Abstract
Foot-and-mouth disease can be controlled by zoo-sanitary measures and vaccination but this is difficult owing to the existence of multiple serotypes of the causative virus, multiple host species including wildlife and extreme contagiousness. Although intolerable to modern high-production livestock systems, the disease is not usually fatal and often not a priority for control in many developing countries, which remain reservoirs for viral dissemination. Phylogenetic analysis of the viruses circulating worldwide reveals seven principal reservoirs, each requiring a tailored regional control strategy. Considerable trade benefits accrue to countries that eradicate the disease but as well as requiring regional cooperation, achieving and maintaining this status using current tools takes a great deal of time, money and effort. Therefore, a progressive approach is needed that can provide interim benefits along the pathway to final eradication. Research is needed to understand and predict the patterns of viral persistence and emergence and to improve vaccine selection. Better diagnostic methods and especially better vaccines could significantly improve control in both the free and the affected parts of the world. In particular, vaccines with improved thermostability and a longer duration of immunity would facilitate control and make it less reliant on advanced veterinary infrastructures.
Keywords: foot-and-mouth disease, virus reservoirs, transboundary disease control, novel vaccines
1. Introduction
Awareness of and attempts to control foot-and-mouth disease (FMD) go back at least several centuries (Blancou 2002) and it was the first mammalian disease for which a viral aetiology, FMD virus (FMDV), was demonstrated by Loeffler and Frosch. There are seven FMDV serotypes (O, A, C, Asia 1, South African Territories 1–3 (SAT 1–3)) and immunity to one does not cross-protect against the others; meanwhile, FMDVs evolve rapidly giving rise to intra-serotypic subtypes that may cross-protect incompletely (Brehm et al. 2008). The virus replicates extremely rapidly, is highly contagious and can affect a wide range of domestic and free-living ungulates (Thomson & Bastos 2002). Along with the occurrence of subclinical forms of the disease, this makes FMD control very difficult. FMD is most severe in high-production modern livestock breeds, particularly dairy cattle and pigs making the disease intolerable to modern farming practices, especially in developed countries. Even under subsistence farming conditions, FMD can result in serious economic losses (Rweyemamu & Leforban 1999). The natural host for the SAT 1–3 serotypes of FMDV is the Cape buffalo (Syncercus cafer) in which the virus replicates and persists with minimal disease pathology (Thomson & Bastos 2002; Vosloo et al. 2002). No natural non-domesticated host species are known for the other serotypes, and although estimates of the time taken for their diversification have been made (Tully & Fares 2008), it is not clear whether they all derive from buffalo-adapted ancestors.
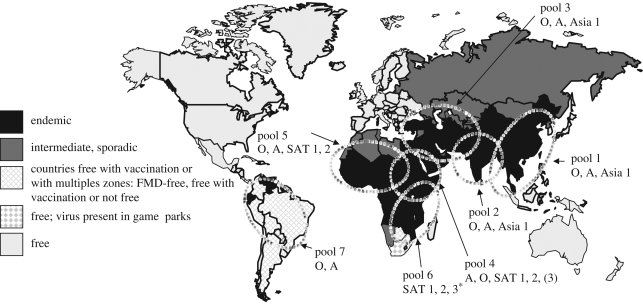
FMDV has been widely distributed in Africa and Eurasia from ancient times, but the New World was probably free of infection until introduction with European livestock during the nineteenth century. The current global burden of FMDV infection is maintained within three continental reservoirs in Asia, Africa and South America that can be further subdivided into seven major virus pools of infection (figure 1; http://www.wrlfmd.org/ref_labs/fmd_ref_lab_reports.htm). Each of these contains at least three serotypes of virus, and because virus circulation is mainly within these regional reservoirs, strains have evolved which are specific to the region and which often (in the case of type A and SAT viruses) require tailored vaccines. An eighth pool of infection, in Western Europe, was present until the 1980s, but has been eradicated through a combination of preventive vaccination and zoo-sanitary measures (Valarcher et al. 2008); the European strains of types O and A are currently maintained only in parts of South America (Rweyemamu et al. 2008a). Serotype C seems to have all but disappeared in the last 10 years. The current worldwide distribution of FMDV mirrors economic development; prosperous countries have eradicated it, whereas developing countries lack the resources and infrastructure to do so. FMD freedom gives rise to significant trade opportunities for countries with livestock export potential, creating an incentive for some countries to invest in veterinary services and FMD control. However, the substantial investment required to guarantee an export market may be either unachievable at present or unlikely to result in sufficient benefits in export earnings, especially if the country is a net importer of livestock and their products. Therefore, many countries have not identified eradication of FMD as a priority. In the case of the Eurasian virus reservoir, the area directly involved straddles the ‘traditional political or economic boundaries’, involving countries in the European, Middle East and West and Central Asian economic groupings. This has the effect that the problem is too big for individual nations to tackle and responsibility is often seen to belong to a third party, while national investment to improve control is hindered by risk of the spread of infection from neighbouring countries. Consequently, over the last 40 years, there has been little change in the prevalence of infection in most of the current reservoirs apart from South America (Correa Melo et al. 2002).
Figure 1.
Conjectured national FMD status in 2008 overlaid with regional FMDV pools and predominant virus serotypes (asterisk, types O and A also in pool 6 where it overlaps pool 4). Adapted from FMD World Reference Laboratory (http://www.wrlfmd.org/). For official information on country status, see http://www.oie.int/wahid-prod/public.php?page=home.
Control of FMD requires strict zoo-sanitary measures and/or vaccination. A slaughter policy with strict movement controls was first applied in the UK in the late nineteenth century, and achieved success, but the scale of slaughter at times overwhelmed the financial or organizational capacity and fresh introductions occurred regularly. Most European countries opted for quarantine policies until mass preventive vaccination became possible from the mid 1950s. Until the late 1960s, epidemics in Europe frequently resulted in thousands of outbreaks in the affected countries. In countries that have achieved FMD-free status, defence of this achievement is through strict zoo-sanitary measures involving import controls on animals and their products from affected countries, early detection and culling of cases, tracing to identify undisclosed sources of infection and onward spread, controls on movements of animals and contaminated materials and intensive surveillance until freedom is re-established. Preventive vaccination, coupled with stamping-out of cases, was adopted by most European countries in the 1950s until 1990, when freedom from FMD allowed vaccination to cease in Europe, with the exception of Turkey and parts of the Russian Federation. Policies based on vaccination, mostly involving quarantine rather than slaughter of cases, have been applied in other regions, such as South America and southern Africa. Vaccination employs type-specific, inactivated vaccines that can be used prophylactically or as an emergency measure, alone or more effectively as an adjunct to zoo-sanitary measures. Prophylactic vaccination seeks to maintain immunity to one or more serotypes of FMD by a programme of regular vaccination and revaccination. Worldwide, of the order of two billion animals are vaccinated annually, but the use of vaccination in endemic countries is highly asymmetric, with many African and south and southeast Asian countries using vaccination to a very limited extent. The majority of vaccine is used in large-scale vaccination programmes in China, South American countries and parts of India and the Middle East. Given the extreme contagiousness of infection, high population immunity is necessary to break transmission, but is often not achieved, and in the absence of effective zoo-sanitary controls, virus can continue to circulate or is introduced readily from remaining pockets of infection or across borders, resulting in continuous or frequent outbreaks.
