Abstract
The fitness consequences of female ornamentation remain little studied and the results are often contradictory. Female ornamentation may be an artefact of a genetic correlation with male ornamentation, but this possibility can be disregarded if the ornament only occurs in females. Female-specific white wing bars in eiders (Somateria mollissima) have been suggested to indicate individual quality, and we studied size variation in this trait in relation to key fitness components and quality attributes. We found that clutch size, body condition, female age, hatching date and success were unrelated to female ornament size; ornament size was explained by its size in the previous year. In contrast, good body condition was associated with hatching success. These results suggest that the breadth of the white wing bars does not indicate individual quality in our study population.
Keywords: female ornament, individual quality, breeding, body condition, eider
1. Introduction
Bright and showy ornaments are typical for males and commonly explained as the result of sexual selection (Darwin 1871; Andersson 1994). Explaining the evolution of ornamented females has proven more difficult. Female ornaments may merely represent a genetically correlated result of sexual selection on males (Lande 1980), but they may also be selected through female competition over mates or resources (Amundsen & Pärn 2006). Owing to this ambiguity regarding the evolution of female ornaments, the functional importance of such ornaments is also debated (Amundsen 2000). Female ornamentation has mainly been studied in birds (Amundsen 2000); some studies have found a connection between female ornaments and phenotypic quality (Møller 1993; Johnsen et al. 1996; Jawor et al. 2004), while others have not (Muma & Weatherhead 1989; Coervo et al. 1996).
Hanssen et al. (2006, 2008) suggested a connection between female ornamentation and phenotypic quality in female eider ducks (Somateria mollissima). These studies found that females with broader and brighter white wing bars had higher immunocompetence, lower mass loss during incubation and marginally larger clutches. Eiders are especially well suited for exploring the role of female ornaments as indicators of individual quality, because the ornament is expressed only in females (Roulin et al. 2001; Hanssen et al. 2006). This feature alleviates the risk of the ornament being a genetic correlate of a trait selected for in males (Amundsen 2000). Furthermore, eider brood-rearing tactics range from lone tending to brood care in female coalitions (Öst et al. 2003a). Hanssen et al. (2006, 2008) therefore proposed that female ornaments may be honest signals of individual quality used not only by courting males, but also in female–female interactions in brood-rearing coalitions.
Body condition underlies variation in brood rearing tactics, and individuals switch between tactics typical of good-condition individuals (lone tending) and tactics employed by individuals in intermediate or poor condition (coalition formation or brood desertion) according to annual fluctuations in their condition (Bustnes & Erikstad 1991; Öst et al. 2003a,b, 2007, 2008a). Body condition strongly reflects phenotypic quality: clutch size and female condition are positively correlated, and the hatch date and body condition of lone-tending females together affect duckling survival (Öst et al. 2008a). If the ornaments of eider females are honest signals of quality, they should show annual fluctuations matching the ones observed in body condition. They may also be expected to respond to previous breeding stress by decreasing in size after a stringent bout of parental care (Hanssen et al. 2006). Assuming that wing bars represent genetic quality, their size would be predicted to be positively correlated with breeding success (Török et al. 2003; Hegyi et al. 2008).
The aim of our study is to revisit the role of female ornamentation as a determinant of individual quality and breeding success in eiders. Using an extensive 4-year dataset, we explore inter- and intra-individual variation in female ornament size in order to establish whether they are size invariant or co-vary with female age or body condition. We also explore the relationship between female ornament size and the timing of breeding, clutch size and nesting success.
2. Material and methods
This study was conducted during 2006–2009 at Tvärminne, western Gulf of Finland (59° N, 23° E). After capturing incubating eiders with hand nets (751 captures of 529 females), we recorded clutch size, hatch date, body condition, breeding experience and hatching success (hatched or destroyed). Hatch date was estimated by egg floatation (Kilpi & Lindström 1997), breeding experience by the years since first capture and standardized body condition indices were calculated according to Öst et al. (2003b). We excluded parasitized clutches exceeding seven eggs (Waldeck et al. 2004) and clutches incubated less than 8 days (laying may be in progress; Öst et al. 2008b). The ornaments of one wing of each female were photographed, and the maximum breadth of the white area was measured on two secondaries and two greater coverts. We photographed 157 females at least twice and 12 females during all 4 years. All measurements were done using the Image J software (http://rsbweb.nih.gov/ij/index.html) and calibrated by a 1 cm measure bar in each photograph. Both double measurements of ornament breadth were strongly positively correlated, suggesting high repeatability (rp1,750 = 0.91, p < 0.001 for both variables). The average breadth of white on the secondaries was strongly positively correlated with the breadth of white on the greater coverts (rp1,750 = 0.62, p < 0.001). The average of all four measures was used as a wing bar index (WBI).
