Abstract
A core requirement for imitation is a capacity to solve the correspondence problem; to map observed onto executed actions, even when observation and execution yield sensory inputs in different modalities and coordinate frames. Until recently, it was assumed that the human capacity to solve the correspondence problem is innate. However, it is now becoming apparent that, as predicted by the associative sequence learning model, experience, and especially sensorimotor experience, plays a critical role in the development of imitation. We review evidence from studies of non-human animals, children and adults, focusing on research in cognitive neuroscience that uses training and naturally occurring variations in expertise to examine the role of experience in the formation of the mirror system. The relevance of this research depends on the widely held assumption that the mirror system plays a causal role in generating imitative behaviour. We also report original data supporting this assumption. These data show that theta-burst transcranial magnetic stimulation of the inferior frontal gyrus, a classical mirror system area, disrupts automatic imitation of finger movements. We discuss the implications of the evidence reviewed for the evolution, development and intentional control of imitation.
Keywords: imitation, mirror system, sensorimotor experience, associative sequence learning, transcranial magnetic stimulation, functional magnetic resonance imaging
In order to imitate an action, the imitator must translate a sensory representation of the action they observe into their own motor program for that action. Despite the apparent ease with which we imitate others—i.e. reproduce the topography of their body movements—this translation poses a significant computational challenge, particularly because the observation of the actor and the execution of an action by an imitator often result in sensory inputs in different modalities and frames of reference. This challenge is known as the correspondence problem (Brass & Heyes 2005). It is most clearly illustrated when the actor performs a perceptually opaque action, such as shrugging the shoulders (Heyes & Ray 2000), an action in which the sensory input the imitator receives from observing the actor is highly dissimilar to that which they receive when performing the action themselves. The problem persists, however, when the actor performs a perceptually transparent action, such as clapping, which yields similar perceptual inputs when observed and executed: the imitator needs to determine which motor commands to use in order to reproduce, from a third-party perspective, the sensory consequences of the actor's movement.
It has been widely assumed that the human ability to solve the correspondence problem is innate (Meltzoff & Moore 1977, 1997; Meltzoff & Decety 2003; Nagy et al. 2005; Nagy 2006). This assumption is based primarily on data suggesting that newborn infants can imitate a range of different movements (discussed below). However, evidence is now accumulating in support of an alternative hypothesis: that the ability to solve the correspondence problem arises as a result of experience, and in particular sensorimotor experience, acquired during development. Heyes & Ray (2000; see also Heyes 2001; Brass & Heyes 2005) outlined an associative sequence learning (ASL) theory of imitation, which proposes that the correspondence problem is solved by a set of excitatory links, or matching vertical associations, each connecting a sensory and a motor representation of the same action. These links are formed in the course of development via standard processes of associative learning (Schultz & Dickinson 2000). Learning of this kind occurs when the individual receives sensorimotor experience in which the observation and execution of a particular action, X, are correlated or contingent; i.e. when a sensory representation of X, activated by movement observation, is more likely to be active at the same time as a motor representation of X than at the same time as a motor representation of any other action. Sensorimotor experience of this kind, i.e. imitogenic experience, comes from direct and mirror-mediated self-observation, from socially synchronous action and from being imitated (Heyes & Ray 2000; Ray & Heyes submitted).
This paper has four parts. The first examines the evidence from behavioural studies (of non-human animals, infants and adults) indicating that imitation depends on sensorimotor learning. The second reports original data showing that, in adult humans, imitation is mediated by the mirror system. These data confirm that the studies reviewed in the third section, reporting experiential effects on the mirror system, provide further support for the view that imitation is made possible by sensorimotor learning. The final section examines the implications of the ASL theory of imitation.
1. Sensorimotor learning and imitation
(a). Non-human animals
Nativist accounts of imitation suggest that specialized processes solve the correspondence problem, that they have been shaped by natural selection for the role that they play in imitation and that they are present only in humans or in closely related species (Meltzoff & Decety 2003; Nagy 2006; Meltzoff 2007). The ASL theory, in contrast, predicts that imitation is likely to occur in a variety of species, to the extent that the sensorimotor experience of the imitated actions was available during development. While early studies of imitation in non-human animals were beset with methodological problems (see Tomasello et al. 1987; Whiten & Ham 1992), recent data (see also Huber et al. 2009) provide compelling evidence for imitation of simple movements across a range of species, including chimpanzees (Custance et al. 1995; Whiten et al. 1996, 2004), marmosets (Bugnyar & Huber 1997; Voelkl & Huber 2007), dogs (Slabbert & Rasa 1997; Range et al. 2007) and several bird species (Lefebvre et al. 1997; Campbell et al. 1999; Mui et al. 2008). For example, dogs will perform a paw-press action to obtain a food reward, rather than the usually preferred mouth action, after observing a demonstrator dog using this action (Range et al. 2007). These data indicate that the ability to solve the correspondence problem is not unique to humans, but they also suggest, consistent with the ASL theory, that this ability is limited to a small range of actions: those actions with which animals are likely to have obtained sensorimotor experience (e.g. Richards et al. 2009).
(b). Infants
Evidence that imitative behaviour is present from birth would clearly support a nativist account of imitation. Since the publication of a seminal paper on neonatal imitation (Meltzoff & Moore 1977), it has been widely assumed that the ability to imitate is innate (see also Meltzoff & Moore 1983, 1989; Kugiumutzakis 1999). However, recent reviews suggest that the only behaviour that is reliably imitated by newborns is tongue protrusion and that this effect is mediated by an innate releasing mechanism or an oral exploratory response, rather than by the mechanisms that support imitation later in development (Anisfeld et al. 2001; Jones 2006, 2009).
Widespread acceptance of the nativist hypothesis has led research on imitation in post-neonatal infancy to be neglected. However, a recent review of studies examining the development of imitation in later infancy found evidence in support of three predictions made by the ASL theory (Ray & Heyes submitted). First, the accuracy of imitation, and the range of behaviours that can be imitated, increase over time as individuals acquire more experience of seeing and doing the same actions (e.g. Abravanel et al. 1976; Killen & Uzgiris 1981; Masur 2006; Jones 2007). Second, imitation of perceptually transparent actions precedes imitation of perceptually opaque actions only to the extent that infants are more likely to have had experience of seeing and doing the former than the latter (e.g. Piaget 1952; Uzgiris 1972; Kaye & Marcus 1978). Third, variation in the development of imitation across infants depends on the amount of imitogenic experience the infants have received, and in particular, on the quality of social interactions in which adult and infant commonly see and do the same action (e.g. Cress et al. 1998; Field et al. 2005; McEwen et al. 2007).
(c). Adults
Experiments using stimulus–response compatibility paradigms have revealed that imitative behaviour can be automatic; we sometimes imitate the actions of others even when this behaviour is contrary to our intentions (Brass et al. 2000; Kilner et al. 2003; Vogt et al. 2003; Press et al. 2005; Bertenthal et al. 2006). For example, in one of the earliest demonstrations of automatic imitation, Stürmer et al. (2000; experiments 1, 5 and 6) showed that adult participants were faster to perform a hand-opening action while viewing a compatible (hand opening) action, than when viewing an incompatible (hand closing) action, and that this effect was reversed for the performance of hand-closing actions. The participants were instructed, and therefore presumably intended, to respond as fast as possible in all trials. Therefore, this result implies that the sight of the action stimulus (opening or closing) primed an imitative response, and that the participants were unable to prevent this from speeding their responses in compatible trials and/or slowing their responses in incompatible trials.
