Abstract
While much attention has been paid to the effects of inbreeding on fitness, this has mostly come from a genetic perspective. Consequently, the interaction between inbreeding and the environment is less well understood. To understand the effects of inbreeding in natural populations where environmental conditions are variable, we need to examine not only how the effects of inbreeding change among environments but also how inbreeding may affect the ability to respond to environmental conditions (i.e. phenotypic plasticity). We reared selfed and outcrossed hermaphroditic snails (Physa acuta) in the presence and absence of chemical cues from predatory crayfish and quantified expression of an inducible defence, an adaptively plastic response to predation risk. Overall, inbred snails exhibited reduced defences, but more importantly, inbreeding reduced the expression of predator-induced adaptive plasticity. Inbreeding depression in defensive morphology was 26 per cent and inbreeding depression in the plasticity of this trait was 48 per cent. Inbreeding depression in adaptive plasticity may be important to understanding the effects of inbreeding in nature.
Keywords: genotype-by-environment interaction, predator–prey interaction, shell thickness
1. Introduction
The effects of inbreeding, and of mating systems in general, on fitness and related traits have been approached from a primarily genetic perspective which has resulted in a rich theory describing, among other things, the evolution of inbreeding depression, heterosis, life histories and mating systems (Charlesworth 2003; Goodwillie et al. 2005). While this perspective has yielded great insights, mounting evidence demonstrates that inbreeding effects are environment-dependent (e.g. Bijlsma et al. 1999; Carr & Eubanks 2002; Ivey et al. 2004; Armbruster & Reed 2005; Waller et al. 2008). There is a great diversity of mating systems in nature (Goodwillie et al. 2005; Jarne & Auld 2006), and the role of environmental conditions in the evolution of mating systems is increasingly discussed (e.g. Steets et al. 2007).
In natural populations, environmental conditions are variable and this can favour the evolution of phenotypic plasticity (Pigliucci 2001). In the same way that inbreeding can affect fitness and fitness-related traits, it may also affect trait plasticity (Maynard Smith et al. 1955). Inbreeding may directly affect plasticity by altering phenotypic expression in one environment or by altering the organism's ability to detect or respond appropriately to different environmental conditions. For example, if inbreeding results in depressed growth or a decreased ability to detect an environmental change, this could affect an organism's ability to produce a phenotype that is well suited to the environment. While several studies have examined how the effects of inbreeding change across environments (e.g. Carr & Eubanks 2002; Henry et al. 2003; Armbruster & Reed 2005), we know very little about how inbreeding may affect the ability to respond adaptively to environmental conditions. If such dynamics occur, they could represent an important yet neglected component of overall inbreeding depression.
In this paper, we examine the effects of inbreeding on the expression of adaptive plasticity (i.e. the expression of an inducible defence in response to predation risk). Previous work has shown that inbreeding depression in fitness is strong in this species (Jarne et al. 2000; Henry et al. 2003), that predators induce thicker shells, and snails with the thickest shells experience the highest survival when exposed to lethal predators (J. R. Auld & R. A. Relyea 2008, unpublished data). Here, we decipher the effects of inbreeding on trait expression and trait plasticity to determine if inbreeding affects the plastic response to predation risk.
2. Material and Methods
We conducted an experiment using Physa acuta snails whose grandparents (G0) were collected from Geneva pond no. 3 in northwestern Pennsylvania. Physa acuta is a self-compatible preferential outcrosser that stores sperm (outcrossing rates more than 90% in natural populations, Wethington & Dillon 1991; Henry et al. 2005), so we assumed that the G1 progeny were outcrossed. Breeding lines were maintained to produce same-aged selfed and outcrossed G2 offspring. Siblings from 10 G1 families were split into two groups to be outcrossed or selfed. To outcross the G1 snails, individuals were reared with a different potential mate (marked with non-toxic paint) that was changed every 24 h for a two-week period. Selfing snails were left alone until they initiated reproduction. While this study was conducted separately, the experimental conditions and protocols for these breeding lines and the subsequent experiment were identical to those reported in Auld & Relyea (2008) including snail feeding (ad libitum Spirulina) and water changes.
