Abstract
Gigantism is widespread among Palaeozoic arthropods, yet causal mechanisms, particularly the role of (abiotic) environmental factors versus (biotic) competition, remain unknown. The eurypterids (Arthropoda: Chelicerata) include the largest arthropods; gigantic predatory pterygotids (Eurypterina) during the Siluro-Devonian and bizarre sweep-feeding hibbertopterids (Stylonurina) from the Carboniferous to end-Permian. Analysis of family-level originations and extinctions among eurypterids and Palaeozoic vertebrates show that the diversity of Eurypterina waned during the Devonian, while the Placodermi radiated, yet Stylonurina remained relatively unaffected; adopting a sweep-feeding strategy they maintained their large body size by avoiding competition, and persisted throughout the Late Palaeozoic while the predatory nektonic Eurypterina (including the giant pterygotids) declined during the Devonian, possibly out-competed by other predators including jawed vertebrates.
Keywords: Eurypterida, Cope's Rule, Romer's theory, extinction, competition, gigantism
1. Introduction
The fossil record of Palaeozoic arthropods reveals that gigantism was widespread among aquatic and terrestrial groups; griffenflies, morphologically similar to dragonflies, attained a wingspan of 70 cm and arthropleurid millipedes were 200 cm long (Dunlop 1995). Such selection for gigantism is often attributed to elevated oxygen levels during the Late Palaeozoic (Berner et al. 2003); however, the presence of gigantism in aquatic arthropods including Ordovician trilobites (Rudkin et al. 2003), and Early Devonian eurypterids (Braddy et al. 2008a) suggests that mechanisms for gigantism selection are more complex.
Eurypterids are extinct chelicerates found in a range of aquatic habitats throughout the Late Palaeozoic. Most are small-medium nektonic predators (Eurypterina), and include the largest arthropods ever to have lived; gigantic pterygotids, with lengths of 250 cm estimated in Jaekelopterus (Braddy et al. 2008a). Stylonurine eurypterids (Stylonurina), which have their posterior legs retained for walking, range from the Late Ordovician to the Late Permian, and also attain gargantuan proportions: Pagea, from the Early Devonian, is around 120 cm long (Plotnick & Elliott 1995) and Cyrtoctenus, from the Carboniferous, is 135 cm long (Waterston et al. 1985). A trackway from Scotland, attributed to Hibbertopterus, indicates an animal 160 cm long (Whyte 2005).
During the Devonian, the diversity of Eurypterina plummeted, whereas the hibbertopterid radiation during the Late Devonian and Carboniferous represents the last major radiation of eurypterids. Romer (1933) proposed that eurypterids and early armoured fish evolved in an ‘arms race’; while this theory has fallen out of favour in recent years, deemed too simplistic, it remains in text books and popular science writing. Analysis of evolutionary trends in eurypterids thus provides an interesting case study to examine whether causal mechanisms for gigantism in Palaeozoic arthropods, and their extinction, were primarily owing to abiotic (e.g. environmental) or biotic (e.g. competition between species) factors.
2. Material and methods
A database of all known eurypterids was compiled from the primary literature and supplemented with size data derived from the maximum recorded specimen size and environmental occurrence data from Plotnick (1999), supplemented with data from the literature (see the electronic supplementary material). Taxa were assigned to benthic assemblages following Braddy (2001). Placoderm generic data follows from Carr (1995), while familial occurrence data follows from Purnell (2001). The phylogenetic topology and family-level assignments of Eurypterina are derived from Tetlie (2007); those of Stylonurina follow Lamsdell et al. (in press). Family- and generic-level eurypterid diversity curves were compiled at series resolution (31 time bins).