Progress towards regional and ultimately global eradication of FMD requires a combination of incentives, resources and international cooperative agreements to inspire and support action. Approaches that can address the regional nature and specificities of each virus reservoir are ongoing in South America, southeast Asia and Europe and are sometimes termed ‘Regional Roadmaps to FMD freedom’. Additional programmes are needed that will include those countries with circulating virus in the remaining major FMDV reservoirs. As well as differences in economic and social development that affect the capability and incentives to resource national control measures, such as the regulation of animal movements, there are significant gaps in knowledge: particularly with regard to the manner in which the viruses persist, spread and evolve, threat prediction and reaction and the problem of how to stimulate a better immune response through vaccination.
2. Epidemiological information gaps
The worldwide and regional distribution of many of the diverse serotypes and subtypes of FMDV has been established, although it is subject to change and has to be continuously monitored (Paton et al. 2005; Rweyemamu et al. 2008a). It has been observed that over the last 40 years, particular genetic subtypes (topotypes) persist within a limited range of neighbouring countries and only periodically and temporarily spread beyond these confines (Kitching 2000; Knowles & Samuel 2003). Viruses found in the South American subcontinent (pool 7) are perhaps the most isolated from other parts of the world and currently do not occur elsewhere, despite having appeared in Europe (UK, 1967), Africa (Angola, 1973) and Asia (Philippines, 1975/1976) at different times over the last 50 years. In the case of the three pools each found in Africa and Asia, the delineation between the pools within each continent are not precise, because some topotypes are restricted to one pool while others occur in multiple overlapping pools. For example, serotype Asia 1 has its reservoir and a continuous presence in the Indian subcontinent (pool 2) but periodically spreads west into the Middle East or north and east into former soviet countries (pool 3) and China (pool 1; Valarcher et al. in press). However, type O viruses, such as the PanAsia-2 strain are more broadly distributed and very similar viruses may be found across all three Asian regional pools. Similarly, some East African topotypes of serotype O have been found almost exclusively within eastern Africa (pool 4), whereas others are shared with West Africa (pool 5) presumably reflecting animal movements between the regions. With regard to the SAT serotypes, their distribution has been linked to that of African buffalo, their presumed natural host, which nowadays exists in geographically isolated pockets surrounded by humans and their livestock. Again there are serotypic differences: SAT 3 viruses rarely emerge into domestic livestock, but SAT 2 viruses do so more frequently and both SAT 1 and 2 may be less dependent on buffalo and have previously spread temporarily beyond the confines of Africa.
Overall, the existence of geographical and genetic clustering of FMDVs suggests ecological adaptation and/or separation, but in many endemically affected areas the temporal and spatial dynamics of infection need to be much more accurately determined by analysis of host animal distributions and contact opportunities, serosurveys to estimate weight of infection and use of the latest available techniques in genetic tracing that have so far been applied to tracking FMDV incursions into disease-free regions (Cottam et al. 2006, 2008). The fact that some FMDVs appear intrinsically more adaptable than others (e.g. O PanAsia that has spread widely contrasted with serotype C that has currently disappeared) might be explained by a wider host preference, shorter-lived immunity, etc. but ignorance of the true selective advantages for such viruses or of their viral determinants inhibits efforts to assess the risks from new variants and to predict the emergence of new threats. Early warning and immediate epidemic response also require rapid typing of FMDV isolates to identify suitable vaccines and detect antigenic shifts. A recent initiative to improve laboratory coverage has been the formation of a network of international FMD reference laboratories (Paton et al. 2005). In endemic regions, insufficient sampling and lack of immediate typing and viral risk analysis affect the capability to rapidly implement control measures, such as avoidance of contact with infectious animals as well as the use by owners or veterinary services of quality vaccines that are appropriate to the epidemic strain. Current serological methods to select vaccines are cumbersome, difficult to standardize and require the generation of monovalent, vaccine-specific, bovine antisera. These tests could be replaced by rapid, gene sequence-based prediction of antigenicity, but this requires further research to determine the contribution and importance of different viral epitopes (Paton et al. 2005). There are information gaps concerning our ability to predict the performance of vaccines, particularly in Africa, where there is the greatest virus diversity coupled with the least incentive to produce tailored vaccines. The repeated appearance in the Middle East, in countries such as Iran, of new serotype A FMDV variants with altered antigenicity is puzzling, as they appear to lack immediate predecessors further to the east (Arrowsmith 1975; Knowles et al. 2009). There is growing evidence that inter- and intra-typic recombination occurs between FMDVs in the field (Tosh et al. 2002) and the role this may have in FMDV antigenic shifts needs further examination. Because mass vaccination campaigns are beyond the means of many countries, improved identification of critical risk control points and better targeting of measures, such as vaccination to high-risk groups and susceptible species, could improve the efficiency of interventions and the use of scarce resources, potentially interrupting epidemic circulation at an earlier point. For example, more emphasis could be placed on vaccination of young stock and of animals prior to movement for trade.
The patterns of excretion of FMDV during infection have been extensively studied, many modalities of transmission between animals have been described (Alexandersen et al. 2003) and recent work has helped narrow the window of maximum infectiousness by contact, revealing that the period is much shorter than the time during which virus can be recovered from various clinical samples (Bankowski et al. 2008). Nevertheless, the precise manner in which virus spreads from farm to farm in the absence of animal movements is frequently not discovered, even where intensive tracing is undertaken (Gibbens et al. 2001), including genetic typing of isolated viruses (Cottam et al. 2008). The possible role of persistently infected animals in initiating new outbreaks also remains highly controversial (Salt 2004) and inability to quantify the real risk posed by such animals is a major impediment to the formulation of proportionate and rational rules on time intervals between disease control and the resumption of international trade (Tenzin et al. 2008). Longitudinal field studies in endemic regions, combined with modern forensic genetic typing could cast light on whether viral carriers do play a role in FMDV transmission. However, failure to demonstrate this in many laboratory-based animal studies implies that transmission will be rare if it occurs and therefore large-scale and/or long-term field studies may be needed to elucidate this question.
3. Development of improved vaccines and antivirals
Traditional vaccines remain the mainstay for emergency and prophylactic uses. These are whole virus preparations prepared by large-scale growth in cell cultures subsequently inactivated, purified and mixed with aqueous or nowadays more usually with oily adjuvants (Doel 2005). The final formulated product is rather labile with a limited shelf life and must be kept cold until administered. The vaccines take several days to elicit immunity and this is type- and sometimes strain-specific and is neither maximal nor maintained without booster doses after primo-vaccination, followed by repeat vaccinations at intervals of between 4 and 12 months.
It is recognized that the ideal vaccine for emergency and prophylactic use may have somewhat different characteristics (Kitching et al. 2007; table 1). For all uses, efficacy is crucial and would be best achieved by a single vaccination, but for emergency use, speed of onset of protection will be crucial and excellent marker characteristics are also essential for use in normally FMD-free countries that will wish to use serology to help substantiate disease freedom as soon as possible (Vannier et al. 2007). With the present killed vaccines, this is achieved by removing viral non-structural proteins (NSP) from the finished product, such that antibodies to these proteins can be taken as indicators of infection but not vaccination. However, it is difficult to remove all of the non-structural viral proteins that are produced during cell culture replication of FMDV and these can elicit antibodies that interfere with marker tests.