The total dataset and data on females captured in subsequent years were analysed separately. All models included year to account for inter-annual variation. We used the total dataset to determine (i) the variance contribution of individual identity in explaining WBI with the overall mean as the only fixed effect, (ii) WBI as a function of female age and condition, (iii) hatch date as a function of WBI and condition, and (iv) clutch size as a function of WBI and condition. The restricted dataset was used to assess (v) WBI in year t as a function of WBI in year t−1, condition in years t and t−1 and hatching success in year t−1 and (vi) hatching success as a function of WBI and condition.
Because some individuals were sampled repeatedly, we performed general linear mixed models (GLMMs) using restricted maximum likelihood and female identity as a random effect. Female experience, nested within individual, was included as a repeated measure when analysing female age. In analysis (vi), however, we used ordinary logistic regression, since female identity, based on penalized quasi-likelihood parameter estimation, explained only a minute fraction (0.005%) of variance, and the analysis of logistic generalized linear mixed models is debatable (Breslow & Lin 1995).
3. Results
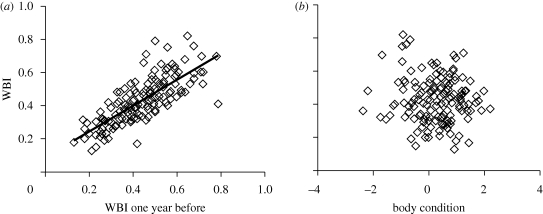
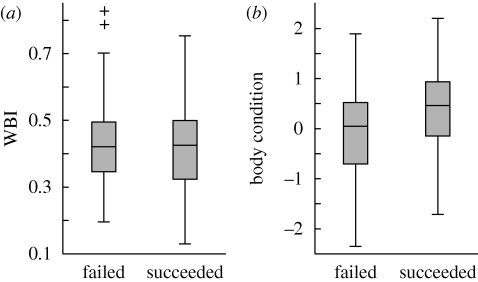
Individual identity explained 75.2 per cent of the variation in WBI. WBI was unaffected by body condition or female breeding experience, but a significant year effect was present (electronic supplementary material, table S1; figure 1a). Hatch date was not explained by WBI, but was significantly affected by body condition and year (table 1). Clutch size was not correlated with WBI, while it significantly increased with increasing condition (table 1). Body condition in year t or t−1 or hatching success in year t−1 did not influence WBI in year t, which was explained by WBI in year t−1 (electronic supplementary material, table S2; figure 1a,b). Hatching success was unrelated to WBI, whereas good condition was associated with higher hatching success (table 2, figure 2).
Figure 1.
WBI in relation to (a) WBI in the preceding year and (b) body condition. Linear trend lines illustrate significant correlations between variables.
Table 1.
GLMM analysis of the effects of the WBI, body condition and year on clutch size and hatch date in eiders (individual identity as random effect).
| variable | clutch size |
hatch date |
||||
|---|---|---|---|---|---|---|
| d.f. | F-value | p-value | d.f. | F-value | p-value | |
| intercept | 1,466 | 12 504.01 | <0.0001 | 1,469 | 79 040.64 | <0.0001 |
| wing bar | 1,198 | 1.80 | 0.18 | 1,202 | 0.88 | 0.35 |
| body condition | 1,198 | 5.38 | 0.02 | 1,202 | 52.40 | <0.0001 |
| year | 1,198 | 2.40 | 0.12 | 1,202 | 226.41 | <0.0001 |
Table 2.
Logistic regression analysis of the effects of the WBI, body condition and year on the hatching success of eiders.
| variable | B | s.e. | Wald | d.f. | p-value | odds ratio |
|---|---|---|---|---|---|---|
| WBI | 1.14 | 1.73 | 0.43 | 1 | 0.51 | 3.13 |
| body condition | 0.73 | 0.29 | 6.41 | 1 | 0.01 | 2.07 |
| year | 0.03 | 0.30 | 0.01 | 1 | 0.93 | 1.03 |
Figure 2.