3. Results
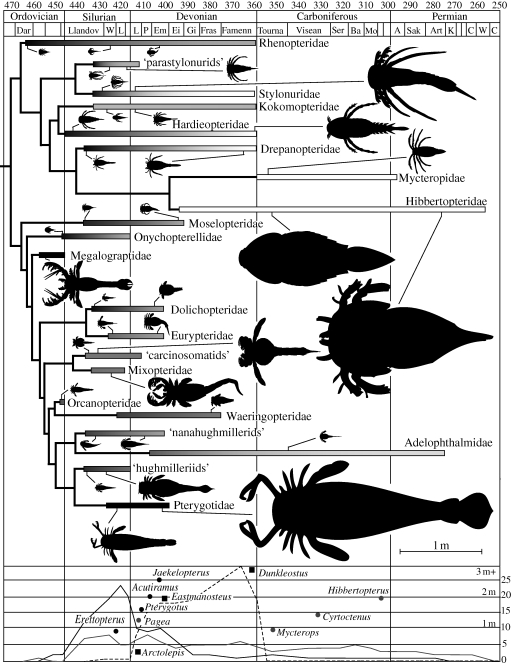
Plotting the temporal ranges of the constituent families of Eurypterida according to their phylogenetic topology (figure 1) reveals that only two families of the Eurypterina (Waeringopteridae and Adelophthalmidae) persist through to the Late Devonian, while only one group of Stylonurina (parastylonurids) go extinct during the Early Devonian. Generic-level diversity curves show a massive decline in Eurypterina during the Early Devonian, losing just over 50 per cent of their diversity in 10 Myr. Stylonurina, by comparison, persist throughout the Devonian with a comparatively consistent diversity, until the Frasnian/Famennian extinction event, when their diversity dropped marginally, coinciding with a changeover to a stylonurine fauna consisting of the highly specialised sweep-feeding Mycteropidae and Hibbertopteridae. Palaeoenvironmental data show that most eurypterid groups originated in marine habitats in the Ordovician or Early Silurian, although the only groups that generally frequented marine shelf environments are the Pterygotidae and Megalograptidae, along with some carcinosomatids; eurypterids were predominantly euryhaline; however, by the Late Devonian the surviving eurypterid groups were confined to freshwater-dominated settings (Braddy 2001). Analysis of size among eurypterid genera shows that basal taxa in both suborders are relatively small (10–20 cm). The taxa in each group tend to increase in size through time, with active predators in some Eurypterina (Megalograptidae, Mixopteridae, carcinosomatids and hughmilleriids) reaching sizes of up to a metre. Pterygotids reach the largest sizes, with individuals reaching 250 cm as their diversity declined during the Devonian. Stylonurina also reached large sizes, with 120 cm recorded from freshwater-inhabiting Stylonuridae and the exclusively freshwater Hibbertopteroidea approaching 200 cm in length.
Figure 1.
Family-level evolutionary tree of the Eurypterida. Bars represent known temporal ranges, and are shaded according to habitat, indicating maximum salinity tolerance (black indicates open marine, grey indicates shallow marine and brackish environments and white indicates a restriction to fresh water). Silhouettes of various taxa are shown to scale with lines indicating their temporal placement within the family. Diversity curves of Eurypterina (black line), Stylonurina (grey line) and Arthrodira (black dashed line) are at generic level. Maximum sizes of several pterygotids (black circles) and stylonurines (grey circles) are overlain, with three placoderm genera (black squares) shown for comparison.
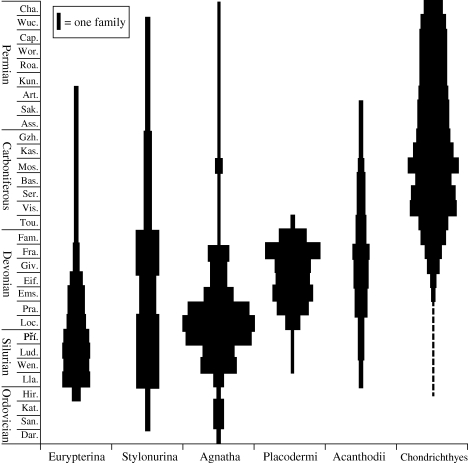
Comparing the family-level diversity of the eurypterid suborders with those of various fish groups (figure 2), the decline of the Eurypterina begins while agnathans are at their acme; however, it coincides with the diversification of placoderms in Europe and North America. Stylonurina family diversity remains largely unaffected except for a slight decrease in the Early Devonian, until a large reduction coinciding with the end-Devonian extinction event, accounting for five of the seven existing stylonurine families. During this time, agnathan diversity decreased and placoderms went extinct. With the beginning of the Carboniferous, chondrichthyans had diversified and sarcopterygian fishes such as rhizodonts had taken over the role of apex predators (Andrews 1985).
