Abstract
Respiratory muscles with dual respiratory and non-respiratory functions (e.g. the pharyngeal and intercostal muscles) show greater suppression of activity in sleep than the diaphragm, a muscle almost entirely devoted to respiratory function. This sleep-related suppression of activity is most apparent in the tonic component of motor activity, which has functional implications of a more collapsible upper airspace in the case of pharyngeal muscles, and decreased functional residual capacity in the case of intercostal muscles. A major source of tonic drive to respiratory motoneurons originates from neurons intimately involved in states of brain arousal, i.e. neurons not classically involved in generating respiratory rhythm and pattern per se. The tonic drive to hypoglossal motoneurons, a respiratory motor pool with both respiratory and non-respiratory functions, is mediated principally by noradrenergic and glutamatergic inputs, these constituting the essential components of the wakefulness stimulus. These tonic excitatory drives are opposed by tonic inhibitory glycinergic and γ-amino butyric acid (GABA) inputs that constrain the level of respiratory-related motor activity, with the balance determining net motor tone. In sleep, the excitatory inputs are withdrawn and GABA release into the brainstem is increased, thus decreasing respiratory motor tone and predisposing susceptible individuals to hypoventilation and obstructive sleep apnoea.
Keywords: sleep, medulla, brainstem, respiratory muscle, neuromodulators
1. Sleep and breathing: from common clinical problems to a focus on respiratory motoneurons
(a). Sleep-related breathing disorders
The Institute of Medicine of the National Academy of Sciences (Colten & Altevogt 2006) has identified sleep disorders as a major public health problem, the most common and serious of which have a failure to maintain adequate breathing during sleep as their root cause. The most prevalent sleep-related breathing disorder is obstructive sleep apnoea, affecting 9 per cent of women and 24 per cent of men in a sample of middle-aged North Americans, while 2–4% of individuals had significant obstructed breathing events during sleep plus symptoms of excessive sleepiness during the day (Young et al. 1993). Obstructive sleep apnoea, however, is not the only breathing disorder that becomes apparent in, or is significantly exacerbated by, the transition from wakefulness to sleep. Restrictive lung diseases (e.g. obesity hypoventilation) and neuromuscular weakness (e.g. partial diaphragm paralysis and amyotrophic lateral sclerosis) also produce significant breathing problems in sleep and a burden of chronic night-time hypoxia that affects clinical course, stability and long-term outcome (Goldstein 1992; Kryger 2000).
(b). Respiratory motoneurons and the ‘wakefulness stimulus’ for breathing
Motoneurons are the ‘final common output pathway’ for the influence of the central nervous system on motor activity, with the level and pattern of motor activity being determined by the net summation of temporally related excitatory and inhibitory drives (Sherrington 1906; Burke 2007). Given this organization, this paper will focus mainly on the control of respiratory motoneurons by those brainstem respiratory networks and sleep-state-dependent neural processes that provide converging excitatory and inhibitory drives to modulate motor outflow and breathing. Nevertheless, attention will turn, as appropriate, to the activity of respiratory pre-motor neurons central to the respiratory network because it is from recordings of such neurons that the neurophysiological principles underlying the concept of a wakefulness stimulus to breathing were first derived (Orem et al. 1985; Orem 1990, 1994).
The concept of a wakefulness stimulus to breathing, i.e. a drive that activates respiratory neurons and motoneurons in wakefulness but not in sleep, has been an important and enduring notion in respiratory medicine (Fink 1961; Phillipson & Bowes 1986; Orem 1990), not least because it is useful in modelling behavioural state effects on breathing and understanding the pathogenesis of sleep-related breathing disorders. Essential to this concept, as related to motor control, is an understanding of the mechanisms underlying both the respiratory and tonic (non-respiratory) inputs to respiratory motoneurons. In the current paper, the role of the tonic inputs is a major focus of discussion as these are critical to modulating the overall level of drive and expression of activity in the respiratory system and are significantly influenced by sleep–wake states. Moreover, the tonic inputs to respiratory motoneurons are envisaged to constitute the essential component of the wakefulness stimulus, and the neuromodulators(s) that mediate the major components of these inputs have recently been identified (Chan et al. 2006; Horner 2008a; Steenland et al. 2008).
2. Overview of the respiratory system
Determining the brainstem mechanisms underlying the control of breathing during sleep essentially encompasses the control of respiratory (pre-motor) neurons and motoneurons across sleep–wake states. The former components of the respiratory network are the source of both the rhythm of breathing and the central respiratory drive potential that activates respiratory motoneurons, while the latter are the final common output pathway for the influence of the central nervous system on the muscles of breathing. The ventral and dorsal respiratory groups contain both bulbo-spinal respiratory pre-motoneurons (i.e. neurons that project to spinal motoneurons, which in turn innervate the respective respiratory pump and abdominal muscles of breathing) and proprio-bulbar neurons (i.e. neurons that project to, and influence the activity of, other medullary respiratory neurons but themselves do not project to motoneurons per se). In addition to the nucleus ambiguus that contains motoneurons innervating the laryngeal and pharyngeal muscles, the brainstem also includes motoneurons of the hypoglossal and trigeminal motor nuclei that innervate the muscles of the tongue and soft palate (figure 1a), i.e. muscles important to the maintenance of an open upper airway for effective breathing (Horner 1996, 2008b). Respiratory-related activity is not restricted to neurons of the ventral and dorsal respiratory groups and cranial motoneurons innervating the pharyngeal and laryngeal muscles, however. Respiratory-related neurons in the pons play an important role in shaping the activity of medullary respiratory neurons during the breathing cycle (St John & Paton 2004; Smith et al. 2007), and the activity patterns of pontine respiratory neurons are altered by sleep–wake states (Lydic & Orem 1979; Orem 1994).