(a) WBI and (b) body condition of female eiders that failed or succeeded in hatching their clutch.
4. Discussion
The breadth of female wing bars reflected individual variation and related neither to breeding variables (hatch date, clutch size, hatching success) nor female state variables (body condition, age). It was noteworthy that ornament size did not co-vary with body condition or hatching success in the previous year. Poor condition could hamper the production of ornaments, and failed breeders should have more time to acquire resources for wing-feather moult, thereby producing bigger ornaments. Our results partly contrast with those of Hanssen et al. (2006, 2008), where broader and brighter wing bars were associated with lower weight loss during incubation, presumably resulting in better body condition at hatching, and marginally larger clutches. We could not establish such connections, suggesting that wing bar breadth is not necessarily an indicator of individual quality in our population, but rather remains stable over time. One explanation could be that the costs of producing depigmented plumage are low (Hanssen et al. 2006), so a relationship between ornament expression and our breeding parameters, many of which are condition dependent, may not exist.
It is unlikely that a connection between ornament breadth and female quality would remain undetected, given our extensive data allowing, for the first time, longitudinal analyses of female ornamentation in eiders. It is therefore intriguing that our study and those of Hanssen et al. (2006, 2008) arrive at partly different conclusions. As our study did not examine female immune responses, the disparity may be related to this aspect of individual quality. The relationship between immunocompetence and body condition, clutch size and laying date in fasting eiders is still unclear (Hanssen et al. 2003; Bourgeon & Raclot 2006).
Clutch size increased with increasing body condition and earlier breeding, and birds in poorer condition experienced a higher probability of nesting failure, agreeing with previous findings (Bustnes & Erikstad 1991; Öst et al. 2003a,b, 2008a). Thus, we are convinced that these variables truly represent female phenotypic quality, but remained unrelated to ornament breadth. Our results imply that courting males may be unable to judge the fecundity (clutch size and hatching success) of their partners by observing the white wing bands.
Different components of a given ornament are often correlated (Jawor et al. 2004; Hanssen et al. 2006). In eiders, the breadth of the white wing bars is an informative feature of the proposed ornament, also correlating with the human perception of ornament brightness (Hanssen et al. 2006). Thereby, our failure to link ornament expression with female quality is unlikely to be a methodological artefact and truly representing the lack of such a relationship in our population. Our results call for further investigation of the relationship between female ornamentation and quality, and of the complex inter-relationships between ornamentation, body condition and immunocompetence.
Acknowledgements
Tvärminne Zoological Station provided facilities and Maj and Tor Nessling Foundation (A.L.) and the Academy of Finland (grant no. 128039 to K.J. and M.Ö.) funding.
References
- Amundsen T.2000Why are female birds ornamented? Trends Ecol. Evol. 15, 149–155 (doi:10.1016/S0169-5347(99)01800-5) [DOI] [PubMed] [Google Scholar]
- Amundsen T., Pärn H.2006Female coloration: a review of functional and nonfunctional hypotheses. In Bird coloration, vol. II: function and evolution (eds Hill G. E., McGraw K. J.), pp. 280–345 Cambridge, MA: Harvard University Press [Google Scholar]
- Andersson M.1994Sexual selection Princeton, NJ: Princeton University Press [Google Scholar]
- Bourgeon S., Raclot T.2006Corticosterone selectively decreases humoral immunity in female eiders during incubation. J. Exp. Biol. 209, 4957–4965 (doi:10.1242/jeb.02610) [DOI] [PubMed] [Google Scholar]
- Breslow N. E., Lin X. H.1995Bias correction in generalized linear mixed models with a single component of dispersion. Biometrika 82, 81–91 (doi:10.1093/biomet/82.1.81) [Google Scholar]