Figure 2.
Family-level diversity of Eurypterida and various aquatic vertebrate groups throughout the Palaeozoic. Diversity of the suborder Eurypterina begins to decline steadily through the Early Devonian, coinciding with the radiation of Placodermi. Stylonurina remain relatively unaffected, but undergo a drop in diversity as part of the end-Devonian mass extinctions, along with agnathans and placoderms.
4. Discussion
(a). Gigantism and Cope's Rule
Gigantism is observed in both eurypterid suborders. Basal taxa in both suborders are relatively small, and more derived taxa tend to increase in size. This trend has been noted specifically in pterygotids (Braddy et al. 2008a), with an increase in length of 1250 per cent, and hibbertopteroids (Lamsdell et al. 2009), with an increase in length of 823 per cent. In this respect, both hibbertopteroids and pterygotids obey Cope's Rule or ‘phyletic gigantism’ (Gould & MacFadden 2004), yet clearly occupied different life habits; the pterygotids were nektonic predators capable of excursions into the open marine realm, while the hibbertopteroids were benthic sweep-feeders limited to freshwater environments from the Late Devonian until their extinction. Another contrast is their longevity; pterygotids appear for only 40 Myr, whereas the Hibbertopteridae alone persist for 140 Myr. Throughout their comparatively short duration, pterygotids achieve a high level of species diversity (although are somewhat taxonomically oversplit (Braddy et al. 2008b)) and undergo a rapid transition to gigantic forms, consistent with a group under strong directional selection, whereas hibbertopterids are known from relatively few species and display a high level of morphological conservatism indicative of inhabiting a stable environment.
Pterygotids increased in size as their overall diversity dropped, apparently coinciding with an increase in the size of arthrodire placoderms (figure 1). It is possible that pterygotids evolved large size as a way of competing with increasingly large, swift vertebrate predators. Gigantism among Stylonurina cannot be explained through competition with vertebrates, as there are no clearly overlapping niche occupations as is the case with vertebrates and pterygotids (Dunlop et al. 2002). It has been suggested that Stylonurina increased in size as a method for drought survival during the arid conditions of the Old Red Sandstone (Rolfe 1985). Salinity may also have been an important factor: hibbertopteroids show an evolutionary trend towards freshwater, possibly amphibious lifestyles, while the giant Stylonuridae Pagea is known from freshwater sediments, and it has been suggested that large size developed in hibbertopteroids as a method for maintaining osmoregulation in fresh water (Lamsdell et al. 2009). Hibbertopterids also show several adaptations for undertaking amphibious excursions. Intrinsic factors including mechanical properties of their exoskeleton and respiratory system limit maximum arthropod size, especially on land. Pterygotids had a thin, unmineralized cuticle and could attain such large size because of their light-weight construction and aquatic lifestyle (Braddy et al. 2008a). Hibbertopterids adopted a more graviportal approach; their cuticle is considerably thicker and they have other adaptations linked to supporting a large body size, including grooves on the podomeres of their load-bearing legs and tubercles or ridges interpreted as muscle attachment sites on the opisthosoma, aiding the function of respiratory organs (Lamsdell et al. 2009). Therefore, while pterygotids were longer, hibbertopterids had the greater mass.
