Abstract
Evidence for a developmental relationship between B cells and macrophages has led to the hypothesis that B cells evolved from a phagocytic predecessor. The recent identification of phagocytic IgM+ cells in fishes and amphibians supports this hypothesis, but raises the question of when, evolutionarily, was phagocytic capacity lost in B cells? To address this, leucocytes were isolated from red-eared sliders, Trachemys scripta, incubated with fluorescent beads and analysed using flow cytometry and confocal microscopy. Results indicate that red-eared slider B cells are able to ingest foreign particles and suggest that ectothermic vertebrates may use phagocytic B cells as part of a robust innate immune response.
Keywords: vertebrate, phagocytosis, ectotherm
1. Introduction
Phagocytosis is a key defensive tool for immune cells of multi-cellular organisms. During phagocytosis, foreign particles bind to specific receptors on the cell membrane of the phagocyte, triggering an alteration of the cytoskeleton which results in extensions forming around the particle (Kwiatkowska & Sobota 1999). These extensions enclose the particle into a phagosome which then fuses with lysosomes to form a phagolysosome, where the internalized particle is then degraded (Coico et al. 2003).
All vertebrates possess cells capable of phagocytosis or the ability to ingest foreign particles. In mammals, phagocytosis is accomplished through several cell types, including macrophages, but mammalian B cells are not considered to be phagocytic (Vidard et al. 1996; Li et al. 2006). B-1 cells are a small subpopulation of B cells and are involved in the production of natural antibodies (Choi & Baumgarth 2008). Why some cells are phagocytic while others are not is unknown, but it has been hypothesized that macrophages and B-1 cells may have evolved from a phagocytic predecessor that has characteristics of both cell types (Katsura 2002). This hypothesis has been supported by the recent discovery in teleost fishes (Onchorhyncus mykiss and Ictalurus punctatus) and an amphibian (Xenopus laevis) of IgM+ cells that were able to engulf 1 µm beads (Li et al. 2006). Identification of a phagocytic B cell in reptiles would further elucidate the evolutionary history of the B cell, as reptiles hold a unique evolutionary position being both ectotherms and amniotes.
Reptile leucocytes include macrophages, monocytes, heterophils, basophils, eosinophils, B cells and T cells. Of these, macrophages, monocytes and heterophils are traditionally considered to be the major phagocytic cell types of reptiles (Jurd 1994); the phagocytic ability of B cells has not been previously examined. Reptilian B cells express membrane IgM (Kawaguchi et al. 1980), and may also express IgD, as this isotype has also been identified in a reptile, the leopard gecko (Eublepharis macularius), with mRNA transcript expression levels and distribution similar to that of IgM (Deza & Espinel 2008). Similar to mammalian responses, reptilian B cells release antibody in response to immunization after a latent period of about one week. However, unlike mammalian responses that peak around two weeks (Coico et al. 2003), the time to peak antibody production in reptiles can be anywhere from six to eight weeks (Grey 1963; Marchalonis et al. 1969; Ingram & Molyneux 1983; Work et al. 2000). After a secondary immunization, the latent period is shortened (Work et al. 2000; Ujvari & Madsen 2005), but there is no increase in binding affinity (Grey 1963) and often no increase in titers compared to the primary response, reminiscent of mammalian B-1 responses (Grey 1963; Marchalonis et al. 1969; Kanakambika & Muthukkaruppan 1972). Thus, despite many similarities, it is clear that the reptilian humoral response differs substantially from the humoral response of mammals, and these differences may affect the functionality of this immune branch in these groups.
As with most reptiles in general, little is known about the immune system of the red-eared slider, Trachemys scripta; however, its general physiology has been extensively studied. Therefore, it makes an excellent model for the reptilian immune system, because studies of its immune responses can be placed in context with other information about this species. In order to investigate the phagocytic ability of reptilian B cells, we incubated leucocytes from adult sliders with 1 µm beads. Ig+ cells were found to have internalized several of the fluorescent particles. These findings of phagocytic B cells in reptiles further support the hypothesis that B cells evolved from a phagocytic predecessor.
2. Material and methods
(a). Isolation of leucocytes
Two adult red-eared sliders were trapped at Banner Marsh State Fish and Wildlife Area and transported to Illinois State University. Turtles were housed in 100 gallon tanks held at a constant water temperature of 27°C with a light cycle of 12 h L : 12 h D. Approximately, 1 ml of blood was taken from the caudal vein of each turtle. Blood was pooled and diluted approximately 1 : 2 with disodium ethylenediamine tetraacetate (EDTA; Labchem, Inc.) and then further diluted to approximately 1 : 4 with RPMI 1640 media (Gibco). Leucocytes were isolated by centrifugation of the blood over an equal volume of Histopaque-1077 (Sigma-Aldrich) at 400 g for 30 min at room temperature. Leucocytes were removed from the density gradient, washed with RPMI and resuspended in 1 ml of RPMI supplemented with 5 per cent foetal bovine serum, 1 per cent penicillin/streptomycin/glutamine, 0.5 per cent 2-mercaptoethanol and 0.5 per cent sodium pyruvate. Thin-film blood smears were prepared and treated with Wright-Giesma staining to validate the presence of lymphocytes (data not shown).