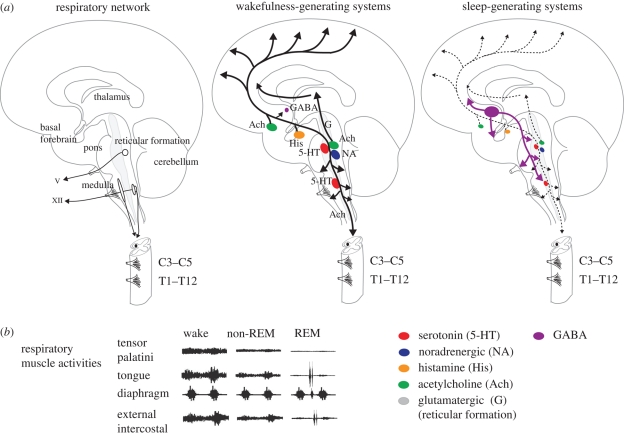
Figure 1.
(a) Saggital section of the brain showing the main neuronal groups and motoneuron pools innervating selected muscles of the upper airway and respiratory pump. Also shown are the main wakefulness and sleep-generating neural systems (adapted from Saper et al. 2005). In sleep, the awake-related neurons are inhibited by γ-amino butyric acid- (GABA-) containing neurons from the ventrolateral pre-optic area of the hypothalamus (lesser activity is shown by dashed line). By their anatomical projections to the pons, medulla and spinal cord, these wake and sleep-promoting neuronal systems are positioned to influence respiratory neurons and motoneurons. The rootlets of cranial nerves V (trigeminal) and XII (hypoglossal) and the cervical (C) and thoracic (T) segments of the spinal cord are shown. (b) Schema to show representative changes in electromyographic activity of selected muscles of the upper airway and respiratory pump across sleep–wake states. See text for further details.
(a). Expression of tonic and respiratory-related activity in respiratory muscles and the functional implications of dual drives
Some respiratory muscles exhibit more respiratory-related activity than others, while other muscles exhibit more tonic activity with little respiratory-related activity (figure 1b). This difference in activity profiles between different respiratory muscles depends on their anatomical location in the respiratory system and their involvement in other functions such as postural support and behavioral motor acts. Importantly, muscles that combine respiratory and non-respiratory functions are most susceptible to decrements in activity in sleep (Phillipson & Bowes 1986; Orem 1990), especially rapid eye movement (REM) sleep (figure 1b).
(i). Pharyngeal muscles
The upper airspace in the region of the pharynx is surrounded by a complex anatomical arrangement of skeletal muscles and soft tissues, an arrangement unlike other regions of the respiratory tract such as the trachea and bronchi that are surrounded by more rigid cartilaginous support. The muscular and soft tissue composition of the pharyngeal airway provides the necessary support for the variety of essential non-respiratory functions such as vocalization, suckling, chewing and swallowing, i.e. behaviours that require dynamic changes in airway size to move air, liquids and solids. However, this property of a collapsible tube compromises the essential respiratory function of the upper airway, i.e. the airway must remain open during breathing, in all postures, to allow for adequate lung ventilation and gas exchange. A generalized suppression of pharyngeal muscle tone in sleep leads to airway narrowing and increased resistance to airflow, and this effect is the main contributor to the normal hypoventilation and increased arterial PCO2 of 3–5 mm Hg typically observed in normal sleeping subjects (Hudgel et al. 1984; Henke et al. 1992). This reduced pharyngeal muscle tone in sleep, by leading to a narrower and more collapsible upper airway, also predisposes to airflow limitation (i.e. hypopnoeas and snoring) and obstructive sleep apnoea in susceptible individuals, such as those with already anatomically narrow upper airways (Horner 2008b). Indeed, in such subjects, the absence of pharyngeal muscle tone can be sufficient to produce an upper airway that is closed, or nearly closed, at pressures close to atmospheric (Kuna & Remmers 2000; Smith & Schwartz 2002; Younes 2008). These observations highlight that some individuals require upper-airway muscle activation to permit adequate inspiratory airflow, and these subjects are therefore highly susceptible to obstructive sleep apnoea and hypopnoea when pharyngeal muscle tone is decreased by sleep or certain drugs (e.g. anaesthesia or sedatives; Kuna & Remmers 2000; Smith & Schwartz 2002; Younes 2008).