Table 1.
Ideal attributes for prophylactic and emergency vaccines.
| importance in different situations |
||
|---|---|---|
| vaccine attribute | prophylactic vaccination | emergency vaccination |
| rapid formulation | low | high |
| thermostable formulation | high | medium |
| rapid onset of immunity | low | high |
| broad antigenic spectrum | medium | medium |
| negative marker | medium | high |
| high efficacy | high | high |
| long-lasting protection | high | low |
| low risk of FMDV release | high | high |
| low production cost | high | medium |
Although the possibility of controlling and eventually eradicating FMD with existing vaccines has been demonstrated in Europe and large parts of South America, the difficulties of achieving this in less developed countries could undoubtedly be reduced if vaccines were available with superior qualities, such as longer duration of immunity and improved thermal stability. A broader spectrum of antigenic cover against different subtypes and even serotypes would be beneficial, but not essential, in all circumstances, given the mainly limited range of viruses circulating in each reservoir. In poor countries, cost and vaccine stability are also likely to be critical. The significant role of antibodies in protection against FMD is well established (McCullough et al. 1992) and various dominant and highly variable epitopes on the virus have been identified (Grubman & Baxt 2004). Identifying conserved, protective epitopes could lead to development of vaccines able to confer a broader spectrum of antigenic protection. Little is known about early innate and T-cell immune responses to FMDV and the reasons why virus persists in some animals termed carriers (Salt 2004). Experimental data on the duration of protection following natural infection is also rather limited and based on studies carried out many years ago (Cunliffe 1964; Doel 2005). Finding methods to trigger more effective immune memory without the use of live FMDV is likely to be crucial in development of improved vaccines.
FMDV causes acute cytopathic infections, resulting in tissue damage in infected tissues that has to be controlled rapidly if the host is to survive. Besides natural resistance mechanisms, such as interferons α and β, rapidly induced neutralizing antibodies are a major protective arm of the immune response. Detailed studies of the host immune response to acute cytopathic virus infections performed in mice have demonstrated the crucial role of the early induction of CD4+ T-cell-independent antibody responses in controlling viraemia and clinical disease. Viral antigens that tend to induce T-cell-independent antibody responses are characterized by having repeating epitopes on their surface (Bachmann & Zinkernagel 1996). These are complex structures, typically rigid two-dimensional arrays comprising epitopes displayed at 5–10 nm intervals, that engage and cross-link the immunoglobulin (Ig) receptors on the surface of B cells generating strong activation signals (Bachmann & Zinkernagel 1996). The induction of IgG after FMDV immunization has been shown to be T-cell-dependent in a murine experimental model (Collen et al. 1989; Ostrowski et al. 2007). The early antibody response to FMDV infection in cattle has recently been studied in detail and specific IgM was detected from 4 days post-infection and specific IgG1 and IgG2 from 5 days post-infection (Juleff et al. 2009). Furthermore, the control of viraemia and clinical signs were not affected by complete depletion of CD4+ T cells after treating the animals with specific monoclonal antibodies.
Unlike current vaccines, FMDV infection in ruminants elicits an immune response that can provide protection for several years (Cunliffe 1964). For a number of viruses, efficient retention of intact viral capsids on follicular dendritic cells within the germinal centres of lymph nodes may be a requirement for sustaining antibody responses relevant for providing protection against challenge. The long-term antibody responses detectable after FMDV infection may be maintained in part by antigen persisting on follicular dendritic cells (Juleff et al. 2008). Establishment of long-term antibody responses usually requires efficient antibody class switching and the development of memory B cells and long-lived plasma cells. This process involves the generation of secondary follicles from primary follicles, which can be observed from day 4 to day 21 post-immunization and leads to the improved affinity of serum antibodies during the course of a humoral response and the generation of plasma cells and memory B cells specific for the immunizing T-dependent antigen (Morrissey et al. 1981; Mond et al. 1995; Bachmann & Zinkernagel 1997; Hangartner et al. 2006). Improving the long-term protective antibody response to FMDV may require enhanced specific T-cell responses. Interestingly, T cells specific for either structural (capsid) or non-structural proteins are equally efficient in their ability to help B cells switch from IgM to IgG antibody secretion.
The integrin cell attachment site in aphthoviruses, including FMDV, is located on the protruding, fully exposed, highly disordered and mobile immunogenic G–H loop (Acharya et al. 1989). The G–H loop is considered highly immunogenic, and immunization of cattle with synthetic peptides representing the loop has been shown to induce neutralizing antibody, although convincing protection against viral challenge has not been demonstrated (Taboga et al. 1997). Recently, studies with VP1 G–H loop substituted chimaeric vaccines indicates that the G–H loop may not be required for producing a strong neutralizing antibody response or a protective immune response following vaccination in cattle (Fowler et al. 2008). Also, CD4+ T-cell depletion of the infected cattle had no discernible effect on the control of viraemia and clinical signs and overall neutralizing antibody response, yet it substantially inhibited the IgG antibody response against the G–H loop peptide. These studies suggest that the repetitive antigenic sites embedded in the stable conformational structure of the viral capsid are essential to stimulate a protective immune response to FMDV.
Development of improved FMD vaccines has been ongoing for many years, but there are few centres with the resources to sustain long-term programmes of vaccine research and evaluation or underpinning scientific enquiry into immune mechanisms of livestock against FMDV. Previously developed attenuated live FMD vaccines have retained or reverted to virulence while subunit vaccines have lacked potency (Grubman & Baxt 2004). A peptide vaccine containing antigenically heterogeneous versions of the G–H loop linked to a promiscuous T-cell immunogen has been shown to protect pigs against FMDV challenge. It is now being produced commercially in China. Such a vaccine would have great advantages in terms of cost and stability, but it has not been shown to be effective in cattle and, based on the evidence above, does not contain the major protective epitopes. A live but replication-defective human adenovirus vector shows promise in pigs and cattle as a delivery agent for the complete FMDV structural proteins (Grubman 2005). Vaccines of this type are being produced against each of the different FMDV serotypes and trialled experimentally in cattle in the USA. The principal advantage over conventional vaccine is that it does not require propagation of live FMDV. It is too early to judge whether it will be superior to conventional types in terms of potency and duration of protection. The same vector is also able to induce an early protective response in pigs through delivery of type 1 interferon and this can be combined with delivery of FMDV proteins to provide innate and adaptive immunity resulting in protection from 1 to 2 days of inoculation. The use of reverse genetics to produce infectious DNA clones of the complete FMDV genome paves the way to produce tailored vaccines with deliberately introduced attenuations, markers or strain-specific structural protein genes. Rescued viruses could be used as either attenuated live viruses or grown and inactivated conventionally as killed vaccines. Deletion of the Lpro gene responsible for host cell shutdown and inhibition of type-I interferon production leads to viral attenuation (Chinsangaram et al. 1998). New knowledge on the immune evasion strategies of FMDV and their viral determinants (Moffat et al. 2007) could be used to engender other mutations or deletions in genes to attenuate the virus. Genetic engineering can also be used to create chimaera vaccines with a common non-structural protein gene constellation but with some or all of the structural protein genes of different serotypes. This may offer a way to overcome difficulties in adapting new strains to growth in cell cultures or lead to viruses with improved marker vaccine potential (Fowler et al. 2008). King et al. (2004) showed that mutation of specific viral amino acid residues from serine to cysteine led to the stabilization of the capsid such that it withstood heat treatment and this could give rise to a vaccine with diminished cold chain requirements and possibly enhanced potency. Other approaches, for instance DNA vaccination, may lead to vaccines that could engender enhanced T-cell responses and thereby complement and sustain the antibody elicited by combined use with conventional vaccines. However, prime-boost strategies may be excessively cumbersome and costly for use in prophylactic vaccination schemes and too slow for emergency vaccination. Few products have yet to be reported that can promote a specific and protective oronasal immunity at what is the most common portal of entry for FMDV.