- Bustnes J. O., Erikstad K. E.1991Parental care in the common eider (Somateria mollissima): factors affecting abandonment and adoption of young. Can. J. Zool. 69, 1538–1545 (doi:10.1139/z91-216) [Google Scholar]
- Cuervo J. J., de Lope F., Møller A. P.1996The function of long tails in female barn swallows (Hirundo rustica): an experimental study. Behav. Ecol. 7, 132–136 (doi:10.1093/beheco/7.2.132) [Google Scholar]
- Darwin C.1871The descent of man, and selection in relation to sex. London, UK: Murrey [Google Scholar]
- Hanssen S. A., Folstad I., Erikstad K. E.2003Reduced immunocompetence and cost of reproduction in common eiders. Oecologia 136, 457–464 (doi:10.1007/s00442-003-1282-8) [DOI] [PubMed] [Google Scholar]
- Hanssen S. A., Folstad I., Erikstad K. E.2006White plumage reflects individual quality in female eiders. Anim. Behav. 71, 337–343 (doi:10.1016/j.anbehav.2005.04.021) [Google Scholar]
- Hanssen S. A., Hasselquist D., Folstad I., Erikstad K. E.2008A label of health: a previous immune challenge is reflected in the expression of a female plumage trait. Biol. Lett 4, 379–381 (doi:10.1098/rsbl.2008.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi G., Rosivall B., Szollosi E., Hargitai R., Eens M., Török J.2008Phenotypic plasticity in a conspicuous female plumage trait: information content and mating patterns. Anim. Behav. 77, 977–989 (doi:10.1016/j.anbehav.2007.08.009) [Google Scholar]
- Jawor J. M., Gray N., Beall S. M., Breitwisch R.2004Multiple ornaments correlate with aspects of condition and behaviour in female northern cardinals Cardinalis cardinalis. Anim. Behav. 67, 875–882 (doi:10.1016/j.anbehav.2003.05.015) [Google Scholar]
- Johnsen T. S., Hengeveld J. D., Blank J. L., Yasukawa K., Nolan V., Jr1996Epaulet brightness and condition in female red-winged blackbirds. Auk 113, 356–362 [Google Scholar]
- Kilpi M., Lindström K.1997Habitat-specific clutch size and cost of incubation in common eiders, Somateria mollissima. Oecologia 111, 297–301 (doi:10.1007/s004420050238) [DOI] [PubMed] [Google Scholar]
- Lande R.1980Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305 (doi:10.2307/2407393) [DOI] [PubMed] [Google Scholar]
- Møller A. P.1993Sexual selection in the barn swallow Hirundo rustica III. Female tail ornaments. Evolution 47, 417–431 (doi:10.2307/2410061) [DOI] [PubMed] [Google Scholar]
- Muma K. E., Weatherhead P. J.1989Male traits expressed in females: direct or indirect sexual selection? Behav. Ecol. Sociobiol 25, 23–31 (doi:10.1007/BF00299707) [Google Scholar]
- Öst M., Ydenberg R., Kilpi M., Lindström K.2003aCondition and coalition formation by brood-rearing common eider females. Behav. Ecol. 14, 311–317 (doi:10.1093/beheco/14.3.311) [Google Scholar]
- Öst M., Ydenberg R., Lindström K., Kilpi M.2003bBody condition and the grouping behavior of brood-caring female common eiders (Somateria mollissima). Behav. Ecol. Sociobiol. 54, 451–457 (doi:10.1007/s00265-003-0641-0) [Google Scholar]
- Öst M., Clark C. W., Kilpi M., Ydenberg R. C.2007Parental effort and reproductive skew in coalitions of brood-rearing female common eiders. Am. Nat. 169, 73–86 (doi:10.1086/510213) [DOI] [PubMed] [Google Scholar]
- Öst M., Smith B. D., Kilpi M.2008aSocial and maternal factors affecting duckling survival in eiders Somateria mollissima. J. Anim. Ecol. 77, 315–325 (doi:10.1111/j.1365-2656.2007.01348.x) [DOI] [PubMed] [Google Scholar]
- Öst M., Wickman M., Matulionis E., Steele B.2008bHabitat-specific clutch size and cost of incubation in eiders reconsidered. Oecologia 158, 205–216 (doi:10.1007/s00442-008-1139-2) [DOI] [PubMed] [Google Scholar]
- Roulin A., Dijkstra C., Riols C., Ducrest A. L.2001Female- and male-specific signals of quality in the barn owl. J. Evol. Biol. 14, 255–266 (doi:10.1046/j.1420-9101.2001.00274.x) [Google Scholar]
- Török J., Hegyi G., Garamszegi L. Z.2003Depigmented wing patch size is a condition-dependent indicator of viability in male collared flycatchers. Behav. Ecol. 14, 382–388 (doi:10.1093/beheco/14.3.382) [Google Scholar]
- Waldeck P., Kilpi M., Öst M., Andersson M.2004Brood parasitism in a population of common eider (Somateria mollissima). Behaviour 141, 725–739 (doi:10.1163/1568539042245132) [Google Scholar]