The genioglossus muscle of the tongue shows both tonic and respiratory-related activity (figure 1b), with the decreased activity of this muscle during sleep being strongly linked to the pathogenesis of obstructive sleep apnoea (Remmers et al. 1978). Tonic genioglossus muscle tone contributes to baseline airway size and stiffness, whereas the increased pharyngeal muscle activity during inspiration (i.e. the respiratory-related activity) further attempts to enlarge and stiffen the airspace to resist the sub-atmospheric inspiratory airway-collapsing pressures (Goh et al. 1986; Horner 1996). In contrast to the genioglossus muscle that exhibits both tonic and respiratory-related activity, the tensor palatini muscle of the soft palate displays mostly tonic activity (figure 1b). This tonic activation enhances stiffness in the segment of the upper airway at the level of the soft palate, a consistent site of airway closure in obstructive sleep apnoea (Horner 1996). Decreases in tonic tensor palatini muscle activity during sleep also contribute to increased upper-airway resistance and the predisposition to hypopneas and obstructive sleep apnea (Anch et al. 1981; Tangel et al. 1991). Importantly, for the tensor palatine muscle, this effect of sleep predominantly affects upper-airway resistance and lung ventilation by an effect on the tonic (i.e. non-respiratory) inputs to these trigeminal motoneurons, which receive little or no respiratory drive at rest.
(ii). Intercostal muscles
Like the different pharyngeal muscles, the intercostal muscles show varying degrees of respiratory-related and tonic activities depending on the relative respiratory and non-respiratory (e.g. postural support and rotational movement) functions of these muscles and their anatomical location in the chest wall (Duron & Marlot 1980; Phillipson & Bowes 1986). Suppression of intercostal muscle activity in REM sleep increases the compliance of the chest wall and contributes to decreased functional residual capacity (Phillipson & Bowes 1986). Of importance clinically, patients with obesity hypoventilation and neuromuscular weakness rely, to varying degrees, on the activation of non-diaphragmatic (i.e. intercostal and accessory) respiratory muscles to help maintain adequate ventilation in wakefulness, but this compensation can be reduced or absent in sleep, especially REM sleep, leading to severe hypoventilation (Phillipson & Bowes 1986; Goldstein 1992).
(iii). Diaphragm versus intercostal muscles
In contrast to those pharyngeal and intercostal muscles that combine both respiratory and non-respiratory functions, the diaphragm is almost entirely devoted to respiratory function, undergoes lesser suppression of activity in non-REM sleep and is largely spared the motor inhibition of REM sleep (figure 1b; Phillipson & Bowes 1986). Of interest, the diaphragm has few (if any) muscle spindles and little inhibition in REM sleep, whereas different intercostal muscles (especially the external inspiratory intercostals) have significant numbers of muscle spindles and profound suppression of activity in REM sleep, with the degree of suppression varying with muscle-spindle density (Duron & Marlot 1980; Phillipson & Bowes 1986). This relationship between the degree of suppression of thoracic respiratory-pump muscle activity in REM sleep and muscle-spindle density is relevant because skeletal muscle tone is reflexly facilitated by the activity of gamma motoneurons, the latter increasing the feedback from muscle spindles to activate spinal alpha motoneurons. Importantly, gamma motor activity is suppressed in REM sleep (Kubota & Tanaka 1966; Morales et al. 1987) because of descending inhibitory inputs from brainstem regions that become active in this state (Siegel 2000; Takakusaki et al. 2001). Accordingly, disfacilitation of alpha motoneuron activity in REM sleep, as a result of suppression of gamma motoneuron activity, can contribute, along with post-synaptic inhibition, to the overall suppression of spinal motor activity in REM sleep (Morales et al. 1987) and can explain how the degree of suppression observed in different thoracic respiratory muscles varies with muscle spindle density (Duron & Marlot 1980; Phillipson & Bowes 1986).
(iv). Summary
Respiratory muscles with dual respiratory and non-respiratory functions (e.g. the pharyngeal and intercostal muscles) show greater suppression of activity in sleep than the diaphragm, the latter a primary respiratory muscle almost entirely devoted to respiratory function. Moreover, this suppression of activity is most apparent in the tonic component of respiratory muscle activity (figure 1b), which has functional implications of a narrower and more collapsible upper airspace in the case of the pharyngeal muscles and decreased functional residual capacity and increased compliance of the chest wall in the case of the intercostal muscles. Importantly, as discussed below, a major source of the tonic drive to respiratory motoneurons originates from neurons intimately involved in states of brain arousal, i.e. neurons not classically involved in generating respiratory rhythm and pattern per se.
(b). Neural basis for tonic and respiratory-related activity in respiratory muscles
(i). Diaphragm and intercostal muscles
During inspiration, the central respiratory drive potential is transmitted to phrenic and intercostal motoneurons via monosynaptic connections from inspiratory pre-motor neurons of the dorsal and ventral respiratory groups (Duffin 2004; figure 2a). Bötzinger complex expiratory neurons have widespread inhibitory connections throughout the brainstem and spinal cord, and these neurons inhibit inspiratory pre-motoneurons and motoneurons during expiration (Duffin 2004; figure 2a). Caudal ventral respiratory-group neurons also increase the excitability of spinal expiratory motoneurons in expiration, although this excitation does not necessarily reach the threshold to manifest as expiratory muscle activity over and above the levels of tonic motor activity.