We randomly chose two G2 snails from each outcrossing and selfing line to be reared individually in 1 l of either freshwater or predator-conditioned water. Therefore, with 10 families, two inbreeding treatments, two predator treatments and two replicate snails, the total potential sample size was 80 snails. However, two of the selfed lines yielded no offspring when we set up the experiment and all offspring of two other selfed lines died before the experiment was completed. Therefore, the final sample size was 68 snails. Individual juvenile G2 snails were added to the containers on 30 May 2006 (age = 29 days; initial mass less than 1 mg); predator-cue treatments began the following day. We produced crayfish-conditioned water by feeding a pond-dwelling crayfish (Procambarus acutus) 150 mg of Physa acuta three times per week. Prior to each feeding, we collected the water in which each crayfish was held, pooled the water from all crayfish (n = 20), removed 400 ml of water from each experimental unit assigned the predator treatment and replaced it with 400 ml of predator-cue water. Water was likewise changed in the no-predator treatment by replacing 400 ml of freshwater. After five weeks, we measured shell thickness to the nearest 0.01 mm at the leading edge of the shell with digital calipers. This time period coincided with the initiation of reproduction and has been shown to be appropriate for quantifying predator-induced shell-thickness plasticity (Auld & Relyea 2008). Additionally, we measured the mass of each snail and, given that preliminary analyses revealed shell thickness was not correlated with mass, we did not mass-adjust our measure of shell thickness.
To analyse the data, we began by analysing the main and interactive effects of the predator and breeding treatments on shell thickness using an analysis of variance. In this analysis, the predator effect represents plasticity in shell thickness, the inbreeding effect represents inbreeding depression in shell thickness, and the interaction term represents inbreeding depression in shell-thickness plasticity. We calculated plasticity within each breeding treatment as the difference between predator and no-predator environments for each family. Inbreeding depression was estimated as (1−xi/xo), where xi was the shell thickness of inbred snails and xo was the shell thickness of outcrossed snails. We also explored the potential for variation in inbreeding depression among families as a family-by-inbreeding interaction, but found that the effect was insignificant and therefore only report the results of the simpler model.
3. Results
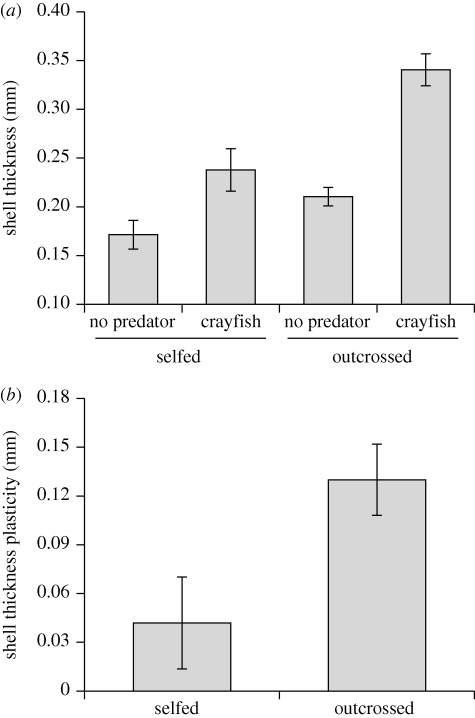
The analysis of variance indicated that shell thickness was affected by inbreeding (F1,64 = 20.08, p < 0.001), predator cues (F1,64 = 44.49, p < 0.001) and their interaction (F1,64 = 4.04, p < 0.05; figure 1a). On average, inbred snails had 26 per cent thinner shells than outcrossed snails and the predator environment induced 54 per cent thicker shells. However, as indicated by the interaction, selfed snails exhibited a weaker predator-induced defence. Indeed, when we quantified the plasticity of the shell thickness, we found that outcrossed snails possessed greater predator-induced shell-thickness plasticity than inbred snails (figure 1b). Based on these data, we estimated inbreeding depression for shell thickness plasticity as 48 per cent.
Figure 1.
(a) The effects of mating system (selfed or outcrossed) and predation risk (no predator or non-lethal crayfish) on shell thickness in Physa acuta (treatment means ± 1 s.e.). (b) Inbreeding depression in predator-induced plasticity is shown as the reduced plasticity of selfed snails compared to outcrossed snails (among-family means ± 1 s.e.).