The ideal antiviral agent would be one that could be given orally and become immediately active in stopping viral replication and spread, preferably without preventing the subsequent slaughter for human consumption of treated animals. Finding orally effective compounds might be feasible for monogastric species such as pigs, but is likely to be more challenging for ruminants. FMDV-specific enzymes involved in RNA replication and protein cleavage are possible targets for inhibition by therapeutic drugs and a variety of compounds are starting to be screened for anti-FMDV activity, although few publications have so far appeared. A viral polymerase inhibitor has been reported to be orally effective in preventing FMDV replication and expression of disease in pigs (Ohashi et al. 2008). Peptides have been shown to be very effective at competitive blockade of viral attachment to cell cultures (Dicara et al. 2008), but currently there are no practical options to deliver an effective dose of such substances to the target tissues of animals on a farm. There is much recent interest in the potential to use silencing RNA to inhibit viral replication (Kim et al. 2008) but further progress is needed in methods of delivery and to overcome the potential for viral escape.
4. Response capability
Options and requirements for the control of FMD differ between FMD-free countries and those with endemic infection. With regard to FMD control following incursions into FMD-free countries much has been learnt from recent experiences in the UK outbreaks of 2001 and 2007. The first of these events in 2001 probably resulted from the introduction of the virus with swill that was not properly heated on-farm before feeding to pigs. The owner then failed to recognize or report illness and the virus probably escaped on the wind to affect sheep that were transported through multiple markets leading to widespread dissemination throughout Britain with 2026 outbreaks and spill over to Northern Ireland, France and The Netherlands (Anderson 2002). Approximately six million animals were culled as part of the slaughter policy that was used to control these outbreaks. The need to act quickly meant that culling decisions had to be made on clinical and epidemiological grounds without the benefit of laboratory testing to confirm diagnoses. In an effort to get ahead of the disease, additional culling was used to remove at-risk livestock even though they were not clinically affected. Since this additional culling was implemented after the epidemic had already peaked, it is highly questionable whether it was necessary (Kitching et al. 2006). There was great difficulty in disposing of carcases and restrictions on movements of animals and people closed down large parts of the countryside for leisure and touristic activities. Controversially, vaccination was not used; the disseminated nature of the outbreak would have required very large vaccine reserves and vaccination teams and there were concerns over the manner in which disease freedom could be re-established thereafter.
Internationally, the biggest change in policy since 2001 for FMD control in FMD-free countries has been the renewed emphasis on vaccination as a supplement to culling and other zoo-sanitary measures. A principal impediment to vaccination in such countries has been the extended waiting period that follows before the earliest restoration of the FMD-free status and resumption of exports. Thus, a country applying a rigorous policy of clinical surveillance with stamping out of all affected herds and flocks could and still can regain the disease-free status in a minimum of three months after the last case of FMD. In contrast, where vaccination was used to help bring outbreaks under control, this waiting period was previously at least 12 months unless all of the vaccinated animals were killed. The longer wait was considered necessary to reduce the risk from ongoing viral circulation and infected ‘carrier’ animals that were not clinically identified because of the effect of vaccination on damping the spread and expression of disease. This waiting period has now been reduced to six months, provided serosurveillance is carried out using marker tests that can identify infected animals within a vaccinated population. Regardless of the use of vaccination, the provisions of the Terrestrial Code on zoning and regionalization have also been updated to improve the prospects for isolating infected regions within a country so that trade can resume as soon as possible from the remainder of its territories (OIE 2008). A new European Directive on FMD control was introduced in 2003 and this outlines a clear pathway for use of vaccination and associated tests differentiating infection from vaccination (DIVA; Anonymous 2003).
In the UK, policy changes have included the banning of swill feeding to pigs, preventing sheep from being moved within 5 days of a previous move and declaring an immediate national standstill on animal movements as soon as the first outbreak is confirmed. Improvements have also been made in the identification of animals and the recording of their locations. Among various measures to strengthen national contingency planning, the stock of vaccine reserves has been updated and detailed preparations made to distribute vaccine and vaccinate animals. More could be done to enhance cooperation between those that maintain antigen banks for different countries, in order to ensure a better coverage of strains and a sufficient overall supply.
Since 2001, many laboratories have strengthened their contingency plans and carried out exercises to test their state of preparedness (Koenen et al. 2007). Improvements in computerized data recording systems, with Internet access, already offer very rapid visualization and analysis of test results by disease control teams. A policy of recording sample details electronically, at the point of collection, would remove a further bottleneck at the laboratory and further enhance testing capacity. A wide range of tests exist for FMDV or virus-induced antibodies and methods are continuously improving as new technologies become available and there is an increased uptake of quality accreditation schemes to ensure the reliability of results achieved. Automated systems for use of real-time RT–PCR tests have been established to speed up the laboratory-based diagnosis and increase the testing capacity for FMD (Reid et al. 2003). Genome sequencing allows increasingly sophisticated tracing of outbreak origins (Cottam et al. 2006, 2008) that may be further improved by next generation sequencing to resolve within-isolate variability and by improved knowledge of evolutionary drivers and bottlenecks (Manrubia et al. 2005). Serological methods include tests for antibodies to viral structural and non-structural proteins, the former being also useful to measure vaccine efficacy and the latter for detecting antibodies induced by infection with all serotypes and regardless of whether the animals have been vaccinated (the so-called marker vaccine or DIVA tests; Vannier et al. 2007). Considerable effort has been expended in developing and determining the performance characteristics of these DIVA tests and in estimating the likely prevalence of infection following the use of emergency vaccination, in order to establish meaningful post-outbreak serosurveillance strategies that facilitate rapid recovery of FMD-free status (Arnold et al. 2008; Parida et al. 2008). Nevertheless, if emergency vaccination is applied effectively, it is expected that virus circulation would rapidly be curtailed and therefore the incidence of infection in the vaccinated population would be very low. It is very difficult to prove absence from a low prevalence of infection-specific antibodies and therefore alternative testing strategies are required to provide assurance that vaccination has been done thoroughly.