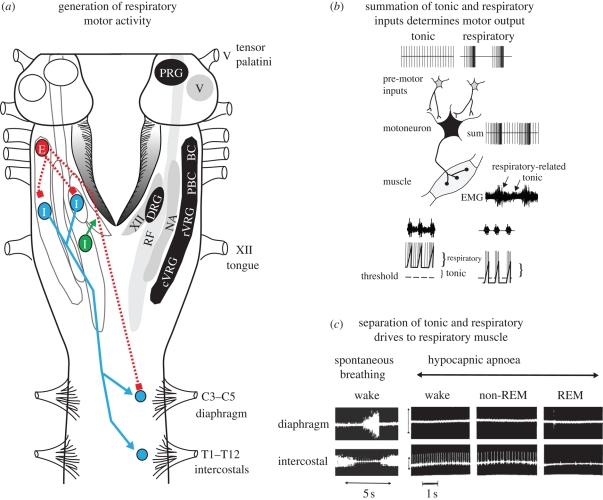
Figure 2.
(a) View of the brainstem (cerebellum removed) showing the main aggregates of respiratory neurons in the dorsal and ventral respiratory groups (DRG and VRG, respectively), the latter also illustrating relative congregations of neurons in the Bötzinger complex (BC), pre-BC (PBC), rostral VRG (rVRG) and caudal VRG (cVRG). The location of expiratory (E) and inspiratory (I) neurons are shown. The location of respiratory-related neurons in the lateral reticular formation (RF) projecting to the hypoglossal motor nucleus (XII) is also shown. The projections of inspiratory and expiratory neurons are depicted as solid and dashed lines, respectively, while excitatory and inhibitory synaptic connections are depicted by arrowhead and square symbols, respectively. The locations of the pontine respiratory group (PRG), nucleus ambiguus (NA) and the trigeminal motor nucleus (V) are also shown. See text for further details on the functional implications of this anatomical organization. (b) Schema to show how converging tonic (e.g. postural, non-respiratory) and respiratory inputs to a motoneuron summate to produce the tonic and respiratory components of electromyographic (EMG) activity. Reduced expression of the tonic and respiratory components of respiratory muscle activity can result from an independent change in tonic drive. In the example shown, respiratory drive is indicated as three depolarizing potentials, each associated with the generation of motoneuron action potentials when the membrane potential exceeds threshold (dashed line). Reduced tonic drive results in reduced tonic muscle activity and decreased expression of respiratory-related activity. (c) Example showing that hypocapnic apnoea, produced by mechanical hyperventilation in a conscious dog preparation, abolishes spontaneous breathing (as indicated by the lack of diaphragm muscle activity), whereas intercostal muscle becomes tonically active. Single intercostal motor-unit activity, representing the discharge of a single respiratory motoneuron, shows decreased activity from wakefulness to non-REM sleep and REM sleep, indicating progressive motoneuron hyperpolarization (see text for further details). The latter trace is from the personal data archive relating to Horner et al. (1994a).
(ii). Pharyngeal muscles
Electrophysiological data from rodents show that the source of inspiratory drive to hypoglossal motoneurons is different from the source of drive to phrenic motoneurons, being predominantly from the reticular formation lateral to the hypoglossal motor nucleus (lateral tegmental field) for the former and from bulbo-spinal dorsal and ventral respiratory-group neurons for the latter (figure 2a; Peever et al. 2001, 2002; Duffin 2004). Of relevance, some reticular formation neurons are tonically active in wakefulness and less active in non-REM sleep (Orem 1994). This feature of their activity probably explains the marked sleep-state dependence of tongue muscle activity compared with the diaphragm and is also probably the basis of the normal increase in upper-airway resistance in sleep and the predisposition to hypoventilation, flow-limitation and obstructive sleep apnea in some individuals.
A further difference in the functional control of pharyngeal and diaphragm muscles is shown by the observation that, unlike phrenic motoneurons, hypoglossal motoneurons are not inhibited in expiration (figure 2a; Woch & Kubin 1995; Peever et al. 2001, 2002; Duffin 2004). Accordingly, the activity of the genioglossus muscle in expiration is simply a manifestation of the prevailing tonic inputs (figure 2b). The significant practical implication of this circuitry is that the overall activation of hypoglossal motoneurons during breathing comprises an inspiratory drive adding to a continuous tonic drive that persists in expiration when the inspiratory activation is withdrawn (figure 2b). Moreover, this tonic drive to the pharyngeal muscles, which contributes to baseline airway size and stiffness, is most prominent in wakefulness but withdrawn in sleep (figure 1b), thus leading to an upper airspace that is more vulnerable to collapse.