Several studies using a paradigm similar to that of Stürmer et al. (2000) have provided support for the ASL theory by showing that sensorimotor learning can enhance, abolish and even reverse automatic imitation. Press et al. (2007) found an enhancement effect in a study comparing the extent to which human and robotic hand movements elicit automatic imitation. At pretest, robotic actions were less potent stimuli for automatic imitation than human action. However, 24 h after a relatively brief period of compatible sensorimotor training with the robotic movements—in which participants responded to robot hand-opening stimuli by opening their hands, and to robot hand-closing stimuli by closing their hands—the robotic movements elicited as much automatic imitation as the human movements. In a complementary way, Heyes et al. (2005) showed that incompatible sensorimotor training with human stimuli—in which participants responded to human hand opening by closing their hands and to human hand closing by opening their hands—abolished automatic imitation. Twenty-four hours after training of this kind, responding in incompatible trials was as fast as responding in compatible trials. Gillmeister et al. (2008) demonstrated a comparable reduction in automatic imitation of hand and foot actions following incompatible sensorimotor experience, while Catmur et al. (2007) showed that, in the case of little- and index-finger abduction movements, incompatible sensorimotor experience can reverse automatic imitation, producing a systematic, involuntary tendency to counter-imitate the observed action (see below). On the basis of associative learning theory, incompatible sensorimotor training would be expected to establish new, non-matching vertical associations, e.g. between a sensory representation of hand opening and a motor representation of hand closing (Elsner & Hommel 2004), and to result in inhibitory learning weakening the effects of the old, matching vertical associations (Schultz & Dickinson 2000).
Each of the foregoing studies isolated the effects of sensorimotor experience (executing a particular action while observing the same or an alternative action) by controlling for the effects of sensory experience (repeated observation of an action) and of motor experience (repeated execution of an action). For example, the performance of a group that received incompatible sensorimotor training (e.g. open stimulus—close response) was compared with that of a group that received compatible sensorimotor training (e.g. open stimulus—open response). Thus, the experimental and control groups saw the stimulus actions equally often, and performed the response actions equally often, but automatic imitation was abolished or reversed only in the groups that received incompatible training. Therefore, these studies show specifically that sensorimotor learning modulates automatic imitation.
2. Imitation and the mirror system
A number of recent studies, reviewed in the next section, have suggested that the human mirror system—a network of brain areas, in particular inferior frontal and inferior parietal cortices, active during both action perception and action execution (see Bastiaansen et al. 2009; Brass et al. 2009; Ferrari et al. 2009)—can be modified by experience. These studies provide support for the ASL theory of imitation only if one assumes that imitation is, at least in part, mediated by the mirror system: that the mirror system is involved in solving the correspondence problem for imitation. Although widely held, this assumption currently lacks direct empirical support. In this section we survey existing evidence linking the mirror system with imitation and present original data confirming that link.
Studies of neuropsychological patients suggest a role for the mirror system in imitation (see also Rumiati et al. 2009), but they do not make clear which areas within the mirror system are critical. Lesions to the inferior parietal lobe, particularly in the left hemisphere, often result in apraxia—a deficit in miming gestures and in imitation (Wheaton & Hallett 2007). Lesions to the inferior frontal cortex in apraxia are not as widely reported as are parietal lesions, and may not always result in imitation deficits: Goldenberg et al. (2007) found impairment in miming gestures following lesions to the left inferior frontal gyrus (IFG), but imitation was preserved in some of these patients. In a different study, imitation of finger movements was impaired following lesions to the left IFG, while left inferior parietal lesions resulted in impaired imitation of hand postures (Goldenberg & Karnath 2006). Thus, the effect of lesions to parts of the mirror system on imitation may depend on lesion location and on the imitation task used.
When using functional magnetic resonance imaging (fMRI) to investigate the neural mechanisms involved in imitation, well-controlled studies compare the blood oxygen level-dependent (BOLD) response during imitation trials (when the performed action matches that which is observed) with the response in non-imitation trials (when the performed action is different from that observed). When imitation trials are instead contrasted with an observation-only control or an execution-only control (Iacoboni et al. 1999; Tanaka & Inui 2002; Koski et al. 2003), any differences in response could be related to whichever element of the task (execution or observation) is absent from the control condition. In studies comparing imitation with non-imitation trials, one would expect the BOLD response to be consistently stronger in imitation trials only if there is a specialized imitation mechanism mediated by the mirror system: if the mirror system is for imitation (Brass & Heyes 2005). However, if the mirror system mediates the kind of general-purpose mechanism postulated by the ASL model—a learned mechanism that can do imitation but is not for imitation—then, across studies, one would not expect consistent differences between imitation and non-imitation trials. In both cases, the BOLD response is likely to summate activation arising from neural populations that are sensitive to action observation (visual), to action execution (motor) and to both observation and execution (visuomotor). In each imitation trial, all three of these sources of activation relate to the same action, A, whereas in non-imitation trials, two of them relate to an alternative action (e.g. visual and visuomotor activation relating to A, and motor activation relating to B). The more concordant activation in imitation trials may contribute to imitative performance, but this would not be reflected in consistent differences between imitation and non-imitation trials because the BOLD response does not distinguish activation relating to different actions, A and B. Consistent with this analysis, the small number of fMRI studies comparing imitation and non-imitation trials have yielded mixed results. Newman-Norlund et al. (2007) found greater BOLD response in the mirror system during non-imitation than imitation trials, whereas Brass et al. (2001) found activity outside the mirror system for this contrast. Williams et al. (2007) did not replicate either of these results, but found more mirror system activity during imitation than non-imitation trials.
Convergent evidence that a cognitive function depends on a particular brain area can be provided by disrupting the functioning of that area using repetitive transcranial magnetic stimulation (rTMS). In several studies, rTMS has been used to interfere with the functioning of the mirror system, in particular by targeting the IFG (Pobric & Hamilton 2006; Avenanti et al. 2007; Urgesi et al. 2007). The IFG is thought to be homologous with area F5, where mirror neurons have been found in the macaque (Rizzolatti & Arbib 1998). Only one experiment, however, has investigated the dependence of imitation on the mirror system using rTMS. Heiser et al. (2003) reported that participants made more response-location errors in a finger movement imitation task than in a control task during rTMS to the IFG, but not during occipital stimulation. Heiser et al. did not find any effects of rTMS of the IFG on more subtle measures of perceptual-motor translation, i.e. response times (RTs), movement kinematics or accuracy of finger selection.
In the experiment reported below, we investigated the role of the mirror system in imitation using a relatively new rTMS protocol, continuous theta-burst stimulation, to disrupt IFG functioning during the performance of an automatic imitation task. Theta-burst stimulation was used because it produces long-lasting effects on the brain after a short period of administration: 20 s of stimulation over the primary motor cortex can reduce cortical excitability for 20 min following stimulation, allowing experiments to be performed subsequent to the administration of rTMS (Huang et al. 2005; Vallesi et al. 2007). In our experiment, we compared the effects of theta-burst rTMS of the IFG, with stimulation of a control site—the posterior parietal cortex (PPC)—and a baseline, no rTMS condition, on automatic imitation performance.
We selected an automatic imitation task, rather than an intentional imitation task of the kind used by Heiser et al. (2003), because automatic imitation tasks isolate the processes involved in perceptual-motor translation by minimizing demands on working memory and other executive processes. In the automatic imitation task used here, participants were required to make an abduction (outward) movement of the index finger or the little finger of their right hand in response to a coloured circle (e.g. orange stimulus—index response; purple stimulus—little response). A task-irrelevant action stimulus, an image of an index- or little-finger abduction movement, was presented at the same time as the coloured circle. In imitatively compatible trials, the action stimulus matched the correct response (e.g. index stimulus—index response), and in imitatively incompatible trials the action stimulus was the alternative to the correct response (e.g. little stimulus—index response). The magnitude of the automatic imitation effect was measured by subtracting RTs in imitatively compatible trials from RTs in imitatively incompatible trials. Our experimental design also varied, orthogonally, the left–right spatial relationship (compatible or incompatible) between the action stimulus and the response. This was in order to prevent participants from using the spatial location of the irrelevant movement stimulus as a response cue.
If perceptual-motor translation for imitation depends on the left IFG, one would expect the automatic imitation effect to be reduced, relative to baseline, following theta-burst rTMS to the IFG, but not following theta-burst rTMS to the PPC.