4. Discussion
This study reveals a novel way in which inbreeding may affect fitness under natural conditions. Inbreeding depression in reproductive fitness has been long known, but here we highlight that inbreeding can also affect the adaptive ability to respond to environmental conditions. Inbreeding may affect plasticity for several reasons, including an overall fitness depression owing to the combined effects of multiple loci (e.g. reduced growth ability), a loss of heterozygosity at overdominant loci involved in trait expression, and/or the expression of a deleterious recessive allele at a particular locus involved in trait expression. Thereby, an adaptively plastic response may be precluded owing to an overall impairment of the ability to detect or respond to the environment that results from many loci or a single locus, where homozygosity at a single locus may directly affect the trait in question or have other more general effects.
While other studies have examined how inbreeding can affect tolerance and/or resistance of plants to herbivores and pathogens (e.g. Ouborg et al. 2000; Carr & Eubanks 2002; Carr et al. 2003; Ivey et al. 2004; Stephenson et al. 2004; Ivey & Carr 2005), these studies have not examined the effects of inbreeding on the inducibility of a trait. Maynard Smith (1956) reared three inbred lines of Drosophila subobscura and their three F1 hybrids under several temperature regimes to test the hypothesis that the inbred lines would be less responsive to heat stress than their F1 hybrids. He found that the inbred lines were indeed less responsive, suggesting that inbreeding effects on plasticity may occur in other systems. Several studies have examined the effects of inbreeding on phenotypic stability to test the hypothesis that heterozygosity and plasticity may be related and that inbreeding may decrease developmental stability (i.e. increase plasticity, Lerner 1954). As an example, Schlichting & Levin (1986) grew Phlox in six environments and found no effect of inbreeding on developmental instability (i.e. no difference in plasticity between inbred and outbred plants). These results contrast with ours and highlight how little we know about the interaction between inbreeding and plasticity.
Inbreeding depression in plasticity is potentially important for understanding selection against inbred individuals in a natural population. Importantly, fitness depression that results from inbreeding may be strong for a particular trait that is under selection in particular environments (e.g. an inducible defence), and thereby the mating system may be under correlated selection through its association with a trait even if that association only exists in certain environments (e.g. with predators). Therefore, depending on the strength and direction of the genetic correlations among traits across environments, the evolution of plasticity may be facilitated or constrained by selection in certain environments (Via & Lande 1985).
While it is known that inbreeding depression can change across environments (e.g. Armbruster & Reed 2005), this study highlights the importance of understanding how inbreeding affects the ability to respond to the environment. Our results point to a novel concern in considering environment-specific inbreeding depression: if inbred organisms not only experience reduced fitness, but also an impaired ability to detect or respond to the environment in an adaptive fashion, this may have implications for understanding the evolution of inbreeding depression and mating systems in natural populations. This highlights the importance of considering the environment in measuring fitness, particularly when the fitness effects of the mating system change across environments.
Acknowledgements
We received funding from the National Science Foundation, A.W. Mellon Foundation, Pymatuning Laboratory of Ecology, Malacological Society of London, and Unitas Malacologica. We thank C. Cox, and D. Jones for help with the experiment and J. Escobar, P. Jarne, and J. Steets for comments on the manuscript.
References
- Armbruster P., Reed D. H.2005Inbreeding depression in benign and stressful environments. Heredity 95, 235–242 (doi:10.1038/sj.hdy.6800721) [DOI] [PubMed] [Google Scholar]
- Auld J. R., Relyea R. A.2008Are there interactive effects of mate availability and predation risk on life history and defense in a simultaneous hermaphrodite? J. Evol. Biol. 21, 1371–1378 (doi:10.1111/j.1420-9101.2008.01562.x) [DOI] [PubMed] [Google Scholar]