(c). Identification of separate tonic and respiratory motor drives to respiratory muscle and the effects of sleep
(i). Separation of tonic and respiratory drives to respiratory muscle
The finding that the tonic motor tone apparent between periodic inspiratory activations for some respiratory muscles is simply the manifestation of a prevailing background tonic drive that becomes apparent in expiration was discussed above in the context of the control of pharyngeal muscle activity. However, this concept was first identified in physiological experiments investigating the control of intercostal muscles. Hypocapnia produced by constant mechanical hyperventilation in anaesthetized (Sears et al. 1982) or conscious (Horner et al. 1994a,b; Orem et al. 2000) animal preparations reveals a background tonic drive to the intercostal nerves and muscles independent of respiratory-related activity. Most relevant to this discussion, these studies showed for the first time that the tonic drive to respiratory muscle could be identified and studied independently of spontaneous respiratory rhythm in an intact conscious organism (Horner et al. 1994a,b). Figure 2b illustrates the concept that the electromyographic activity in a given respiratory muscle is critically dependent on the overall sum of the respiratory and non-respiratory (i.e. tonic) inputs to the motoneurons innervating that muscle, such that abolition of spontaneous respiratory rhythm leaves only the prevailing tonic drive (figure 2c).
(ii). Independent effect of sleep on tonic drive to respiratory motoneurons
The significant role of non-respiratory tonic drives in determining the activity of respiratory motoneurons has clear physiological relevance because when identified experimentally (e.g. in the absence of spontaneous respiratory rhythm during hypocapnia), the tonic drive to respiratory motoneurons is reduced from wakefulness to non-REM sleep (figure 2c; Horner et al. 1994a; Orem et al. 2000). This independent effect of sleep on tonic activity will contribute to sleep-related decreases in respiratory muscle activity and subsequent hypoventilation. Tonic drive to respiratory motoneurons can also be further reduced in REM sleep (figure 2c), although time-varying fluctuations in this tonic drive produces transient increases and decreases in respiratory motor activity (Horner et al. 1994a; Orem & Anderson 1996; Orem et al. 2000). This variation in tonic drive in REM sleep will contribute to variable changes in lung ventilation in REM sleep by a mechanism independent of effects on the respiratory-related inputs (Horner et al. 1994a; Orem & Anderson 1996; Orem et al. 2000).
3. Neurobiology of sleep–wake states
A brief overview of the neurobiology of sleep and wakefulness is presented below because some details are pertinent to respiratory motor control in sleep and identification of neuromodulators(s) that mediate the wakefulness stimulus to respiratory muscle.
(a). Wakefulness
Figure 1 also shows some of the main neuronal groups contributing to the ascending arousal system from the brainstem that promotes wakefulness. This ascending arousal system includes the cholinergic laterodorsal and pedunculopontine tegmental nuclei that promote cortical activation via excitatory thalamocortical projections (Jones 2000). The aminergic arousal system originates from brainstem neuronal groups principally containing serotonin (dorsal raphé nuclei), noradrenaline (locus coeruleus), histamine (tuberomammillary nucleus) and dopamine (ventral periaqueductal grey). Orexin neurons from the perifornical region of the hypothalamus and cholinergic neurons from the basal forebrain also contribute to this ascending arousal system (Jones 2000). Overall, multiple neuronal systems contribute to cortical arousal and wakefulness. Importantly, these neuronal systems are also positioned to influence respiratory neurons and motoneurons via their anatomical projections to the pons, medulla and spinal cord (figure 1; Horner 2008a).
(b). Non-rapid eye movement sleep
Sleep is actively generated by neurons in the ventrolateral pre-optic area, anterior hypothalamus and basal forebrain (figure 1; Jones 2000). These neurons become active in non-REM sleep, leading to a direct suppression of cortical arousal via ascending inhibitory cortical projections, and inhibition of the aforementioned brainstem arousal neurons via release of γ-amino butyric acid (GABA) and galanin (McGinty & Szymusiak 2000; Saper et al. 2005). This effect of GABA explains the sedative-hypnotic effects of barbiturates, benzodiazepines and imidazopyridine compounds that enhance GABA-mediated neuronal inhibition via interactions with binding sites on the GABAA receptor (Mendelson 2000). GABAA receptors are also strongly implicated in respiratory control and are present throughout the respiratory network, excessive stimulation of which can promote respiratory depression (Robinson & Zwillich 2000). In summary, sleep onset is triggered by increased sleep-state-dependent GABAergic neuronal activity, and this is accompanied by a massed and coordinated withdrawal of activity of brainstem arousal neurons comprising serotonergic, noradrenergic, histaminergic and cholinergic neurons. Given the widespread projections of these sleep-state-dependent neuronal groups, these changes in neuronal activity in sleep are also positioned to influence respiratory neurons and motoneurons (figure 1; Horner 2008a).