(a). Methods
Table 1 shows the stimuli presented and the responses made in trials of each type defined by imitative and spatial compatibility. Further information about the sequence of events in each trial and block composition is provided in figure 1; see the electronic supplementary material for detailed description of the methods.
Table 1.
Stimuli presented (columns) and responses made (rows) in trials of each type. Different shading is used to indicate the four principal trial types: spatially compatible, imitatively compatible; spatially compatible, imitatively incompatible; spatially incompatible, imitatively compatible; spatially incompatible, imitatively incompatible. Imitative compatibility refers to the identity of the finger making the movement (index or little), while spatial compatibility refers to the direction of the movement (to the left or right). Note that responses were always made with the right hand.
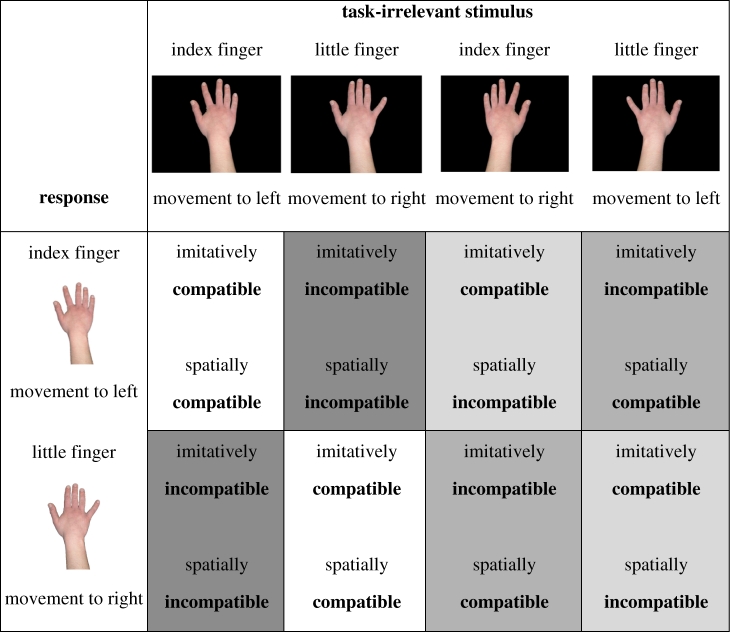 |
Figure 1.
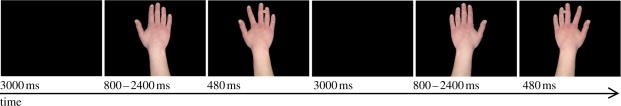
An example of two successive trials in the automatic imitation task. A blank screen was followed by a neutral hand stimulus on which the eventual location of the coloured circle was indicated by a white outline of a circle. The coloured circle instructing the response appeared at the same time as the irrelevant finger action stimulus. For participants given orange to index finger and purple to little finger stimulus–response mappings, the first trial is spatially and imitatively compatible, while the second is spatially compatible but imitatively incompatible. Prior to theta-burst stimulation, 144 baseline trials were presented in two blocks (preceded by 24 practice trials). After theta-burst stimulation, 288 trials were presented in four blocks. Each of the four trial types (as listed in table 1) was presented an equal number of times in each block in a pseudo-random order.
(b). Results
The mean RT for the first block of trials in each condition (baseline, IFG, PPC), where the disruptive effects of theta-burst stimulation are maximal, was calculated for each of the four trial types (spatially compatible, imitatively compatible; spatially compatible, imitatively incompatible; spatially incompatible, imitatively compatible; spatially incompatible, imitatively incompatible), collapsing across the two response movements (index- and little-finger movements); see electronic supplementary material, table S1, for the RT data.
A repeated-measures ANOVA was performed on the RT data with within-subjects factors of rTMS condition (baseline, IFG, PPC), spatial compatibility (compatible, incompatible) and imitative compatibility (compatible, incompatible). A significant main effect of the rTMS condition was observed: participants were fastest in the IFG condition (473 ± 27 ms), followed by the PPC condition (486 ± 20 ms) and the baseline condition (517 ± 24 ms; F2,14 = 6.5, p = 0.01). Post hoc t-tests (Bonferroni corrected: α = 0.0167) showed a trend towards a difference in RTs between the baseline and two rTMS conditions (baseline versus IFG, t7 = 3.0, p = 0.02; baseline versus PPC, t7 = 2.6, p = 0.04). This is probably owing to a generalized arousal effect of rTMS (Wassermann et al. 1999; Koren et al. 2001; Drager et al. 2004). Significant main effects of spatial compatibility (F1,7 = 93.7, p < 0.001) and imitative compatibility (F1,7 = 7.5, p = 0.03) were also observed. None of the two-way interactions reached significance.
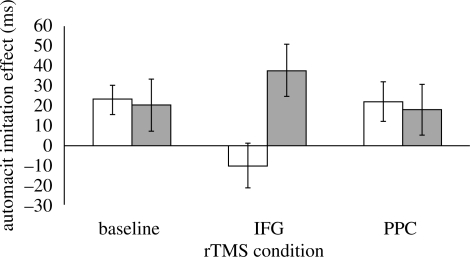
Of principal interest, there was a significant three-way interaction between rTMS condition, spatial compatibility and imitative compatibility (F2,14 = 4.7, p = 0.03; see figure 2). Simple interaction analysis revealed a significant two-way interaction between spatial and imitative compatibility in the IFG stimulation condition (F1,7 = 7.9, p = 0.03), but not in the other two conditions. This indicates that the automatic imitation effect was abolished under IFG stimulation in spatially compatible, but not in spatially incompatible, trials. In spatially compatible trials, the automatic imitation effect was significantly smaller in the IFG stimulation condition than in the baseline (t7 = 2.4, p = 0.05) and PPC (t7 = 3.4, p = 0.01) conditions.
Figure 2.
Mean ± s.e.m. of automatic imitation effects (RT in imitatively incompatible − RT in imitatively compatible trials) for spatially compatible (white bars) and spatially incompatible (grey bars) trials in each of the three rTMS conditions.
(c). Discussion
The results of this experiment provide support for the hypothesis that the left IFG plays a causal role in perceptual-motor translation for imitation. Theta-burst rTMS of the left IFG abolished the automatic imitation effect in trials where the correct response was spatially compatible with the irrelevant movement stimulus. Comparable stimulation of the PPC had no effect on automatic imitation in either spatially compatible or spatially incompatible trials.
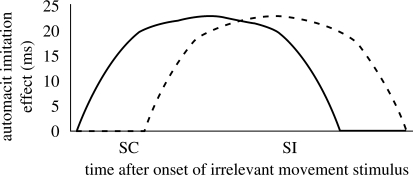
The absence of an effect of rTMS of left IFG on the size of the automatic imitation effect in spatially incompatible trials was unexpected, but can be understood if one considers that spatially incompatible trials are associated with slower RTs than spatially compatible trials (see electronic supplementary material, table S1). This raises the possibility that, rather than blocking perceptual-motor translation entirely, the effect of rTMS of the left IFG is to delay this translation process. Supporting this hypothesis, a recent study found that 1 Hz rTMS to the parietal cortex delayed the onset of the rubber-hand illusion (Kammers et al. 2009). The result of delaying perceptual-motor translation in an imitation task would be a reduction in the automatic imitation effect in fast, i.e. spatially compatible, trials, but a preserved automatic imitation effect in slower, spatially incompatible, trials. Figure 3 illustrates the anticipated outcome of such a delay, in terms of the build-up and decay of the automatic imitation effect over time.
Figure 3.