New technologies also herald the prospect of reducing dependency on centralized laboratories that may not be affordable or else may be far from affected farms and slow the diagnostic process. In some developed countries, efforts have also been made to set up regional laboratories to carry out real-time RT–PCR for diseases such as FMD, both to increase the test capacity and to reduce the sample shipping times (table 2). Digital cameras and cellular phones make it possible to get distant advice on the interpretation of clinical signs and lesions, while infrared cameras can help screen stock for those with fevers or inflamed feet, possibly even from the air (Bashiruddin et al. 2006; Dunbar et al. 2008). Disposable chromatographic strip tests perform with similar accuracy to antigen detection ELISAs used in laboratories and can confirm the presence of FMDV antigens in vesicular epithelium or fluid within 20 min of sample collection, at the animal's side (Ferris et al. 2009). Even more sensitive portable nucleic acid-based equipment is now becoming available (King et al. 2008) and it may soon be possible to locate air sampling devices in strategic positions to detect airborne virus emissions remotely (Ryan et al. 2009). However, a practical way of screening imported animal products for FMDV is so far not available.
Table 2.
Regional progress towards FMD control.
| region | virus pool | eradication schemes | International FMD Reference Laboratory Network member |
|---|---|---|---|
| Asia: East | 1 | coordination of eight southeast Asia countries and vigorous national schemes | Lanzhou, China; Pakchong, Thailand |
| Asia: South | 2 | weak or nascent national schemes | Mukteshwar, India |
| Eurasia | 3 | mainly National with some attempts at coordination | Vladimir, Russia; Pirbright, UKa; Brussels, Belgiuma |
| Africa: East | 4 | no | no |
| Africa: West | 5 | no | no |
| Africa: South | 6 | national and zonal in the most developed countries | Gabarone, Botswana; Ondestepoort, South Africa |
| America: South | 7 | advanced regional | Rio de Janeiro, Brazil; Buenos Aires, Argentina |
aNot located within affected region.
The UK 2007 outbreaks were caused by release of FMDV from the Pirbright site where both the FMD World Reference Laboratory and the Merial FMD Vaccine Production Unit are situated. Although it is not known which facility caused the release, the incident highlights the dangers of handling very large quantities of live, virulent FMDV such as occur during vaccine production providing further impetus to efforts aimed at alternative methods, for example, a non-infectious subunit of FMDV. Unlike the 2001 epidemic, the 2007 outbreaks were few and confined to one area of southern England. However, much of the economic loss in 2007 was due to this ban and better decision-support tools might help to define the most appropriate size of restriction zones at different stages of epidemics (Schley et al. 2009). The UK 2007 outbreaks also saw the first use of laboratory-based, real-time RT–PCR to screen animals for viral carriage prior to the presentation of clinical signs of FMD (Reid et al. 2009). In the first instance, the method was used to test animals that had already been slaughtered because they were under common ownership with confirmed cases. This identified two premises with clinically unapparent, early infections. Later in the course of the outbreaks, the method was used to proactively screen animals in at-risk herds as an alternative to pre-emptive culling. Although the approach was successful, ramping up this capability in the case of a much larger series of outbreaks would be challenging without advanced planning and investment in resources.
The feasibility of extending FMD control to currently endemic regions of Asia and Africa has been questioned, given the logistic and economic difficulties to achieve herd immunity at the required scale for sufficient periods with conventional vaccines. In all regions, especially parts of Africa faced with SAT virus challenge from wildlife, capability is needed to rapidly detect emergent viruses that threaten to break through vaccination programmes and mount an early response using, where possible, vaccines antigenically matched to the epidemic virus. Given the antigenic diversity of FMDV, suitable commercially available vaccines do not exist for all antigenic strains; the time required to develop a new vaccine seed virus from an epidemic FMDV isolate, to meet the required OIE standards, is at least three months, and more probably six months to pass regulatory checks. The lack of suitable vaccines for all FMDV currently circulating and delays to supply suitable vaccines to counter epidemic spread of some FMDV strains are gaps that need to be addressed. Vaccine production capability is also a limiting factor to sudden up-scaling of vaccine for emergency use, and global production will have to rise greatly if the enormous animal populations in Asia and Africa are to be included in routine programmes. This will require many years of investment in new production plants, unless alternatives to current vaccine production technologies can be developed. Capability to deliver routine vaccination is also a limiting factor in some regions, given that current vaccines require a cold chain and the economic and organization difficulty to reach the populations at risk.
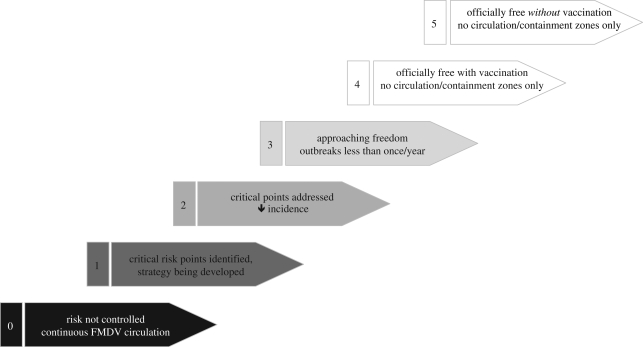
Given the extreme contagiousness of FMD, it is very unlikely that control will be achieved by collective private action, and coordination of public and private actions has been key to FMD control in South America. Veterinary services able to direct and deliver services including emergency responses are therefore prerequisites for achieving freedom from FMD. The experience of eradicating animal diseases is also lacking in many countries, where control is aimed at suppressing clinical disease to an acceptable level. Given the scale of human, financial and technical resources required to reach and maintain FMD freedom, what are the options for endemic regions? One option, being promoted by the Food and Agriculture Organization (FAO), is a progressive, risk-based approach (progressive FMD control pathway). The approach has four stages of activities that lead towards FMD freedom (figure 2), with the first stage being risk identification and strategy development, through management of critical transmission risk points, to the point where circulation of FMDV is discontinuous, and new outbreaks are managed as emergencies with follow-up measures to assure lack of virus transmission. An advantage of the approach is that the initial activities are feasible for most countries and create a better understanding of FMD epidemiology and transmission, introduce the concept of risk-based control strategies and provide some of the key information necessary for decisions on investing scarce resources in targeted or national FMD prevention actions. A further advantage is that the approach enables countries and regional bodies to assess their progress with comparative indicators, creating an incentive for routine serosurveillance to assess progress. At worst, if countries do not progress beyond stage 1, the information collected would assist risk assessment and be of use to neighbours. Regional roadmaps, in which countries agree to progress their activities along a risk management pathway towards a common vision, should be facilitated by application of the progressive pathway approach, and such roadmaps would enable pressure or support to be exerted on countries that fail or choose not to progress. Long-term visions for FMD control have been developed for southeast Asia (SEAFMD 2020 programme), and a process to develop a roadmap for FMD control to 2020 has been initiated among 14 countries in West Eurasia (FAO 2009). Further regional consultations are expected in Africa and South Asia in 2009, as part of the development of an international initiative against FMD that is based on long-term regional efforts (‘roadmaps’) in the seven major FMDV pools.
Figure 2.
Pathway for progressive FMD control. Stages 0–3, infected countries/zones.