(c). Rapid eye movement sleep
Decreased serotonergic and noradrenergic activity preceding and during REM sleep withdraws inhibition of the laterodorsal and pedunculopontine tegmental nuclei (Jones 2000; Saper et al. 2005), leading to increased acetylcholine release into the pontine reticular formation to trigger REM sleep (Kubin et al. 1998; Lydic & Baghdoyan 2005). Exogenous application of cholinergic agonists into the pontine reticular formation in anaesthetized or decerebrate animals is used to mimic this process experimentally, i.e. the ‘carbachol model of REM sleep’ (Kubin et al. 1998; Lydic & Baghdoyan 2005). Pontine carbachol, however, does not produce the whole range of respiratory events that characterize natural REM sleep, such as respiratory rate increases and periods of transient motor excitation, and so is an incomplete model of the state (Orem 1994; Kubin et al. 1998). Moreover, other states such as wakefulness and non-REM sleep cannot be studied with such reduced animal models.
A significant component of the motor suppression of REM sleep is mediated by descending pathways involving activation of medullary reticular formation relay neurons (Siegel 2000) that are inhibitory to spinal motoneurons via release of glycine and GABA (Chase & Morales 2000). Despite the strong experimental support for the interaction of pontine monoaminergic and cholinergic neurons as being primarily responsible for the initiation and maintenance of REM sleep, recent evidence has implicated a glutamatergic–GABAergic mechanism (Luppi et al. 2006; Fuller et al. 2007). One of the key differences between the aminergic–cholinergic and the glutamatergic–GABAergic hypotheses of REM sleep generation is that the motor atonia is produced by different pathways, i.e. the latter framework does not require a relay in the medullary reticular formation (Fuller et al. 2007). Rather, in the glutamatergic–GABAergic mechanism of REM sleep induction, the REM sleep-active pontine neurons are thought to lead to suppression of spinal motoneuron activity via long glutamatergic projections to the ventral horn of the spinal cord, which then activate local glycinergic interneurons to inhibit motor activity (Fuller et al. 2007). Such a mechanism is probably involved in the strong inhibition of spinal intercostal motoneurons in REM sleep, but whether collaterals from these specific, long, descending glutamatergic projections also synapse onto glycinergic inhibitory interneurons in cranial (e.g. hypoglossal and trigeminal) motor pools is not established.
4. Model system to determine mechanisms underlying sleep–wake-dependent respiratory motor control
It was not until 2001 that the central neurotransmitter systems underlying the modulation of a respiratory motoneuron pool began to be investigated across states of wakefulness and natural sleep following the development of appropriate freely behaving animal models (Jelev et al. 2001). Studies using such a model were initially focused on the hypoglossal motor nucleus, as illustrated in figure 3. These studies of respiratory motor control in intact animals across natural states of behaviour provided the necessary extension of the prior fundamental studies of the cellular and network control of hypoglossal motoneurons performed in vitro (Berger 2000; Rekling et al. 2000), and the in vivo studies performed in decerebrate or anaesthetized animals in pharmacologically induced ‘sleep-like’ states (Kubin et al. 1998). In contrast to this focus on pharyngeal motoneurons, there is a lack of studies investigating the control of intercostal and phrenic motoneurons in intact naturally sleeping animal models, largely owing to technical difficulties in accessing the respective motor pools. Nevertheless, studies of spinal motoneurons have provided important information regarding the control of postural motoneurons across sleep–wake states (Chase & Morales 2000). Given that intercostal motoneurons perform both postural and respiratory functions, the mechanisms identified at spinal postural motoneurons probably have close similarities to the mechanisms controlling the non-respiratory component of intercostal motor activity.
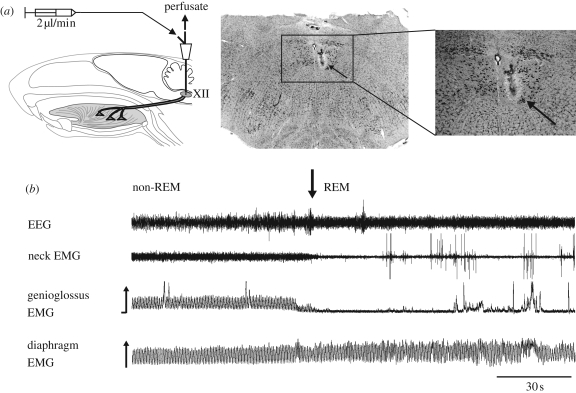
Figure 3.
Freely behaving animal preparation for in vivo microdialysis of the caudal medulla to modulate neurotransmission at the hypoglossal motor nucleus (XII) across states of wakefulness and natural sleep. Chronically implanted electrodes are used to record the electroencephalogram (EEG) and neck electromyogram (EMG) for the determination of sleep–wake states, and genioglossus and diaphragm electrodes for respiratory muscle recordings. (a) The tip of the microdialysis probe is inserted into the hypoglossal motor nucleus where diffusion of neurochemicals across a semi-permeable membrane modulates the hypoglossal motor pool, the source of motor outflow to the genioglossus muscle of the tongue. The histological sections show that the microdialysis probe is located within the cluster of motoneurons that innervate the genioglossus muscle, as shown by retrograde transport of fluorescent probe injected at the site of the recording electrodes. The location of the lesion site left by the microdialysis probe in the hypoglossal motor nucleus is shown by the arrow. (b) Sample recordings at the transition from non-REM to REM sleep showing suppression of genioglossus muscle activity at the onset of REM sleep.
