braiman M. S., Stern L. J., Chao B. H., Khorana H. G. Structure-function studies on bacteriorhodopsin. IV. Purification and renaturation of bacterio-opsin polypeptide expressed in Escherichia coli. J Biol Chem. 1987 Jul 5;262(19):9271–9276. [PubMed] [Google Scholar]
- Braiman M., Mathies R. Resonance Raman spectra of bacteriorhodopsin's primary photoproduct: evidence for a distorted 13-cis retinal chromophore. Proc Natl Acad Sci U S A. 1982 Jan;79(2):403–407. doi: 10.1073/pnas.79.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard M., Gerwert K., Hess B., Kreutz W., Siebert F. Light-driven protonation changes of internal aspartic acids of bacteriorhodopsin: an investigation by static and time-resolved infrared difference spectroscopy using [4-13C]aspartic acid labeled purple membrane. Biochemistry. 1985 Jan 15;24(2):400–407. doi: 10.1021/bi00323a024. [DOI] [PubMed] [Google Scholar]
- Hackett N. R., Stern L. J., Chao B. H., Kronis K. A., Khorana H. G. Structure-function studies on bacteriorhodopsin. V. Effects of amino acid substitutions in the putative helix F. J Biol Chem. 1987 Jul 5;262(19):9277–9284. [PubMed] [Google Scholar]
- Karnik S. S., Nassal M., Doi T., Jay E., Sgaramella V., Khorana H. G. Structure-function studies on bacteriorhodopsin. II. Improved expression of the bacterio-opsin gene in Escherichia coli. J Biol Chem. 1987 Jul 5;262(19):9255–9263. [PubMed] [Google Scholar]
- Khorana H. G. Bacteriorhodopsin, a membrane protein that uses light to translocate protons. J Biol Chem. 1988 Jun 5;263(16):7439–7442. [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W., Bogomolni R. A., Hwang S., Stoeckenius W. Kinetics and stoichiometry of light-induced proton release and uptake from purple membrane fragments, Halobacterium halobium cell envelopes, and phospholipid vesicles containing oriented purple membrane. Biochim Biophys Acta. 1976 Sep 13;440(3):545–556. doi: 10.1016/0005-2728(76)90041-4. [DOI] [PubMed] [Google Scholar]
- Marinetti T. Abrupt onset of large scale nonproton ion release in purple membranes caused by increasing pH or ionic strength. Biophys J. 1987 Jun;51(6):875–881. doi: 10.1016/S0006-3495(87)83415-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti T. Large scale nonproton ion release and bacteriorhodopsin's state of aggregation in lipid vesicles. I. Monomers. Biophys J. 1987 Jul;52(1):115–121. doi: 10.1016/S0006-3495(87)83195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti T., Mauzerall D. Absolute quantum yields and proof of proton and nonproton transient release and uptake in photoexcited bacteriorhodopsin. Proc Natl Acad Sci U S A. 1983 Jan;80(1):178–180. doi: 10.1073/pnas.80.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti T., Mauzerall D. Large transient nonproton ion movements in purple membrane suspensions are abolished by solubilization in Triton X-100. Biophys J. 1986 Sep;50(3):405–415. doi: 10.1016/S0006-3495(86)83476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti T. Nonproton ion release by purple membranes exhibits cooperativity as shown by determination of the optical cross-section. Biophys J. 1988 Aug;54(2):197–204. doi: 10.1016/S0006-3495(88)82948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi T., Stern L. J., Hackett N. R., Khorana H. G. Bacteriorhodopsin mutants containing single tyrosine to phenylalanine substitutions are all active in proton translocation. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5595–5599. doi: 10.1073/pnas.84.16.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi T., Stern L. J., Marti T., Chao B. H., Khorana H. G. Aspartic acid substitutions affect proton translocation by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4148–4152. doi: 10.1073/pnas.85.12.4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassal M., Mogi T., Karnik S. S., Khorana H. G. Structure-function studies on bacteriorhodopsin. III. Total synthesis of a gene for bacterio-opsin and its expression in Escherichia coli. J Biol Chem. 1987 Jul 5;262(19):9264–9270. [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Zagaeski M., Cantore W. A. Conformational changes of bacteriorhodopsin detected by Fourier transform infrared difference spectroscopy. Biochem Biophys Res Commun. 1981 Nov 30;103(2):483–489. doi: 10.1016/0006-291x(81)90478-2. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Xie A. H., Nagle J. F., Lozier R. H. Flash spectroscopy of purple membrane. Biophys J. 1987 Apr;51(4):627–635. doi: 10.1016/S0006-3495(87)83387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]