However, the lack of drivers for progressive control will continue to be an issue, unless clear benefits are attainable for those progressing along the pathway of risk reduction (Rweyemamu et al. 2008b). At present, most benefits are seen only in export-driven countries after attaining official freedom; the process may take between 2 and 20 years, depending on the capacity of veterinary services and risk situation. Earlier attainment of benefits after investing in FMD control would promote further uptake of control measures; if the benefits are seen at livestock-owner level, then their support and private investment should follow. Possibilities for earlier benefits include compartmentalization, where FMD prevention and control in high health production units or producer groups could create market access, and linkage of FMD control at animal level with measures at slaughter to reduce the risk of FMDV in livestock products to an acceptable level to meet importers requirements—a commodity trading approach (Thomson et al. 2006). A benefit of these two approaches is that it bypasses the need to attain zonal or country freedom and places emphasis on private investment to maintain FMD control in the market chain. A disadvantage could be that FMD control is practised only by a few and that measures for the rest of the population are not taken owing to lack of incentives.
At the international level, there is a need to create the conditions for investment in FMD control. International standards are needed that facilitate trade of animals and animal products in a way that creates incentives for stakeholders to participate in FMD control even in endemic regions. There is also a need to coordinate and support regional efforts, with an international role to monitor progress in each region and provide resources that will assist countries to progress actions along a defined pathway. Monitoring progress also requires a change in the way FMD control is measured, with more emphasis on routine serosurveillance and/or active searching for cases to measuring incidence in risk populations, enabling comparative figures for FMD incidence between countries, which should assist both in opening up trade between countries as well as regions to address hotspots of high risk, with international support as required. Central to the risk-based approach will be sufficient laboratory services at national, regional (table 2) and global levels, with every laboratory eventually part of quality assurance schemes to build confidence in reported data. In parallel, country capacity to measure progress and to take effective local decisions to control new risks must be built; regional technical support will be needed for several years until the skills required are in place and used on a routine basis. These two elements could be efficiently built up through international support given to regional roadmaps in the seven major regions; the main technical tools (ELISA tests for NSP serosurveillance, risk mapping and geographical information systems—GIS) exist but require transfer and training to national facilities; the real question relates to the application of control measures, which will require enormous investment by public or private stakeholders, except in the rare circumstances where a ‘knock-out blow’ to FMD could be achieved by a short-term (1–2 years)-targeted programme. Incentives for investment will be a key issue, as will the related issue of reducing the costs of control with new generation vaccines; the latter will be enormously important in bringing effective controls within reach for less economically advanced regions.
Acknowledgements
Thanks to Don King for comments on the manuscript. D.J.P. and B.C. are Jenner Institute Investigators.
Footnotes
One contribution of 12 to a Theme Issue ‘Livestock diseases and zoonoses’.
References
- Acharya R., Fry E., Stuart D., Fox G., Rowlands D., Brown F.1989The three-dimensional structure of foot-and-mouth disease virus at 2.9 A resolution. Nature 337, 709–716 (doi:10.1038/337709a0) [DOI] [PubMed] [Google Scholar]
- Alexandersen S., Zhang Z., Donaldson A. I., Garland A. J.2003The pathogenesis and diagnosis of foot-and-mouth disease. J. Comp. Pathol. 129, 1–36 (doi:10.1016/S0021-9975(03)00041-0) [DOI] [PubMed] [Google Scholar]
- Anderson I.2002Foot and mouth disease. 2001: Lessons to be learned inquiry report .Report to the Prime Minister and the Secretary of State for Environment Food and Rural Affairs, London, UK [Google Scholar]
- Anonymous 2003Council Directive 2003/85/EC on Community measures for the control of foot-and-mouth disease repealing Directive 85/511/EEC and Decisions 89/531/EEC and 96/665/EEC and amending Directive 92/46/EEC. Article 21 & 45. Off. J. Eur. Union, L306 [Google Scholar]
- Arnold M. E., Paton D. J., Ryan E., Cox S. J., Wilesmith J. W.2008Modelling studies to estimate the prevalence of foot-and-mouth disease carriers after reactive vaccination. Proc. Biol. Sci. 275, 107–115 (doi:10.1098/rspb.2007.1154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrowsmith A. E.1975Variation among strains of type A foot-and-mouth disease virus in the Eastern Mediterranean region 1964–1972. J. Hyg. 75, 387–397 (doi:10.1017/S0022172400024451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann M. F., Zinkernagel R. M.1996The influence of virus structure on antibody responses and virus serotype formation. (Review). Immunol. Today 17, 553–558 (doi:10.1016/S0167-5699(96)10066-9) [DOI] [PubMed] [Google Scholar]
- Bachmann M. F., Zinkernagel R. M.1997Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 15, 235–270 (doi:10.1146/annurev.immunol.15.1.235) [DOI] [PubMed] [Google Scholar]
- Bankowski B. M., Juleff N., Gibson D. G., Gloster J., Doel C., Cox S. J., Barnett P. V., Woolhouse M. J., Charleston B.2008Defining the period of infectiousness in cattle naturally infected with foot-and-mouth disease virus. Report of the Open Session of the EuFMD Standing Technical Committee, Erice, Sicily, 14–16 October 2008, Appendix 53, pp. 293–300 [Google Scholar]
- Bashiruddin J. B., Mann J., Fitch R., Zhang Z., Paton D.2006Preliminary study of the use of thermal imaging to assess surface temperatures during foot-and-mouth disease virus infection in cattle, sheep and pigs. Report of the Session of the Research Group of the Standing Technical Committee of EuFMD, Paphos, Cyprus, 17–20 October 2006, Appendix 46, pp. 304–308 [Google Scholar]
- Blancou J.2002History of the control of foot and mouth disease. Comp. Immunol. Microbiol. Inf. Dis. 25, 283–296 (doi:10.1016/S0147-9571(02)00026-7) [DOI] [PubMed] [Google Scholar]
- Brehm K. E., Kumar N., Thulke H. H., Haas B.2008High potency vaccines induce protection against heterologous challenge with foot-and-mouth disease virus. Vaccine 26, 1681–1687 (doi:10.1016/j.vaccine.2008.01.038) [DOI] [PubMed] [Google Scholar]