Illustration of the delay hypothesis. ‘SC’ and ‘SI’ indicate time of response selection in spatially compatible and spatially incompatible trials, respectively. As time (left to right along the x-axis) passes after the onset of the irrelevant movement stimulus, the automatic imitation effect builds up and then declines. In the baseline condition (solid line), the perceptual-motor translation process is not delayed and therefore the build-up begins immediately; in the rTMS to IFG condition (dashed line), the translation process is delayed and thus the build-up of the automatic imitation effect begins later. SC and SI represent the time points at which responses are selected in spatially compatible and spatially incompatible trials, respectively. In the baseline condition (solid line), response selection at both of these times will result in automatic imitation effects of similar sizes. However, in the delayed IFG condition (dashed line), response selection in spatially compatible trials (early) will result in a smaller automatic imitation effect than response selection in spatially incompatible trials (late).
We carried out a time-bin analysis to test this hypothesis. Trials in the three rTMS conditions were ordered by RT and divided into quintiles. ANOVA with factors of imitative compatibility (compatible, incompatible) and quintile (1, fastest, to 5, slowest) revealed an interaction between quintile and imitative compatibility in the IFG condition (F4,28 = 3.4, p = 0.02), such that the automatic imitation effect recovered with increasing RT. This interaction was not present in the baseline (F4,28 = 0.1, p = 0.97) or PPC (F4,28 = 2.0, p = 0.13) conditions.
We also conducted a behavioural follow-up experiment to test the delay hypothesis, in which the irrelevant movement stimulus was presented either simultaneously with, or 40 ms after, the coloured circle (see electronic supplementary material for further details of the method). The 40 ms condition simulated the effects of the hypothesized delay in processing of the irrelevant movement induced by rTMS to IFG. Consistent with the delay hypothesis, analysis of the RT data from this follow-up study revealed an interaction between delay condition and spatial compatibility (F1,7 = 5.4, p = 0.05): the automatic imitation effect was smaller in spatially compatible trials in the 40 ms than the simultaneous condition (11 ± 6 ms compared with 22 ± 7 ms), but no effect of delay was observed on the automatic imitation effect in spatially incompatible trials (17 ± 5 ms compared with 15 ± 9 ms).
Thus, the results of our experiment using theta-burst rTMS suggest that interference with the left IFG, a classical mirror area, delays perceptual-motor translation in an automatic imitation task, and thereby provides stronger evidence than was previously available that the mirror system plays a causal role in generating imitative behaviour.
3. Sensorimotor learning and the mirror system
Having confirmed that imitation depends on the mirror system, we now turn to research in cognitive neuroscience that has used fMRI and TMS to investigate the effects of experience on the development of the mirror system.
(a). Functional magnetic resonance imaging
Several studies have used fMRI to examine the effects of expertise and learning on mirror system activity during action observation. Most of these studies provide compelling evidence that experience modulates the activity of the mirror system, but do not tell us whether sensory experience, motor experience or sensorimotor experience is critical. For example, Haslinger et al. (2005) contrasted the observation of piano playing and non-piano playing finger movements in professional pianists and control participants and showed that training as a pianist enhanced the BOLD response to the observation of piano-playing stimuli in areas including the left IFG and bilateral inferior parietal cortices. Similarly, Margulis et al. (2009) found greater BOLD response in left premotor and inferior parietal cortices when musicians (flute or violin players) listened to a musical excerpt played on their instrument of expertise, compared with the other instrument. As musicians have extensive experience of seeing and hearing their instrument being played (sensory experience), and playing their instrument (motor experience), and of seeing and hearing it played while performing the actions that produce these sensory inputs (sensorimotor experience), these effects could have been due to sensory, motor or sensorimotor experience.
Vogt et al. (2007) investigated the observation and imitative performance of practiced and non-practiced guitar chords in expert and novice guitar players. In contrast with the previous studies (Haslinger et al. 2005; Margulis et al. 2009), they found that the observation of practiced chords produced less activity in mirror system areas than the observation of non-practiced chords. This may have been due to the attentional demands of the task used by Vogt et al.: their participants were required to imitate the observed actions after observing them, and may therefore have paid more attention to the difficult-to-imitate non-practiced chords. Providing less ambiguous evidence of an effect of experience (sensory, motor or sensorimotor), the study by Vogt et al. also indicated that, during chord observation, the guitarists showed greater activity than the non-guitarists in dorsal premotor cortex. (The dorsal premotor cortex is not a classical mirror system area, but in this study it was active during both the observation and the production of chords, suggesting that it has mirror properties.)
Focusing on dance rather than musical expertise, Cross et al. (2006) trained dancer participants to perform a new modern dance piece, and found, over five weeks of rehearsal and brain scanning sessions, that BOLD response in ventral premotor and inferior parietal areas during the observation of sequences from the piece was correlated with the dancers' reported ability to perform the sequences. This interesting result was taken to indicate that participants' motor ability, which is likely to be an indicator of their motor experience, influences the mirror system response to observed actions. However, during rehearsals, the dancers received sensorimotor as well as motor experience—they not only performed the movements, but observed the visual consequences of their movements—and therefore it is not clear whether one or both of these types of experience were critical in modulating the mirror system response.
Across two further studies of dance, Calvo-Merino et al. (2005, 2006) investigated the differences between visual and motor experience of a complex action in mirror system responses. Participants in these studies were capoeira dancers and male and female ballet dancers. Initially, the contrast was made between observing an action with which the participant was familiar and one that was unfamiliar to them: so capoeira dancers observed capoeira actions (familiar), contrasted with a visually similar ballet action (unfamiliar), while the contrast for the ballet dancers was the reverse. BOLD response in premotor and parietal areas was higher when observing the familiar movement than when observing the unfamiliar movement. However, this design confounds motor and visual familiarity: ballet dancers will have more visual experience, as well as more motor experience, of ballet moves. Therefore, the second study contrasted male and female ballet moves: both genders would be equally visually familiar with both types of move, but each would have motor experience only of their own gender-specific moves. Left premotor cortex, as well as parietal and cerebellar areas, showed a greater BOLD response when participants viewed their own gender's movements than when viewing those of the other gender. This suggests that visual experience of an action is less important than motor experience and/or sensorimotor experience in modulating mirror system responses to observation of that action. However, like previous fMRI studies, this one did not isolate the roles of motor and sensorimotor experience in mirror system development. During training, dancers use mirrors and observe other members of their troupe engaging in actions synchronized with their own. Therefore, dancers receive more sensorimotor experience, as well as more motor experience, of own-gender than of other-gender moves as they acquire their expertise.
Catmur et al. (2008) showed that sensorimotor experience, rather than pure sensory or pure motor experience, is the critical type of experience for altering mirror system properties. Incompatible sensorimotor training, where participants performed a hand response to a foot stimulus and a foot response to a hand stimulus, was compared with compatible sensorimotor training (hand stimulus—hand response; foot stimulus—foot response). Incompatible sensorimotor training resulted in a reversal of the normal dominance for hand over foot actions during action observation in the mirror system (premotor and parietal cortices). As both groups received equal sensory and motor experience of the movements, this study confirmed the importance of sensorimotor learning in modifying mirror system responses.
(b). Transcranial magnetic stimulation
Studies using single-pulse TMS have revealed that the human mirror system matches observed with executed actions at a remarkably high level of specificity. Single-pulse TMS to the primary motor cortical representation of a muscle produces a motor-evoked potential (MEP) in that muscle. Passive observation of an action increases the size of MEPs in precisely those muscles that would be involved in performance of the observed action, demonstrating increased activity of those muscles as a result of action observation (e.g. Fadiga et al. 1995; Strafella & Paus 2000). However, only two TMS studies have investigated the role that experience of action observation and execution plays in modulating MEPs during action observation.
In the first study, by D'Ausilio et al. (2006), amateur pianists were asked to learn the left-hand part of a piece of piano music. MEPs from a left-hand muscle were measured before and after the learning period, while participants were listening to either the piano piece or a control flute piece. After the learning period, there was a significant increase in MEP size when participants listened to the learned piece but not to the control piece. This result suggests that the auditory-motor experience received during practice established sensorimotor associations between the sound of the piece and left-hand muscle activity. However, this conclusion is not secure because the participants did not listen to the flute piece during the training period, and therefore auditory experience of the two pieces was not controlled.