5. Tonic excitatory drives modulating respiratory motor tone across sleep–wake states
Neurons of the aminergic arousal system provide an important source of tonic drive to the respiratory system (figure 1), and changes in tonic drives are positioned to modulate both the tonic components of respiratory muscle activity and the magnitude of respiratory-related activity (figure 2b). Serotonin- and noradrenaline-containing neurons have been of particular interest experimentally because these neurons send excitatory projections to respiratory motoneurons and show their highest activity in wakefulness, reduced activity in non-REM sleep and minimal activity in REM sleep, i.e. a pattern that may contribute to reduced respiratory muscle activity in sleep via withdrawal of tonic excitation (Kubin et al. 1998; Horner 2008a).
Studies in intact naturally sleeping rats show that an endogenous noradrenergic drive to the hypoglossal motor pool contributes to both the tonic and respiratory-related components of genioglossus muscle activity in wakefulness, and the residual expression of respiratory-related activity that persists in non-REM sleep as the tonic drive is withdrawn (Chan et al. 2006; figure 4a). Moreover, this noradrenergic contribution to genioglossus muscle tone was minimal in REM sleep, thus explaining, at least in part, the periods of genioglossus muscle hypotonia during REM sleep (Chan et al. 2006; figure 4a). This latter result also fits with similar findings from the carbachol model of REM sleep in anaesthetized rats (Fenik et al. 2005).
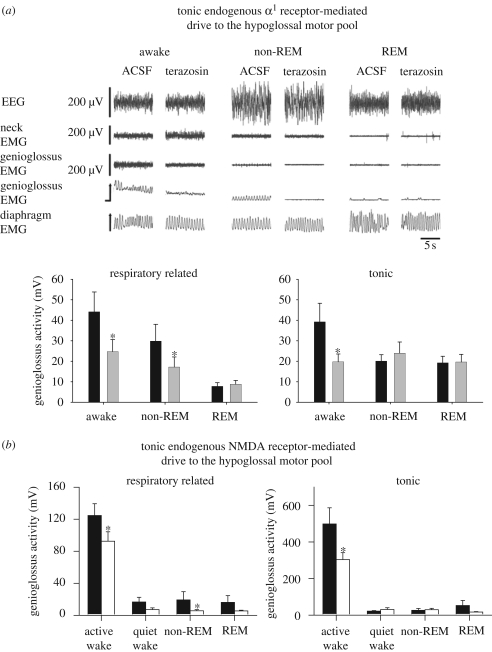
Figure 4.
(a) Example and group data showing the effects of α1 receptor antagonism with terazosin at the hypoglossal motor nucleus in intact freely behaving rats in wakefulness and natural sleep. Compared with artificial cerebrospinal fluid (ACSF) controls, terazosin reduced respiratory-related and tonic genioglossus muscle activities in wakefulness, and also reduced respiratory-related genioglossus activity in non-REM sleep. Genioglossus (GG) activity was the lowest in REM sleep and there was no subsequent effect of terazosin. See text for further details. Adapted from Chan et al. (2006). Black bar, ACSF; grey bar, terazosin (α1 antagonist, 1 mM). (b) Studies in intact naturally sleeping rats also show that an endogenous N-methyl-d-aspartate (NMDA) receptor-mediated drive to the hypoglossal motor nucleus contributes to both the tonic and respiratory-related components of genioglossus muscle activity in active wakefulness and non-REM sleep. Adapted from Steenland et al. (2008). Asterisk indicates a significant difference from the respective ACSF controls. Black bar, ACSF; white bar, D-APv (NMDA receptor antagonist, 1 mM).
The identification of an endogenous excitatory noradrenergic drive that contributes to the levels of tonic motor activity and expression of respiratory-related activity in wakefulness, but with this drive being withdrawn in sleep, is significant. The first point of significance is that this result was the first identification of a neural drive contributing to the sleep-state-dependent activity of a respiratory muscle, with this drive also acting as a neural substrate for the wakefulness stimulus. Also of relevance, the hypoglossal motor nucleus innervates the muscles of the tongue, relaxation of which is central to the pathogenesis of obstructive sleep apnoea in humans (Remmers et al. 1978; Horner 1996). Accordingly, a further point of significance is that since the first clinical description and mechanistic insight into obstructive sleep apnoea (Gastaut et al. 1969), this was the first identification of a neural drive contributing to the sleep-state-dependent activity of a respiratory muscle that is central to this disorder. Given the widespread anatomical projections of brainstem noradrenergic neurons, these neurons are also positioned to provide an endogenous input to other components of the respiratory network and so influence respiratory-pump muscle activity and ventilation across sleep–wake states (Fenik et al. 2002; Li & Nattie 2006).