- Chinsangaram J., Mason P. W., Grubman M. J.1998Protection of swine by live and inactivated vaccines prepared from a leader proteinase-deficient serotype A12 foot-and-mouth disease virus. Vaccine 16, 1516–1522 (doi:10.1016/S0264-410X(98)00029-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen T., Pullen L., Doel T. R.1989T cell-dependent induction of antibody against foot-and-mouth disease virus in a mouse model. J. Gen. Virol. 70, 395–403 (doi:10.1099/0022-1317-70-2-395) [DOI] [PubMed] [Google Scholar]
- Correa Melo E., Saraiva V., Astudillo V.2002Review of the status of foot and mouth disease in countries of South America and approaches to control and eradication. Rev. Sci. Tech. 21, 429–436 [DOI] [PubMed] [Google Scholar]
- Cottam E. M., Haydon D. T., Paton D. J., Gloster J., Wilesmith J. W., Ferris N. P., Hutchings G. H., King D. P.2006Molecular epidemiology of the foot-and-mouth disease virus outbreak in the United Kingdom in 2001. J. Virol. 80, 11 274–11 282 (doi:10.1128/JVI.01236-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottam E. M., et al. 2008Transmission pathways of foot-and-mouth disease virus in the United Kingdom in 2007. PLoS Pathogens 4, 1–8 (doi:10.1371/journal.ppat.1000050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe H. R.1964Observations on the duration of immunity in cattle after experimental infection with foot-and-mouth disease virus. Cornell Vet. 54, 501–510 [PubMed] [Google Scholar]
- Dicara D., Burman A., Clark S., Berryman S., Howard M. J., Hart I. R., Marshall J. F., Jackson T.2008Foot-and-mouth disease virus forms a highly stable, EDTA-resistant complex with its principal receptor, integrin alphavbeta6: implications for infectiousness. J. Virol. 82, 1537–1546 (doi:10.1128/JVI.01480-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doel T. R.2005Natural and vaccine induced immunity to FMD. Curr. Top. Microbiol. Immunol. 288, 103–131 (doi:10.1007/3-540-27109-0_5) [DOI] [PubMed] [Google Scholar]
- Dunbar M. R., Johnson S. R., Rhyan J. C., McCollum M.2008Use of infrared thermography to detect signs of foot-and-mouth disease in wild and domestic ungulates. Report of the Open Session of the EuFMD Standing Technical Committee, Erice, Sicily, 14–16 October 2008, Appendix 35, p. 209 [DOI] [PubMed] [Google Scholar]
- FAO 2009Development of a roadmap for the progressive control of foot-and-mouth disease in West Eurasia. Report of a Workshop held in Shiraz, Islamic Republic of Iran, 9–13 November 2008, FAO of the UN, Rome, Italy [Google Scholar]
- Ferris N. P., et al. 2009Development and laboratory validation of a lateral flow device for the detection of foot-and-mouth disease virus in clinical samples. J. Virol. Methods 155, 10–17 (doi:10.1016/j.jviromet.2008.09.009) [DOI] [PubMed] [Google Scholar]
- Fowler V. L., Paton D. J., Rieder E., Barnett P. V.2008Chimeric foot-and-mouth disease viruses: evaluation of their efficacy as potential marker vaccines in cattle. Vaccine 26, 1982–1989 (doi:10.1016/j.vaccine.2008.02.012) [DOI] [PubMed] [Google Scholar]
- Gibbens J. C., Sharpe C. E., Wilesmith J. W., Mansley L. M., Michalopoulou E., Ryan J. B., Hudson M.2001Descriptive epidemiology of the 2001 foot-and-mouth disease epidemic in Great Britain: the first five months. Vet. Rec. 149, 729–743 [PubMed] [Google Scholar]
- Grubman M. J.2005Development of novel strategies to control foot-and-mouth disease: marker vaccines and antivirals. Biologicals 33, 227–234 (doi:10.1016/j.biologicals.2005.08.009) [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Baxt B.2004Foot-and-mouth disease. Clin. Microbiol. Rev. 17, 465–493 (doi:10.1128/CMR.17.2.465-493.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangartner L. R., Zinkernagel M., Hengartner H.2006Antiviral antibody responses: the two extremes of a wide spectrum. Nat. Rev. Immunol. 6, 231–243 (doi:10.1038/nri1783) [DOI] [PubMed] [Google Scholar]
- Juleff N., Windsor M., Reid E., Seago J., Zhang Z., Monaghan P., Morrison I. W., Charleston B.2008Foot-and-mouth disease virus persists in the light zone of germinal centres. PLoS ONE 3, e3434 (doi:10.1371/journal.pone.0003434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juleff N., et al. 2009Foot-and-mouth disease virus can induce a specific and rapid CD4+ T cell-independent neutralising and isotype class switched antibody response in naive cattle. J. Virol. 83, 3626–3636 (doi:10.1128/JVI.02613-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. M., Lee K. N., Park J. Y., Ko Y. J., Joo Y. S., Kim H. S., Park J. H.2008Therapeutic application of RNA interference against foot-and-mouth disease virus in vitro and in vivo. Antivir. Res. 80, 178–184 (doi:10.1016/j.antiviral.2008.06.001) [DOI] [PubMed] [Google Scholar]
- King A. M. Q., Burman A. J., Audonnet J.-C. F., Lombard M. F. A. Vaccine against foot-and-mouth disease. 2004. United States Patent application 20040001864. [Google Scholar]
- King D. P., Dukes J. P., Reid S. M., Ebert K., Shaw A. E., Mills C. E., Boswell L., Ferris N. P.2008Prospects for rapid diagnosis of foot-and-mouth disease in the field using reverse transcriptase-PCR. Vet. Rec. 162, 315–316 [DOI] [PubMed] [Google Scholar]
- Kitching R. P.2000The role of the World Reference Laboratories for foot-and-mouth disease and for rinderpest. Ann. NY Acad. Sci. 916, 139–146 [DOI] [PubMed] [Google Scholar]
- Kitching R. P., Thrusfield M. V., Taylor N. M.2006Use and abuse of mathematical models: an illustration from the 2001 foot and mouth disease epidemic in the United Kingdom. Rev. Sci. Tech. 25, 293–311 [DOI] [PubMed] [Google Scholar]
- Kitching P., Hammond J., Jeggo M., Charleston B., Paton D., Rodriguez L., Heckert R.2007Global FMD control—is it an option? Vaccine 25, 5660–5664 (doi:10.1016/j.vaccine.2006.10.052) [DOI] [PubMed] [Google Scholar]
- Knowles N. J., Samuel A. R.2003Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 91, 65–80 (doi:10.1016/S0168-1702(02)00260-5) [DOI] [PubMed] [Google Scholar]
- Knowles N. J., et al. 2009Recent spread of a new strain (A-Iran-05) of foot-and-mouth disease virus type A in the Middle East. Transbound. Emerg. Dis. 56, 157–169 (doi:10.1111/j.1865-1682.2009.01074.x) [DOI] [PubMed] [Google Scholar]
- Koenen F., Uttenthal A., Meindl-Böhmer A.2007Real-time laboratory exercises to test contingency plans for classical swine fever: experiences from two national laboratories. Rev. Sci. Tech. 26, 629–638 [PubMed] [Google Scholar]
- Manrubia S. C., Escarmís C., Domingo E., Lázaro E.2005High mutation rates, bottlenecks, and robustness of RNA viral quasispecies. Gene 347, 273–282 (doi:10.1016/j.gene.2004.12.033) [DOI] [PubMed] [Google Scholar]