In the second study, Catmur et al. (2007) showed specifically that sensorimotor experience can reverse the perceptual-motor matching properties of the mirror system. The participants in this study were given incompatible sensorimotor training in which the observation of an index-finger movement was followed by the performance of a little-finger movement, and vice versa. Compared with controls, who received compatible training, the incompatibly trained participants showed a reversal of the muscle-specific enhancement of MEP size during action observation. For example, after incompatible training, MEPs in the little-finger muscle were larger when observing index-finger movements than when observing little-finger movements. As the control and incompatible training groups saw the action stimuli and performed the responses with equal frequency, this reversal must have been due to the contingency between observation and execution experienced by the incompatible training group, i.e. to sensorimotor learning.
4. Implications
The correspondence problem is at the heart of imitation. Any system that can imitate must have a way of translating perceptual input into matching motor output. The studies reviewed in this paper suggest that the correspondence problem is solved, not by specialized, innate cognitive processes, but by sensorimotor learning. This hypothesis, generated by the ASL model of imitation (Heyes & Ray 2000), is consistent with the results of recent behavioural studies showing that animals can imitate a range of simple body movements; that infants begin to imitate only when they have had the opportunity to learn the appropriate sensorimotor contingencies; and that automatic imitation in human adults can be enhanced, eliminated and reversed by sensorimotor experience. Given the evidence, presented here and in previous studies, that imitation depends on the mirror system, this hypothesis is also supported by research using fMRI and TMS showing that experience, and especially sensorimotor experience, modulates the action-matching properties of the mirror system.
The ASL theory of the origins of imitation and the mirror system is related in a number of ways to each of the principal targets of contemporary research in this field: to questions about the evolution, development and intentional control of imitation. With respect to evolution, the ASL theory clearly implies continuity between the imitative abilities of human and non-human animals. It suggests that phylogenetically ancient mechanisms of associative learning solve the correspondence problem in humans and in a wide range of other taxa (Huber et al. 2009; Whiten et al. 2009). It also suggests that the imitative abilities of other animals are constrained, not by the absence of cognitive processes adapted for the solution of the correspondence problem, but by the limited amount of sensorimotor experience received by other animals in the course of their development. Much of the sensorimotor, imitogenic experience of human infants and children comes from sociocultural sources that are not normally available to other animals; from intensive face-to-face interaction with adults, from mirrors and from the kind of play, dance and exercise programmes that encourage and reward synchronous action. Therefore, the ASL theory predicts that, if non-human animals were given this kind of experience—using training procedures carefully designed to accommodate species-specific perceptual, attentional and motor characteristics—they would develop the capacity to imitate the trained actions.
Although the ASL theory denies that there are cognitive processes dedicated to solving the correspondence problem, it allows that biological evolution may have contributed to human imitative competence, not only by providing basic mechanisms of associative learning, but also by enhancing input mechanisms (Heyes 2003): processes that increase the range and volume of sensorimotor experience received in the course of development. The canalized Hebbian learning hypothesis (del Giudice et al. 2009) makes a non-specific proposal of this kind. It suggests that the perceptual and motor characteristics of human infants have been favoured by natural selection, not because they promote the development of imitation or the mirror system specifically, but because they facilitate the acquisition of visuomotor control. The ASL theory is also compatible with the suggestion that imitation contributes to a cultural inheritance system (Shea 2009), but it adds a twist to this proposal. If the ASL model is correct, human imitation is not only a channel, but also a product of cultural inheritance.
Turning from evolution to development, the ASL theory is obviously congruent with research showing that the ontogeny of imitation in infancy depends on experience (Jones 2009), and with the suggestion that, like imitation, emotional simulation depends on Hebbian learning (Bastiaansen et al. 2009). However, the Hebbian hypothesis (Keysers & Perrett 2004) and the ASL hypothesis (Heyes & Ray 2000) are not identical. The former implies that the learning that gives rise to imitation and the mirror system depends exclusively on contiguity—on observing and executing the same action at the same time—whereas the latter suggests that contingency—experiencing a predictive relationship between observation and execution—is also important (Cook et al. submitted). This distinction may prove critical as further attempts are made to estimate the extent and timing of the imitogenic experience received in the course of typical human ontogeny. Over time, the execution of any given action is likely to be paired with observation of a range of other actions, but there will be a strong contingent relationship between the execution and observation of the same action, owing to the sources of experience detailed above. Therefore, a sensorimotor learning mechanism, based on contiguity and contingency, is more likely than a Hebbian learning mechanism, based on contiguity alone, to yield matching vertical associations, rather than to link, for example, a motor representation of hand opening with perceptual representations of a range of stimuli.
The ideomotor account of imitation apparently stresses a third principle, neither contiguity nor contingency, but similarity (Massen & Prinz 2009), giving the misleading impression that the ideomotor and ASL models are in conflict (Brass & Heyes 2005). In fact, they differ only in emphasis. The ideomotor theory focuses on the on-line control of imitation—on the way in which common codes function once they have become established, whereas the ASL model is also concerned with the origins of these common codes or matching vertical associations. However, the ideomotor theory assumes that common codes originate in sensorimotor learning based on contiguity and contingency (Elsner & Hommel 2004) and, in a complementary way, the ASL theory assumes that, once they have been learned, matching vertical associations function on the basis of similarity or, as it is called in the literature on associative learning, stimulus generalization (Pearce 1987); the degree to which an incoming stimulus excites the sensory component of a matching vertical association depends on the degree of physical similarity between the incoming stimulus and the stimulus represented by the sensory component (Press et al. 2005).
The ASL model may also appear to represent a departure from other views regarding the on-line control of imitative behaviour. Whereas most contemporary theories emphasize the importance of mindsets (van Baaren et al. 2009), or of strategic (Rumiati et al. 2009), intentional (Massen & Prinz 2009) or rational (Gergely et al. 2002) processes in guiding imitative behaviour, the ASL model stresses automaticity. It suggests that, once a vertical association has been formed between a sensory and a motor representation of action, activation of the sensory component inevitably results in activation of the motor component (Heyes & Bird 2007). In fact, however, the ASL model is wholly compatible with the guidance of imitative performance by higher level cognitive processes; it simply suggests that these processes are extrinsic with respect to the core mechanisms of imitation, those that solve the correspondence problem. For example, the ASL model suggests that the imitation inhibition mechanisms identified by Brass et al. (2009) may prevent imitation by reducing the probability that the sensory component of a vertical association will be activated, or by inhibiting the behavioural consequences of activation of the motor component, but not by interfering directly with the propagation of activation from the sensory to the motor component of a vertical association.
The ASL theory is supported by a range of recent findings in cognitive neuroscience and in cognitive, developmental, comparative and social psychology. These studies, reviewed in this paper, suggest that the clever capacity to imitate is based on dumb associative learning.
Acknowledgements
This work was supported by funding from the European Community's Sixth Framework Programme (to C.C. and C.H.) under contract number NEST 012929, and the ESRC Centre for Economic Learning and Social Evolution (to C.H. and C.C.). V.W. is supported by the Royal Society.
Footnotes
Present address: Department of Experimental Psychology, University of Oxford, Oxford OX1 3UD, UK.
Present address: All Souls College, University of Oxford, Oxford OX1 4AL, UK.
One contribution of 13 to a Theme Issue ‘Evolution, development and intentional control of imitation’.