(b). Patterns of extinction and Romer's theory
Romer (1933) proposed that eurypterids evolved in an ‘arms race’ with early vertebrates; various Silurian and Early Devonian jawless fishes evolved dermal armour specifically as defence against eurypterids, and the decline of eurypterids during the Devonian was because of the increasing dominance of faster-swimming, jawed fishes. Eurypterids are commonly associated with fishes in around one-third of Silurian and Early Devonian localities (Dunlop et al. 2002); pterygotids invariably dominate, except for the stylonurine-dominated faunas in Scotland.
Romer's theory has been criticized as over-simplified (Gee 1999); an alternative interpretation of the ‘ostracoderm’ dermal armour is as a phosphate store (Donoghue & Aldridge 2001), originating in the Ordovician and maintained long after the decline of the Eurypterida, and as such, most recent workers consider there to be no clear link between the evolution of early armoured vertebrates and eurypterids (Briggs et al. 1988). However, previous studies have treated eurypterids as a single group; if they are separated into two suborders/niches (i.e. Stylonurina, benthic scavengers and sweep-feeders, and Eurypterina, active nektonic predators), the decline in eurypterid diversity is restricted to Eurypterina, coinciding with the radiation of arthrodire placoderms (figure 1). Evolving towards an ecological niche distinct from the nektonic Eurypterina, Stylonurina would have avoided competing with these more manoeuvrable paddled forms. Stylonurina lack the anteriorly placed eyes of active predatory eurypterids such as carcinosomatids, mixopterids and pterygotids, indicating that they were not adapted to direct prey capture. While it is unlikely that predatory pressure from pterygotids led to the evolution of dermal armour in vertebrates, biotic competition from jawed vertebratee and other predators (e.g. cephalopods, which show changes in diversity in response to predation pressure from first eurypterids, then gnathostomes (Kröger 2005)) could explain the drastic decline in the diversity of Eurypterina during the Devonian, whereas sweep-feeding Stylonurina survived, less affected by such competition. Hibbertopterids survived until the end-Permian, when they went extinct with an estimated 90 per cent of marine species owing to abiotic environmental change (Erwin 1994).
References
- Andrews S. M.1985Rhizodont crossopterygian fish from the Dinantian of Foulden, Berwickshire, Scotland, with a re-evaluation of this group. Trans. R. Soc. Edin. 76, 67–95 [Google Scholar]
- Berner R. A., Beerling D. J., Dudley R., Robinson J. M., Wildman R. A., Jr2003Phanerozoic atmospheric oxygen. Annu. Rev. Earth Planet. Sci. 31, 105–134 (doi:10.1146/annurev.earth.31.100901.141329) [Google Scholar]
- Braddy S. J.2001Eurypterid palaeoecology: palaeobiological, ichnological and comparative evidence for a ‘mass-moult-mate’ hypothesis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 172, 115–132 (doi:10.1016/S0031-0182(01)00274-7) [Google Scholar]
- Braddy S. J., Poschmann M., Tetlie O. E.2008aGiant claw reveals the largest ever arthropod. Biol. Lett. 4, 106–109 (doi:10.1098/rsbl.2007.0491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddy S. J., Poschmann M., Tetlie O. E.2008bReply: giant claws and big bodies. Biol. Lett. 4, 281 (doi:10.1098/rsbl.2008.t0116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs D. E. G., Fortey R. A., Clarkson E. N. K.1988Extinction and the fossil record of arthropods. In Extinction and survival in the fossil record (ed. Larwood G. P.), pp. 171–209 Oxford, UK: Clarendon Press [Google Scholar]