- McCullough K. C., De Simone F., Brocchi E., Capucci L., Crowther J. R., Kihm U.1992Protective immune response against foot-and-mouth disease. J. Virol. 66, 1835–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat K., Knox C., Howell G., Clark S. J., Yang H., Belsham G. J., Ryan M., Wileman T.2007Inhibition of the secretory pathway by foot-and-mouth disease virus 2BC protein is reproduced by coexpression of 2B with 2C, and the site of inhibition is determined by the subcellular location of 2C. J. Virol. 81, 1129–1139 (doi:10.1128/JVI.00393-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Lees A., Snapper C. M.1995T cell-independent antigens type 2. Annu. Rev. Immunol. 13, 655–692 (doi:10.1146/annurev.iy.13.040195.003255) [DOI] [PubMed] [Google Scholar]
- Morrissey P. J., Boswell H. S., Scher I., Singer A.1981Role of accessory cells in B cell activation. IV. Ia+ accessory cells are required for the in vitro generation of thymic independent type 2 antibody responses to polysaccharide antigens. J. Immunol. 127, 1345–1347 [PubMed] [Google Scholar]
- Ohashi S., Sakamoto K., Fukai K., Morioka K., Yamazoe R., Takahashi K., Furuta Y.2008An antiviral agent, T-1105 prevents virus excretion from pigs infected with porcinophilic foot-and-mouth disease virus. Report of the Open Session of the EuFMD Standing Technical Committee, Erice, Sicily, 14–16 October 2008, Appendix 70, pp. 393–398 [Google Scholar]
- OIE 2008OIE Terrestrial Animal Health Code, vol. 2, ch. 8.5, pp. 347–369, 17th edn Paris, France: World Organisation for Animal Health [Google Scholar]
- Ostrowski M., Vermeulen M., Zabal O., Zamorano P. I., Sadir A. M., Geffner J. R., Lopez O. J.2007The early protective thymus-independent antibody response to foot-and-mouth disease virus is mediated by splenic CD9+ B lymphocytes. J. Virol. 81, 9357–9367 (doi:10.1128/JVI.00677-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parida S., Fleming L., Oh Y., Mahapatra M., Hamblin P., Gloster J., Paton D. J.2008Emergency vaccination of sheep against foot-and-mouth disease: significance and detection of subsequent sub-clinical infection. Vaccine 26, 3469–3479 (doi:10.1016/j.vaccine.2008.04.026) [DOI] [PubMed] [Google Scholar]
- Paton D. J., Valarcher J.-F., Bergmann I., Matlho O. G., Zakharov V. M., Palma E. L., Thomson G. R.2005Selection of foot-and-mouth disease vaccine strains—a review. Sci. Tech. Rev. 24, 981–993 [PubMed] [Google Scholar]
- Reid S. M., Grierson S. S., Ferris N. P., Hutchings G. H., Alexandersen S.2003Evaluation of automated RT–PCR to accelerate the laboratory diagnosis of foot-and-mouth disease virus. J. Virol. Methods 107, 129–139 (doi:10.1016/S0166-0934(02)00210-0) [DOI] [PubMed] [Google Scholar]
- Reid S. M., et al. 2009Performance of real-time RT–PCR for the detection of foot-and-mouth disease virus during field outbreaks in the United Kingdom in 2007. J. Vet. Diagn. Invest. 21, 321–330 [DOI] [PubMed] [Google Scholar]
- Rweyemamu M. M., Leforban Y.1999Foot-and-mouth disease and international development. Adv. Virus Res. 53, 111–126 (doi:10.1016/S0065-3527(08)60345-0) [DOI] [PubMed] [Google Scholar]
- Rweyemamu M., Roeder P., Mackay D., Sumption K., Brownlie J., Leforban Y., Valarcher J. F., Knowles N. J., Saraiva V.2008aEpidemiological patterns of foot-and-mouth disease worldwide. Transbound. Emerg. Dis. 55, 57–72 [DOI] [PubMed] [Google Scholar]
- Rweyemamu M., Roeder P., MacKay D., Sumption K., Brownlie J., Leforban Y.2008bPlanning for the progressive control of foot-and-mouth disease worldwide. Transbound Emerg Dis. 55, 73–87 [DOI] [PubMed] [Google Scholar]
- Ryan E., Wright C., Gloster J.2009Measurement of airborne foot-and-mouth disease virus: preliminary evaluation of two portable air sampling devices. Vet. J. 179, 458–461 (doi:10.1016/j.tvjl.2007.10.008) [DOI] [PubMed] [Google Scholar]
- Salt J.2004Persistence of foot-and-mouth disease. In Foot and mouth disease; current perspectives, ch. 6 (eds Sobrino F., Domingo E.), pp. 103–143 Wymondham, UK: Horizon Bioscience [Google Scholar]
- Schley D., Gubbins S., Paton D. J.2009Quantifying the risk of localised animal movement bans for foot-and-mouth disease. PLoS ONE 4, e5481 (doi:10.1371/journal.pone.0005481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taboga O., et al. 1997A large-scale evaluation of peptide vaccines against foot-and-mouth disease: lack of solid protection in cattle and isolation of escape mutants. J. Virol. 71, 2606–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenzin , Dekker A., Vernooij H., Bouma A., Stegeman A.2008Rate of foot-and-mouth disease virus transmission by carriers quantified from experimental data. Risk. Anal. 28, 303–309 (doi:10.1111/j.1539-6924.2008.01020.x) [DOI] [PubMed] [Google Scholar]
- Thomson G. R.1994Foot and mouth disease. In Infectious diseases of livestock with special reference to Southern Africa (eds Coetzer J. A. W., Thomson G. R., Tustin R. C.), pp. 825–852 Cape Town, South Africa: Oxford University Press [Google Scholar]
- Thomson G. R., Bastos A. D. S.2002Foot-and-mouth disease. In Infectious diseases of livestock with special reference to southern Africa (eds Coetzer J. A. W., Tustin R. C.), pp. 1324–1365, 2nd edn Cape Town, South Africa: Oxford University Press [Google Scholar]
- Thomson G. R., Perry B. D., Catley A., Leyland T. J., Penrith M. L., Donaldson A. I.2006Certification for regional and international trade in livestock commodities: the need to balance credibility and enterprise. Vet. Rec. 159, 53–57 [DOI] [PubMed] [Google Scholar]
- Tosh C., Hemadri D., Sanyal A.2002Evidence of recombination in the capsid-coding region of type A foot-and-mouth disease virus. J. Gen. Virol. 83, 2455–2460 [DOI] [PubMed] [Google Scholar]
- Tully D. C., Fares M. A.2008The tale of a modern animal plague: tracing the evolutionary history and determining the time-scale for foot and mouth disease virus. Virology 382, 250–256 (doi:10.1016/j.virol.2008.09.011) [DOI] [PubMed] [Google Scholar]
- Valarcher J. F., Leforban Y., Rweyemamu M., Roeder P. L., Gerbier G., Mackay D. K., Sumption K. J., Paton D. J., Knowles N.2008Incursions of foot-and-mouth disease virus into Europe between 1985 and 2006. Transbound. Emerg. Dis. 55, 14–34 [DOI] [PubMed] [Google Scholar]
- Valarcher J.-F., et al. In press Multiple origins of a foot-and-mouth disease virus serotype Asia 1 epidemic. Emerg. Infect. Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannier P., Capua I., Le Potier M. F., Mackay D., Muylkens B., Parida S., Paton D. J., Thiry E.2007Marker vaccines and impact of their use on diagnostic and prophylactic measures. OIE Sci. Tech. Rev. 26, 351–372 [PubMed] [Google Scholar]
- Vosloo W., Bastos A. D. S., Sangare O., Hargreaves S. K., Thomson G. R.2002Review of the status and control of foot and mouth disease in sub-Saharan Africa. OIE Sci. Tech. Rev. 21, 437–449 [DOI] [PubMed] [Google Scholar]