References
- Abravanel E., Levan-Goldschmidt E., Stevenson M. B.1976Action imitation: the early phase of infancy. Child Dev. 47, 1032–1044 (doi:10.2307/1128440) [PubMed] [Google Scholar]
- Anisfeld M., Turkewitz G., Rose S. A., Rosenberg F. R., Sheiber F. J., Couturier-Fagan D. A., Ger J. S., Sommer I.2001No compelling evidence that newborns imitate oral gestures. Infancy 2, 111–122 (doi:10.1207/S15327078IN0201_7) [DOI] [PubMed] [Google Scholar]
- Avenanti A., Bolognini N., Maravita A., Aglioti S. M.2007Somatic and motor components of action simulation. Curr. Biol. 17, 2129–2135 (doi:10.1016/j.cub.2007.11.045) [DOI] [PubMed] [Google Scholar]
- Bastiaansen J. A. C. J., Thioux M., Keysers C.2009Evidence for mirror systems in emotions. Phil. Trans. R. Soc. B 364, 2391–2404 (doi:10.1098/rstb.2009.0058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertenthal B. I., Longo M. R., Kosobud A.2006Imitative response tendencies following observation of intransitive actions. J. Exp. Psychol. Hum. Percept. Perform. 32, 210–225 (doi:10.1037/0096-1523.32.2.210) [DOI] [PubMed] [Google Scholar]
- Brass M., Heyes C.2005Imitation: is cognitive neuroscience solving the correspondence problem? Trends Cogn. Sci. 9, 489–495 (doi:10.1016/j.tics.2005.08.007) [DOI] [PubMed] [Google Scholar]
- Brass M., Bekkering H., Wohlschlager A., Prinz W.2000Compatibility between observed and executed finger movements: comparing symbolic, spatial, and imitative cues. Brain Cogn. 44, 124–143 (doi:10.1006/brcg.2000.1225) [DOI] [PubMed] [Google Scholar]
- Brass M., Zysset S., von Cramon D. Y.2001The inhibition of imitative response tendencies. NeuroImage 14, 1416–1423 (doi:10.1006/nimg.2001.0944) [DOI] [PubMed] [Google Scholar]
- Brass M., Ruby P., Spengler S.2009Inhibition of imitative behaviour and social cognition. Phil. Trans. R. Soc. B 364, 2359–2367 (doi:10.1098/rstb.2009.0066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnyar T., Huber L.1997Push or pull: an experimental study on imitation in marmosets. Anim. Behav. 54, 817–831 (doi:10.1006/anbe.1996.0497) [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B., Glaser D. E., Grezes J., Passingham R. E., Haggard P.2005Action observation and acquired motor skills: an fMRI study with expert dancers. Cereb. Cortex 15, 1243–1249 (doi:10.1093/cercor/bhi007) [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B., Grezes J., Glaser D. E., Passingham R. E., Haggard P.2006Seeing or doing? Influence of visual and motor familiarity in action observation. Curr. Biol. 16, 1905–1910 (doi.10.1016/j.cub.2006.07.065) [DOI] [PubMed] [Google Scholar]
- Campbell F. M., Heyes C. M., Goldsmith A. R.1999Stimulus learning and response learning by observation in the European starling, in a two-object/two-action test. Anim. Behav. 58, 151–158 (doi:10.1006/anbe.1999.1121) [DOI] [PubMed] [Google Scholar]
- Catmur C., Walsh V., Heyes C.2007Sensorimotor learning configures the human mirror system. Curr. Biol. 17, 1527–1531 (doi:10.1016/j.cub.2007.08.006) [DOI] [PubMed] [Google Scholar]
- Catmur C., Gillmeister H., Bird G., Liepelt R., Brass M., Heyes C.2008Through the looking glass: counter-mirror activation following incompatible sensorimotor learning. Eur. J. Neurosci. 28, 1208–1215 (doi:10.1111/j.1460-9568.2008.06419.x) [DOI] [PubMed] [Google Scholar]
- Cook R., Press C., Dickinson A., Heyes C. Is the acquisition of automatic imitation dependent on sensorimotor contingency? doi: 10.1037/a0019256. Submitted. [DOI] [PubMed] [Google Scholar]
- Cress C. J., Andrews T. A., Reynolds C. D.1998Gestural imitation and contingent parent responses in nonspeaking children with physical impairment. Atlanta, GA: Poster at ICIS [Google Scholar]
- Cross E. S., Hamilton A. F., Grafton S. T.2006Building a motor simulation de novo: observation of dance by dancers. NeuroImage 31, 1257–1267 (doi:10.1016/j.neuroimage.2006.01.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custance D. M., Whiten A., Bard K. A.1995Can young chimpanzees (Pan troglodytes) imitate arbitrary actions? Hayes & Hayes (1952) revisited. Behaviour 132, 837–859 (doi:10.1163/156853995X00036) [Google Scholar]
- D'Ausilio A., Altenmuller E., Olivetti B. M., Lotze M.2006Cross-modal plasticity of the motor cortex while listening to a rehearsed musical piece. Eur. J. Neurosci. 24, 955–958 (doi:10.1111/j.1460-9568.2006.04960.x) [DOI] [PubMed] [Google Scholar]
- del Giudice M., Manera V., Keysers C.2009Programmed to learn? The ontogeny of mirror neurons. Dev. Sci. 12, 350–363 (doi.10.1111/j.1467-7687.2008.00783.x) [DOI] [PubMed] [Google Scholar]
- Drager B., Breitenstein C., Helmke U., Kamping S., Knecht S.2004Specific and nonspecific effects of transcranial magnetic stimulation on picture-word verification. Eur. J. Neurosci. 20, 1681–1687 (doi:10.1111/j.1460-9568.2004.03623.x) [DOI] [PubMed] [Google Scholar]
- Elsner B., Hommel B.2004Contiguity and contingency in action-effect learning. Psychol. Res. 68, 138–154 (doi:10.1007/s00426-003-0151-8) [DOI] [PubMed] [Google Scholar]
- Fadiga L., Fogassi L., Pavesi G., Rizzolatti G.1995Motor facilitation during action observation: a magnetic stimulation study. J. Neurophysiol. 73, 2608–2611 [DOI] [PubMed] [Google Scholar]
- Ferrari P. F., Bonini L., Fogassi L.2009From monkey mirror neurons to primate behaviours: possible ‘direct’ and ‘indirect’ pathways. Phil. Trans. R. Soc. B 364, 2311–2323 (doi:10.1098/rstb.2009.0062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T., Hernandez-Reif M., Vera Y., Gil K., Diego M., Bendell D., Yando R.2005Anxiety and anger effects on depressed mother-infant spontaneous and imitative interactions. Infant Behav. Dev. 28, 1–9 (doi:10.1016/j.infbeh.2004.06.003) [Google Scholar]
- Gergely G., Bekkering H., Kiraly I.2002Developmental psychology: rational imitation in preverbal infants. Nature 415, 755 (doi:10.1038/415755a) [DOI] [PubMed] [Google Scholar]
- Gillmeister H., Catmur C., Liepelt R., Brass M., Heyes C.2008Experience-based priming of body parts: a study of action imitation. Brain Res. 1217, 157–170 (doi:10.1016/j.brainres.2007.12.076) [DOI] [PubMed] [Google Scholar]
- Goldenberg G., Karnath H. O.2006The neural basis of imitation is body part specific. J. Neurosci. 26, 6282–6287 (doi:10.1523/JNEUROSCI.0638-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G., Hermsdorfer J., Glindemann R., Rorden C., Karnath H. O.2007Pantomime of tool use depends on integrity of left inferior frontal cortex. Cereb. Cortex 17, 2769–2776 (doi:10.1093/cercor/bhm004) [DOI] [PubMed] [Google Scholar]
- Haslinger B., Erhard P., Altenmuller E., Schroeder U., Boecker H., Ceballos-Baumann A. O.2005Transmodal sensorimotor networks during action observation in professional pianists. J. Cogn. Neurosci. 17, 282–293 (doi:10.1162/0898929053124893) [DOI] [PubMed] [Google Scholar]