(b). Phagocytosis assay
To assay phagocytic ability of the cells, we followed the method described by Li et al. (2006). Briefly, 500 µl of cell suspension (3.45 × 106 leucocytes) and 40 µl of 1 µm fluorescent beads (6.4 × 107 beads; Fluoresbrite Plain Yellow Green Microspheres, Polysciences) were placed in each well of a 24-well plate and incubated for 3 h at 27°C. The cell suspension was removed from the wells, and empty wells were washed with 40 µl well−1 of EDTA-trypsin (Mediatech-Cellgro) for 5 min at 37°C to remove any remaining cells. The cell suspension was centrifuged and resuspended in 400 µl phosphate buffered saline (PBS). Cells were centrifuged at 1500 g for 5 min at 4°C over a cushion of 3 per cent BSA 4.5 per cent dextrose-PBS to remove non-phagocytosed beads.
(c). Flow cytometry
Whole blood was stained for flow cytometric analysis with saturating dilutions of anti-turtle light chain mAb conjugated to biotin (HL673, University of Florida Hybridoma Facility) for 15 min on ice. Bound antibodies were visualized with streptavidin-phycoerythrin (Southern Biotech). Samples included 10 per cent normal rat serum to prevent non-specific binding. Cells were analysed immediately on a Becton Dickinson FACSCalibur flow cytometer using CellQuest Pro software (San Jose, CA, USA) with 10 000 events collected.
(d). Confocal microscopy
Cells from the phagocytic assay were stained with Alexa 633-Wheat Germ Agglutinin, Alexa 568-Phalloidin, SYBR Green and/or anti-turtle light chain mAb detected with rhodamine labelled goat anti-mouse IgG + IgM (Kirkegaard & Perry Laboratories). Cells were fixed onto a chambered slide with 2 per cent formaldehyde in PBS for 15 min, washed twice with PBS, washed twice with PBS + 2 per cent Triton X-100 (Sigma) and mounted using Vectashield (Vector). Cells were visualized using a Leica SP2 confocal microscope. As a control, cells were incubated with the secondary antibody only and minimal background staining was detected.
3. Results
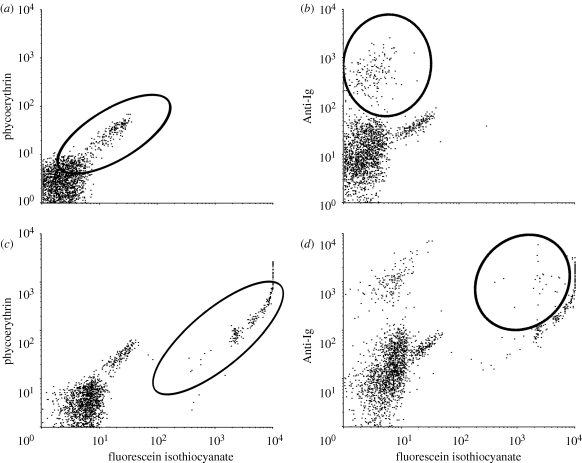
Flow cytometric analysis of cells was performed, and using forward and side scatter, leucocytes were gated away from larger contaminating red blood cells (RBCs) and smaller free beads. In the absence of anti-Ig antibody, the gated population showed a small contaminating population of auto-florescent red blood cells with the leucocytes (figure 1a). Nonetheless, anti-Ig staining clearly identified a population of B cells, which was roughly 10 per cent of the leucocytes (figure 1b). Following the phagocytic assay, we were able to detect a population of cells associated with beads (figure 1c). However, the brightness of the beads limited our ability to positively identify anti-Ig stained cells with beads, even with maximal fluorescence compensation (figure 1d). We were also unable to confirm that the beads were truly internalized using this methodology.
Figure 1.
Flow cytometry analysis of leucocytes (a) unstained cells without beads, with the contaminating population of red blood cells circled, (b) anti-Ig staining without beads, with population of B cells circled, (c) unstained cells with beads and (d) anti-Ig staining with beads, indicating population of B cells associated with beads.