- Bijlsma R., Bundgaard J., Van Putten W. F.1999Environmental dependence of inbreeding depression and purging in Drosophila melanogaster. J. Evol. Biol. 12, 1125–1137 (doi:10.1046/j.1420-9101.1999.00113.x) [Google Scholar]
- Carr D. E., Eubanks M. D.2002Inbreeding alters resistance to insect herbivory and host plant quality in Mimulus guttatus (Scrophulariaceae). Evolution 56, 22–30 [DOI] [PubMed] [Google Scholar]
- Carr D. E., Murphy J. F., Eubanks M. D.2003Susceptibility and response of inbred and outbred Mimulus guttatus to infection by Cucumber mosaic virus. Evol. Ecol. 17, 85–103 (doi:10.1023/A:1022439432213) [Google Scholar]
- Charlesworth D.2003Effects of inbreeding on the genetic diversity of populations. Phil. Trans. R. Soc. Lond. B 358, 1051–1070 (doi:10.1098/rstb.2003.1296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwillie C., Kalisz S., Eckert C. G.2005The evolutionary enigma of mixed mating in plants: occurrence, theoretical explanations, and empirical evidence. Ann. Rev. Ecol. Evol. Syst. 36, 47–79 (doi:10.1146/annurev.ecolsys.36.091704.175539) [Google Scholar]
- Henry P.-Y., Pradel R., Jarne P.2003Environment-dependent inbreeding depression in a hermaphroditic freshwater snail. J. Evol. Biol. 16, 1211–1222 (doi:10.1046/j.1420-9101.2003.00629.x) [DOI] [PubMed] [Google Scholar]
- Henry P.-Y., Bousset L., Sourrouille P., Jarne P.2005Partial selfing, ecological disturbance, and reproductive assurance in an invasive freshwater snail. Heredity 95, 428–436 (doi:10.1038/sj.hdy.6800731) [DOI] [PubMed] [Google Scholar]
- Ivey C. T., Carr D. E.2005Effects of herbivory and inbreeding on the pollinators and mating system on Mimulus guttatus (Phrymaceae). Am. J. Bot. 92, 1641–1649 (doi:10.3732/ajb.92.10.1641) [DOI] [PubMed] [Google Scholar]
- Ivey C. T., Carr D. E., Eubanks M. D.2004Effects of inbreeding in Mimulus guttatus on tolerance to herbivory in natural environments. Ecology 85, 567–574 (doi:10.1890/02-0730) [Google Scholar]
- Jarne P., Auld J. R.2006Animals mix it up too: the distribution of self-fertilization among hermaphroditic animals. Evolution 60, 1816–1824 [DOI] [PubMed] [Google Scholar]
- Jarne P., Perdieu M.-A., Pernot A.-F., Delay B., David P.2000The influence of self-fertilization and grouping on fitness attributes in the freshwater snail Physa acuta: population and individual inbreeding depression. J. Evol. Biol. 13, 645–655 (doi:10.1046/j.1420-9101.2000.00204.x) [Google Scholar]
- Lerner I. M.1954Genetic homeostasis New York, NY: Dover Publishing [Google Scholar]
- Maynard Smith J.1956Acclimatization to high temperatures in inbred and outbred Drosophila subobscura. J. Genet. 54, 497–505 [DOI] [PubMed] [Google Scholar]
- Maynard Smith J., Clarke J. M., Hollingsworth M. J.1955The expression of hybrid vigor in Drosophila subobscura. Proc. R. Soc. Lond. B 144, 159–171(doi:10.1098/rspb.1955.0042) [DOI] [PubMed] [Google Scholar]
- Ouborg N. J., Biere A., Mudde C. L.2000Inbreeding effects on resistance and transmission-related traits in the Silene-Microbotryum pathosystem. Ecology 81, 520–531 [Google Scholar]
- Pigliucci M.2001Phenotypic plasticity: beyond nature and nurture Baltimore, MD: The Johns Hopkins University Press [Google Scholar]
- Schlichting C. D., Levin D. A.1986Effects of inbreeding on phenotypic plasticity in cultivated Phlox. Theor. Appl. Genet. 72, 114–119 [DOI] [PubMed] [Google Scholar]
- Steets J. A., Wolf D. E., Auld J. R., Ashman L.2007The role of natural enemies in the expression and evolution of mixed mating in hermaphroditic plants and animals. Evolution 61, 2043–2055 (doi:10.1111/j.1558-5646.2007.00184.x) [DOI] [PubMed] [Google Scholar]
- Stephenson A. G., Leyshon B., Travers S. E., Hayes C. N., Windsor J. A.2004Interrelationships among inbreeding, herbivory, and disease on reproduction in a wild gourd. Ecology 85, 3023–3034 (doi:10.1890/04-0005) [Google Scholar]
- Via S., Lande R.1985Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522 (doi:10.2307/2408649) [DOI] [PubMed] [Google Scholar]
- Waller D. M., Dole J., Bersch A. J.2008Effects of stress and phenotypic variation on inbreeding depression in Brassica rapa. Evolution 62, 917–931 (doi:10.1111/j.1558-5646.2008.00325.x) [DOI] [PubMed] [Google Scholar]
- Wethington A. R., Dillon R. T.1991Sperm storage and evidence for multiple insemination in a natural population of the freshwater snail, Physa. Am. Malacol. Bull. 9, 99–102 [Google Scholar]