- Heiser M., Iacoboni M., Maeda F., Marcus J., Mazziotta J. C.2003The essential role of Broca's area in imitation. Eur. J. Neurosci. 17, 1123–1128 (doi:10.1046/j.1460-9568.2003.02530.x) [DOI] [PubMed] [Google Scholar]
- Heyes C.2001Causes and consequences of imitation. Trends Cogn. Sci. 5, 253–261 (doi:10.1016/S1364-6613(00)01661-2) [DOI] [PubMed] [Google Scholar]
- Heyes C.2003Four routes of cognitive evolution. Psychol. Rev. 110, 713–727 (doi:10.1037/0033-295X.110.4.713) [DOI] [PubMed] [Google Scholar]
- Heyes C. M., Bird G.2007Mirroring, association and the correspondence problem. In Sensorimotor foundations of higher cognition, attention & performance XX (eds Haggard P., Rossetti Y., Kawato) M. Oxford, UK: Oxford University Press [Google Scholar]
- Heyes C. M., Ray E. D.2000What is the significance of imitation in animals? Adv. Study Behav. 29, 215–245 (doi:10.1016/S0065-3454(08)60106-0) [Google Scholar]
- Heyes C., Bird G., Johnson H., Haggard P.2005Experience modulates automatic imitation. Cogn. Brain Res. 22, 233–240 (doi:10.1016/j.cogbrainres.2004.09.009) [DOI] [PubMed] [Google Scholar]
- Huang Y. Z., Edwards M. J., Rounis E., Bhatia K. P., Rothwell J. C.2005Theta burst stimulation of the human motor cortex. Neuron 45, 201–206 (doi:10.1016/j.neuron.2004.12.033) [DOI] [PubMed] [Google Scholar]
- Huber L., Range F., Voelkl B., Szucsich A., Virányi Z., Miklosi A.2009The evolution of imitation: what do the capacities of non-human animals tell us about the mechanisms of imitation? Phil. Trans. R. Soc. B 364, 2299–2309 (doi:10.1098/rstb.2009.0060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M., Woods R. P., Brass M., Bekkering H., Mazziotta J. C., Rizzolatti G.1999Cortical mechanisms of human imitation. Science 286, 2526–2528 (doi:10.1126/science.286.5449.2526) [DOI] [PubMed] [Google Scholar]
- Jones S. S.2006Exploration or imitation? The effect of music on 4-week-old infants' tongue protrusions. Infant Behav. Dev. 29, 126–130 (doi:10.1016/j.infbeh.2005.08.004) [DOI] [PubMed] [Google Scholar]
- Jones S. S.2007Imitation in infancy: the development of mimicry. Psychol. Sci. 18, 593–599 (doi:10.1111/j.1467-9280.2007.01945.x) [DOI] [PubMed] [Google Scholar]
- Jones S. S.2009The development of imitation in infancy. Phil. Trans. R. Soc. B 364, 2325–2335 (doi:10.1098/rstb.2009.0045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammers M. P., Verhagen L., Dijkerman H. C., Hogendoorn H., de Vignemont F., Schutter D. J.2009Is this hand for real? Attenuation of the rubber hand illusion by transcranial magnetic stimulation over the inferior parietal lobule. J. Cogn. Neurosci. 21, 1311–1320 (doi:10.1162/jocn.2009.21095) [DOI] [PubMed] [Google Scholar]
- Kaye K., Marcus J.1978Imitation over a series of trials without feedback: age six months. Infant Behav. Dev. 1, 141–155 (doi:10.1016/S0163-6383(78)80025-3) [Google Scholar]
- Keysers C., Perrett D. I.2004Demystifying social cognition: a Hebbian perspective. Trends Cogn. Sci. 8, 501–507 (doi:10.1016/j.tics.2004.09.005) [DOI] [PubMed] [Google Scholar]
- Killen M., Uzgiris I. C.1981Imitation of actions with objects: the role of social meaning. J. Genet. Psychol. 138, 219–229 [Google Scholar]
- Kilner J. M., Paulignan Y., Blakemore S. J.2003An interference effect of observed biological movement on action. Curr. Biol. 13, 522–525 (doi:10.1016/S0960-9822(03)00165-9) [DOI] [PubMed] [Google Scholar]
- Koren D., Shefer O., Chistyakov A., Kaplan B., Feinsod M., Klein E.2001Neuropsychological effects of prefrontal slow rTMS in normal volunteers: a double-blind sham-controlled study. J Clin. Exp. Neuropsychol. 23, 424–430 (doi:10.1076/jcen.23.4.424.1225) [DOI] [PubMed] [Google Scholar]
- Koski L., Iacoboni M., Dubeau M. C., Woods R. P., Mazziotta J. C.2003Modulation of cortical activity during different imitative behaviors. J. Neurophysiol. 89, 460–471 (doi:10.1152/jn.00248.2002) [DOI] [PubMed] [Google Scholar]
- Kugiumutzakis J.1999Genesis and development of early infant mimesis to facial and vocal models. In Imitation in infancy (eds Nadel J., Butterworth G.), pp. 36–59 Cambridge, MA: Cambridge University Press [Google Scholar]
- Lefebvre L., Templeton J., Brown K., Koelle M.1997Carib grackles imitate conspecific and Zenaida dove tutors. Behaviour 134, 1003–1017 (doi:10.1163/156853997X00368) [Google Scholar]
- Margulis E. H., Mlsna L. M., Uppunda A. K., Parrish T. B., Wong P. C.2009Selective neurophysiologic responses to music in instrumentalists with different listening biographies. Hum. Brain Mapp. 30, 267–275 (doi:10.1002/hbm.20503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massen C., Prinz W.2009Movements, actions, and tool-use actions: an ideomotor approach to imitation. Phil. Trans. R. Soc. B 364, 2349–2358 (doi:10.1098/rstb.2009.0059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur E. F.2006Vocal and action imitation by infants and toddlers during dyadic interactions. In Imitation and the social mind: autism and typical development (eds Rogers S. J., Williams J. H. G.). New York, NY: Guildford Press [Google Scholar]
- McEwen F., Happe F., Bolton P., Rijsdijk F., Ronald A., Dworzynski K., Plomin R.2007Origins of individual differences in imitation: links with language, pretend play, and socially insightful behavior in two-year-old twins. Child Dev. 78, 474–492 (doi:10.1111/j.1467-8624.2007.01010.x) [DOI] [PubMed] [Google Scholar]
- Meltzoff A. N.2007‘Like me’: a foundation for social cognition. Dev. Sci. 10, 126–134 (doi:10.1111/j.1467-7687.2007.00574.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff A. N., Decety J.2003What imitation tells us about social cognition: a rapprochement between developmental psychology and cognitive neuroscience. Phil. Trans. R. Soc. Lond. B 358, 491–500 (doi:10.1098/rstb.2002.1261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff A. N., Moore M. K.1977Imitation of facial and manual gestures by human neonates. Science 198, 74–78 (doi:10.1126/science.897687) [DOI] [PubMed] [Google Scholar]
- Meltzoff A. N., Moore M. K.1983Newborn infants imitate adult facial gestures. Child Dev. 54, 702–709 (doi:10.2307/1130058) [PubMed] [Google Scholar]
- Meltzoff A. N., Moore M. K.1989Imitation in newborn infants: exploring the range of gestures imitated and the underlying mechanisms. Dev. Psychol. 25, 954–962 (doi:10.1037/0012-1649.25.6.954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff A. N., Moore M. K.1997Explaining facial imitation: a theoretical model. Early Dev. Parent. 6, 179–192 (doi:10.1002/(SICI)1099-0917(199709/12)6:3/4<179::AID-EDP157>3.0.CO;2-R) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mui R., Haselgrove M., Pearce J., Heyes C.2008Automatic imitation in budgerigars. Proc. R. Soc. B 275, 2547–2553 (doi:10.1098/rspb.2008.0566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy E.2006From imitation to conversation: the first dialogues with human neonates. Infant Child Dev. 15, 223–232 (doi:10.1002/icd.460) [Google Scholar]