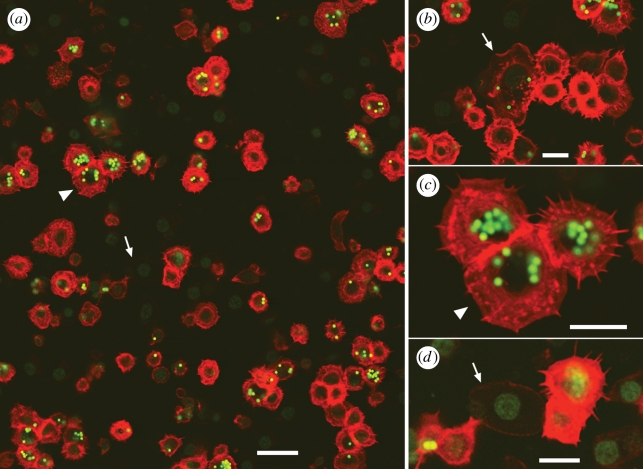
To determine whether the B cells had indeed phagocytosed beads, we used confocal microscopy to optically section the cells (see the electronic supplementary material). We successfully identified cells with lymphocyte morphology (large nuclei and little cytoplasm) (figure 2a). We found some cells to be Ig+ and ingest multiple beads (figure 2a,c), but interestingly, not all of the lymphocytes had phagocytosed beads (figure 2b). Some contaminating RBCs were identified in our sample, but did not hinder our ability to identify B cells (figure 2d). Thus, red-eared slider B cells exhibit phagocytic properties.
Figure 2.
Confocal sections through a population of blood cells following the phagocytosis assay. Anti-Ig is in red; both beads and SYBR Green DNA stain are green. (a) Overview. Scale bar 20 µm. (b–d) Closeups. Scale bars 10 µm. Arrowhead indicates the same cluster of cells in (a) and (c). Arrow in (b) indicates a macrophage. Arrow indicates the same RBC in (a) and (d). For (a–c), the original contrast is shown in the red channel; for (d), the image was brightened considerably to make the RBC visible, and (c) highlights cytoplasmic projections present on the surface of Ig+ cells.
4. Discussion
In mammals, there are several major classes of phagocytes that engulf foreign particles, but phagocytic activity in B cells has not been described. We report phagocytic activity of B cells in a reptile. Interestingly, as seen in both figures 1 and 2, not all B cells engulfed beads. Further studies are needed to determine the mechanism that triggers a B cell to become phagocytic, which may explain the lack of such activity in other groups.
A developmental relationship between mammalian B-1 cells and macrophages has been proposed, which has led to the hypothesis that B cells evolved from a phagocytic predecessor (Katsura 2002). This idea has been supported by the recent identification of phagocytic B cells in both fishes and amphibians (Li et al. 2006). Our finding of phagocytic activity in reptilian B cells extends this function into the amniotes and indicates that the loss of phagocytic activity in B cells may be a relatively recent evolutionarily event. Unfortunately, because nothing is known about the phagocytic capacity (or lack thereof) of avian B cells, we cannot discern whether the loss of activity is limited to mammals, but it is clear that at least some amniotes have retained this ancestral characteristic.
In comparison to mammals, the antibody responses of ectothermic vertebrates are of lower affinity, do not increase in affinity over time and often do not increase in titer upon a secondary exposure. This has generally been attributed to the lack of germinal centres in these taxa (Hsu 1998). Ectothermic vertebrates may rely more heavily on innate immune responses, and indeed, several studies of innate immunity in ectotherms indicate that responses can be stronger and of much broader range than their mammalian counterparts. For example, the complement system of fishes contains components with multiple isoforms, which results in a more functionally diverse response that expands the recognition capabilities of the innate immune system (Sunyer et al. 1998). Serum from the American alligator, Alligator mississippiensis, demonstrated complement-mediated bactericidal activity against multiple strains that human serum was unable to effectively kill (Merchant et al. 2003). Ranid frogs produce a number of structurally diverse antimicrobial peptides, some of which are effective against a broad range of human pathogens (Conlon et al. 2004). Phagocytic B cells would form another component in the innate immune system of ectothermic vertebrates that could be used instead of relying on adaptive immune responses that are often slower and less robust.
Acknowledgements
Animals were collected under Illinois DNR Permit NH08.2084 and the experiment was conducted under Illinois State University Care and Use of Animals Protocol 01-2008.
We thank Ryan Paitz for collecting turtles. Research was supported by NSF IOS-0758505 to R.M.B. and L.A.V. and a College of Arts & Sciences University Research Grant to R.M.B.