- Carr R. K.1995Placoderm diversity and evolution. In Studies on early vertebrates (7th International Symposium on Lower Vertebrates, Miguasha, Quebec) (eds Arsenault M., Lelièvre H., Janvier P.), pp. 85–125 Paris, Bull. Mus. Natl. Hist. Nat. Section C, 17 [Google Scholar]
- Donoghue P. C. J., Aldridge R. J.2001Origin of a mineralized skeleton. In Major events in vertebrate evolution (ed. Ahlberg P. E.), pp. 85–105 London, UK: Taylor & Francis [Google Scholar]
- Dunlop J. A.1995Gigantism in arthropods. Am. Tarantula Soc. 4, 145–147 [Google Scholar]
- Dunlop J. A., Braddy S. J., Tetlie O. E.2002The Early Devonian eurypterid Grossopterus overathi (Gross 1933) from Overath, Germany. Mitt. Mus. Naturkde. Berlin, Geowiss. Reihe 5, 93–104 (doi:10.1002/mmng.4860050107) [Google Scholar]
- Erwin D. H.1994The Permo-Triassic extinction. Nature 367, 231–235 (doi:10.1038/367231a0) [Google Scholar]
- Gee H.1999In search of deep time New York, NY: Free Press [Google Scholar]
- Gould G. C., MacFadden B. J.2004Gigantism, dwarfism, and Cope's rule: ‘nothing in evolution makes sense without a phylogeny’. Bull. Am. Mus. Nat. Hist. 285, 219–237 (doi:10.1206/0003-00902004285!0219:CO2.0.CO;2) [Google Scholar]
- Kröger B.2005Adaptive evolution in Paleozoic coiled cephalopods. Paleobiology 31, 253–268 (doi:10.1666/0094-8373(2005)031[0253:AEIPCC]2.0.CO;2) [Google Scholar]
- Lamsdell J. C., Braddy S. J., Tetlie O. E.2009Redescription of Drepanopterus abonensis (Chelicerata: Eurypterida: Stylonurina) from the Late Devonian of Portishead, UK. Palaeontology 52, 1113–1139 (doi:10.1111/j.1475-4983.2009.00902.x) [Google Scholar]
- Lamsdell J. C., Braddy S. J., Tetlie O. E.In press The systematics and phylogeny of the Stylonurina (Arthropoda: Chelicerata: Eurypterida). J. Syst. Palaeontol. [Google Scholar]
- Plotnick R. E.1999Habitat of Llandoverian-Lochkovian eurypterids. In Paleocommunities: a case study from the Silurian and Lower Devonian (eds Boucot A. J., Lawson J.), pp. 106–131 Cambridge, UK: Cambridge University Press [Google Scholar]
- Plotnick R. E., Elliott D. K.1995A lower Devonian stylonurid eurypterid from Arctic Canada. J. Paleontol. 69, 399–402 [Google Scholar]
- Purnell M. A.2001Scenarios, selection, and the ecology of early vertebrates. In Major events in early vertebrate evolution (ed. Ahlberg P. E.), pp. 188–208 London, UK: Taylor & Francis [Google Scholar]
- Rolfe W. D. I.1985Aspects of the Carboniferous terrestrial arthropod community. Neuvieme Cong. Int. Stratig. Geol. Carbonif. Compte Rendu 5, 303–316 [Google Scholar]
- Romer A. S.1933Eurypterid influence on vertebrate history. Science 78, 114–117 (doi:10.1126/science.78.2015.114) [DOI] [PubMed] [Google Scholar]
- Rudkin D. M., Young G. A., Elias R. J., Dobrzanski E. P.2003The world's biggest trilobite-Isotelus rex new species from the Upper Ordovician of northern Manitoba, Canada. J. Paleont. 77, 99–112 (doi:10.1666/0022-3360(2003)077!0099:TWBTIRO2.0.CO;2) [Google Scholar]
- Tetlie O. E.2007Distribution and dispersal history of Eurypterida (Chelicerata). Palaeogeogr. Palaeoclimatol. Palaeoecol. 252, 557–574 (doi:10.1016/j.palaeo.2007.t05.011) [Google Scholar]
- Waterston C. D., Oelofsen B. W., Oosthuizen R. D. F.1985Cyrtoctenus wittebergensis sp. nov. (Chelicerata: Eurypterida), a large sweep-feeder from the Carboniferous of South Africa. Trans. R. Soc. Edin. 76, 339–358 [Google Scholar]
- Whyte M. A.2005A gigantic fossil arthropod trackway. Nature 438, 576 (doi:10.1038/438576a) [DOI] [PubMed] [Google Scholar]