- Nagy E., Compagne H., Orvos H., Pal A., Molnar P., Janszky I., Loveland K. A., Bardos G.2005Index finger movement imitation by human neonates: motivation, learning, and left-hand preference. Pediatr. Res. 58, 749–753 (doi:10.1203/01.PDR.0000180570.28111.D9) [DOI] [PubMed] [Google Scholar]
- Newman-Norlund R. D., van Schie H. T., van Zuijlen A. M., Bekkering H.2007The mirror neuron system is more active during complementary compared with imitative action. Nat. Neurosci. 10, 817–818 (doi:10.1038/nn1911) [DOI] [PubMed] [Google Scholar]
- Pearce J. M.1987A model for stimulus generalization in Pavlovian conditioning. Psychol. Rev. 94, 61–73 (doi:10.1037/0033-295X.94.1.61) [PubMed] [Google Scholar]
- Piaget J.1952Play, dreams and imitation in childhood. New York, NY: Norton [Google Scholar]
- Pobric G., Hamilton A. F.2006Action understanding requires the left inferior frontal cortex. Curr. Biol. 16, 524–529 (doi:10.1016/j.cub.2006.01.033) [DOI] [PubMed] [Google Scholar]
- Press C., Bird G., Flach R., Heyes C.2005Robotic movement elicits automatic imitation. Cogn. Brain Res. 25, 632–640 (doi:10.1016/j.cogbrainres.2005.08.020) [DOI] [PubMed] [Google Scholar]
- Press C., Gillmeister H., Heyes C.2007Sensorimotor experience enhances automatic imitation of robotic action. Proc. Biol. Sci. 274, 2509–2514 (doi:10.1098/rspb.2007.0774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Range F., Viranyi Z., Huber L.2007Selective imitation in domestic dogs. Curr. Biol. 17, 868–872 (doi:10.1016/j.cub.2007.04.026) [DOI] [PubMed] [Google Scholar]
- Ray E., Heyes C.Submitted Imitation in infancy: the wealth of the stimulus. [DOI] [PubMed] [Google Scholar]
- Richards C., Mottley K., Pearce J., Heyes C. M.2009Imitative pecking in budgerigars, Melopsittacus undulatus, over a 24-hour delay. Anim. Behav. 77, 1111–1118 (doi:10.1016/j.anbehav.2009.01.019) [Google Scholar]
- Rizzolatti G., Arbib M. A.1998Language within our grasp. Trends Neurosci. 21, 188–194 (doi:10.1016/S0166-2236(98)01260-0) [DOI] [PubMed] [Google Scholar]
- Rumiati R. I., Carmo J. C., Corradi-Dell'Acqua C.2009Neuropsychological perspectives on the mechanisms of imitation. Phil. Trans. R. Soc. B 364, 2337–2347 (doi:10.1098/rstb.2009.0063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W., Dickinson A.2000Neuronal coding of prediction errors. Annu. Rev. Neurosci. 23, 473–500 (doi:10.1146/annurev.neuro.23.1.473) [DOI] [PubMed] [Google Scholar]
- Shea N.2009Imitation as an inheritance system. Phil. Trans. R. Soc. B 364, 2429–2443 (doi:10.1098/rstb.2009.0061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabbert J. M., Rasa O. A.1997Observational learning of an acquired maternal behaviour pattern by working dog pups: an alternative training method? Appl. Anim. Behav. Sci. 53, 309–316 (doi:10.1016/S0168-1591(96)01163-X) [Google Scholar]
- Strafella A. P., Paus T.2000Modulation of cortical excitability during action observation: a transcranial magnetic stimulation study. Neuroreport 11, 2289–2292 [DOI] [PubMed] [Google Scholar]
- Stürmer B., Aschersleben G., Prinz W.2000Correspondence effects with manual gestures and postures: a study of imitation. J. Exp. Psychol. Hum. Percept. Perform. 26, 1746–1759 (doi:10.1037/0096-1523.26.6.1746) [DOI] [PubMed] [Google Scholar]
- Tanaka S., Inui T.2002Cortical involvement for action imitation of hand/arm postures versus finger configurations: an fMRI study. Neuroreport 13, 1599–1602 (doi:10.1097/00001756-200209160-00005) [DOI] [PubMed] [Google Scholar]
- Tomasello M., Davis-Dasilva M., Camak L., Bard K.1987Observational learning of tool-use by young chimpanzees. Hum. Evol. 2, 175–183 (doi:10.1007/BF02436405) [Google Scholar]
- Urgesi C., Candidi M., Ionta S., Aglioti S. M.2007Representation of body identity and body actions in extrastriate body area and ventral premotor cortex. Nat. Neurosci. 10, 30–31 (doi:10.1038/nn1815) [DOI] [PubMed] [Google Scholar]
- Uzgiris I. C.1972Patterns of vocal and gestural imitation in infants. In Determinants of behavioural development (eds Monks F. J., Hartup W. W., de Witt J.), pp. 467–471 New York, NY: Academic Press [Google Scholar]
- Vallesi A., Shallice T., Walsh V.2007Role of the prefrontal cortex in the foreperiod effect: TMS evidence for dual mechanisms in temporal preparation. Cereb. Cortex 17, 466–474 (doi:10.1093/cercor/bhj163) [DOI] [PubMed] [Google Scholar]
- van Baaren R., Janssen L., Chartrand T. L., Dijksterhuis A.2009Where is the love? The social aspects of mimicry. Phil. Trans. R. Soc. B 364, 2381–2389 (doi:10.1098/rstb.2009.0057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkl B., Huber L.2007Imitation as faithful copying of a novel technique in marmoset monkeys. PLoS ONE 2, e611 (doi:10.1371/journal.pone.0000611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt S., Taylor P., Hopkins B.2003Visuomotor priming by pictures of hand postures: perspective matters. Neuropsychologia 41, 941–951 (doi:10.1016/S0028-3932(02)00319-6) [DOI] [PubMed] [Google Scholar]
- Vogt S., et al. 2007Prefrontal involvement in imitation learning of hand actions: effects of practice and expertise. NeuroImage 37, 1371–1383 (doi:10.1016/j.neuroimage.2007.07.005) [DOI] [PubMed] [Google Scholar]
- Wassermann E. M., Blaxton T. A., Hoffman E. A., Berry C. D., Oletsky H., Pascual-Leone A., Theodore W. H.1999Repetitive transcranial magnetic stimulation of the dominant hemisphere can disrupt visual naming in temporal lobe epilepsy patients. Neuropsychologia 37, 537–544 (doi:10.1016/S0028-3932(98)00102-X) [DOI] [PubMed] [Google Scholar]
- Wheaton L. A., Hallett M.2007Ideomotor apraxia: a review. J. Neurol. Sci. 260, 1–10 (doi:10.1016/j.jns.2007.04.014) [DOI] [PubMed] [Google Scholar]
- Whiten A., Ham R.1992On the nature and evolution of imitation in the animal kingdom: a reappraisal of a century of research. Adv. Stud. Behav. 21, 239–283 (doi:10.1016/S0065-3454(08)60146-1) [Google Scholar]
- Whiten A., Custance D. M., Gomez J. C., Teixidor P., Bard K. A.1996Imitative learning of artificial fruit processing in children (Homo sapiens) and chimpanzees (Pan troglodytes). J. Comp. Psychol. 110, 3–14 (doi:10.1037/0735-7036.110.1.3) [DOI] [PubMed] [Google Scholar]
- Whiten A., Horner V., Litchfield C. A., Marshall-Pescini S.2004How do apes ape? Learn. Behav. 32, 36–52 [DOI] [PubMed] [Google Scholar]
- Whiten A., McGuigan N., Marshall-Pescini S., Hopper L. M.2009Emulation, imitation, over-imitation and the scope of culture for child and chimpanzee. Phil. Trans. R. Soc. B 364, 2417–2428 (doi:10.1098/rstb.2009.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. H., Whiten A., Waiter G. D., Pechey S., Perrett D. I.2007Cortical and subcortical mechanisms at the core of imitation. Soc. Neurosci. 2, 66–78 (doi:10.1080/17470910701268059) [DOI] [PubMed] [Google Scholar]