References
- Choi Y. S., Baumgarth N.2008Dual role for B-1a cells in immunity to influenza virus infection. J. Exp. Med. 205, 3053–3064 (doi:10.1084/jem.20080979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coico R., Sunshine G., Benjamini E.2003Immunology: a short course, 5th edn Wilmington, DE: Wiley-Liss [Google Scholar]
- Conlon J. M., Kolodziejek J., Nowotny N.2004Antimicrobial peptides from ranid frogs: taxonomic and phylogenetic markers and a potential source of new therapeutic agents. Biochem. Biophys. Acta 1696, 1–14 [DOI] [PubMed] [Google Scholar]
- Deza F., Espinel C. S.2008IgD in the reptile leopard gecko. Mol. Immunol. 45, 3470–3476 [DOI] [PubMed] [Google Scholar]
- Grey H.1963Phylogeny of the immune response: studies on some physical, chemical, and serologic characteristics of antibody produced in the turtle. J. Immunol. 91, 819–825 [PubMed] [Google Scholar]
- Hsu E.1998Mutation, selection, and memory in B lymphocytes of exothermic vertebrates. Immunol. Rev. 162, 25–36 (doi:10.1111/j.1600-065X.1998.tb01426.x) [DOI] [PubMed] [Google Scholar]
- Ingram G. A., Molyneux D. H.1983The humoral immune response of the spiny-tailed agamid lizard (Agama Caudospinosum) to injection with Leishmania agamae promastigotes. Vet. Immunol. Immunopathol. 4, 479–491 (doi:10.1016/0165-2427(83)90008-9) [DOI] [PubMed] [Google Scholar]
- Jurd R. D.1994Reptiles and birds. In Immunology: a comparative approach (ed. Turner R. J.), pp. 137–172 Chichester, UK: John Wiley & Sons, Ltd [Google Scholar]
- Kanakambika P., Muthukkaruppan V. R.1972The immune response to sheep erythrocytes in the lizard Calotes versicolor. J. Immunol. 109, 415–420 [PubMed] [Google Scholar]
- Katsura Y.2002Redefinition of lymphoid progenitors. Nat. Rev. Immunol. 2, 1–6 [DOI] [PubMed] [Google Scholar]
- Kawaguchi S., Hiruki T., Harada T., Morikawa S.1980Frequencies of cell-surface or cytoplasmic IgM-bearing cells in the spleen, thymus and peripheral blood of the snake Elaphe quadrivirgata. Dev. Comp. Immunol. 4, 559–563 (doi:10.1016/S0145-305X(80)80057-7) [DOI] [PubMed] [Google Scholar]
- Kwiatkowska K., Sobota A.1999Signaling pathways in phagocytosis. BioEssays 21, 422–431 (doi:10.1002/(SICI)1521-1878(199905)21:5<422::AID-BIES9>3.0.CO;2-#) [DOI] [PubMed] [Google Scholar]
- Li J., Barreda D. R., Zhang Y., Boshra H., Gelman A. E., LaPatra S., Tort L., Sunyer J. O.2006B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat. Immunol. 7, 1116–1124 (doi:10.1038/ni1389) [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Ealey E. H. M., Diener E.1969Immune response of the tuatara, Sphenodon punctatum. Aust. J. Exp. Biol. Med. Sci. 47, 367–380 (doi:10.1038/icb.1969.40) [DOI] [PubMed] [Google Scholar]
- Merchant M., Roche C., Elsey R. M., Prudhomme J.2003Antibacterial properties of serum from the American alligator (Alligator mississippiensis). Comp. Biochem. Physiol. B 136, 505–513 (doi:10.1016/S1096-4959(03)00256-2) [DOI] [PubMed] [Google Scholar]
- Sunyer J. O., Zarkadis I. K., Lambris J. D.1998Complement diversity: a mechanism for generating immune diversity? Immunol. Today 19, 519–523 [DOI] [PubMed] [Google Scholar]
- Ujvari B., Madsen T.2005Age, parasites, and condition affect humoral immune response in tropical pythons. Behav. Ecol. 17, 20–24 (doi:10.1093/beheco/ari091) [Google Scholar]
- Vidard L., Kovacsovics-Bankowski M., Kraeft S. K., Chen L. B., Benacerraf B., Rock K. L.1996Analysis of MHC Class II presentation of particulate antigens by B lymphocytes. J. Immunol. 156, 2809–2818 [PubMed] [Google Scholar]
- Work T. M., Balazs G. H., Rameyer R. A., Chang S. P., Berestecky J.2000Assessing humoral and cell-mediated immune response in Hawaiian green turtles Chelonia mydas. Vet. Immunol. Immunopathol. 74, 179–194 (doi:10.1016/S0165-2427(00)00168-9) [DOI] [PubMed] [Google Scholar]