More recent data also implicate a role for endogenous glutamatergic inputs in mediating another tonic excitatory drive to the hypoglossal and trigeminal motor pools that increases pharyngeal muscle activity in wakefulness, the withdrawal of which contributes to reduced activity in sleep (Burgess et al. 2008; Horner 2008a; Steenland et al. 2008). This latter result constitutes the identification of a second neural substrate for the wakefulness stimulus to respiratory muscle that modulates the level of tonic motor activity and the expression of respiratory-related activity (figure 4b). In contrast to these functionally active tonic excitatory noradrenergic and glutamatergic drives, endogenous serotonin at the hypoglossal motor nucleus plays a lesser role in modulating hypoglossal motor activity across natural sleep–wake states (Sood et al. 2005, 2006, 2007). Serotonergic inputs are more active in modulating hypoglossal motor activity in reduced preparations that are vagotomized (Sood et al. 2003, 2005; Fenik et al. 2005).
6. Tonic inhibitory drive modulating respiratory motor tone across sleep–wake states
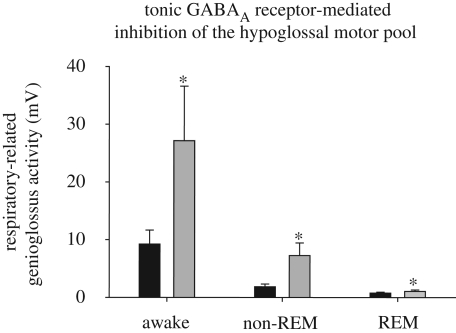
Glycine and GABA are the main inhibitory neurotransmitters in the central nervous system. Glycine and GABAA receptor stimulation at the hypoglossal motor pool in vivo suppresses respiratory-related genioglossus muscle activity (Morrison et al. 2002; Liu et al. 2003), whereas antagonism of these receptors increases respiratory-related activity in wakefulness and non-REM sleep, with a small but consistent effect also in REM sleep (Morrison et al. 2003a,b; figure 5). This augmentation of respiratory-related motor activity with the application of antagonists for these inhibitory neurotransmitters fits with the notion of a tonic inhibitory tone that constrains the rhythmic activation of respiratory neurons and motoneurons via gain modulation (Zuperku & McCrimmon 2002). This augmentation of respiratory-related activity at hypoglossal motoneurons was probably caused by blockade of end-inspiratory inhibition (Withington-Wray et al. 1988; Woch & Kubin 1995; Peever et al. 2001) mediated by glycine and GABA (Shao & Feldman 1997; Singer et al. 1998; O’Brien & Berger 1999; Berger 2000; Donato & Nistri 2000). The lack of effect on tonic motor activity in expiration (Morrison et al. 2003a,b) also fits with the concept that hypoglossal motoneurons are not actively inhibited in expiration (figure 2a; Woch & Kubin 1995; Peever et al. 2001, 2002; Duffin 2004). It has also been recently demonstrated that, like the hypoglossal motor nucleus, the trigeminal motor pool is also under a tonic inhibitory glycinergic and GABAergic inhibition (Brooks & Peever 2008).
Figure 5.
Studies in intact naturally sleeping rats show that an endogenous inhibitory GABAA receptor-mediated tone constrains the level of respiratory-related genioglossus muscle activity across sleep–wakes, as shown by the significant increase in activity with application of a GABAA receptor antagonist to the hypoglossal motor pool. Data re-drawn from Morrison et al. (2003a). Asterisk indicates a significant difference from the respective ACSF controls. Black bar, ACSF; grey bar, bicuculline (GABAA receptor antagonist, 100 µM).
The inhibitory effect of GABA at respiratory motoneurons is also clinically relevant given the widespread use of sedative hypnotic drugs in modern society. Benzodiazepine and imidazopyridine drugs are commonly prescribed as sedative hypnotics (e.g. lorazepam and zolpidem, respectively), and both these classes of sedatives promote sleep by enhancing GABA-mediated neuronal inhibition via interactions with binding sites on GABAA receptors (Mendelson 2000). However, the presence of lorazepam and zolpidem at the hypoglossal motor pool also leads to inhibition of genioglossus muscle activity (Horner 2008a). This inhibitory effect of sedative hypnotics at respiratory motor nuclei may underlie a component of the respiratory depression observed clinically with excessive GABAA receptor stimulation, and the predisposition of some individuals to obstructive sleep apnoea with sedatives (Robinson & Zwillich 2000). The role of GABA and glycine in the inhibition of cranial versus spinal motor activity in REM sleep is outside the scope of the current paper addressing tonic excitatory and inhibitory drives in respiratory motor control, and the interested reader is referred to a recent review (Horner 2008a).
7. Summary
Since 2001, there have been significant developments in determining the basis for modulation of respiratory motoneuron activity across states of wakefulness and natural sleep (Horner 2008a). Current information obtained from intact naturally sleeping animal models demonstrate that for the hypoglossal motor nucleus, a motor pool with dual respiratory and non-respiratory functions, motor outflow is the net result of a balance between tonic excitatory and inhibitory drives. The tonic excitatory drives, mediated principally by noradrenergic and glutamatergic inputs, modulate the levels of tonic motor activity and the expression of respiratory-related activity, while the tonic inhibitory GABA inputs constrain the levels of respiratory-related activity. Importantly, the tonic excitatory drives are prominent in wakefulness and withdrawn in sleep, thus contributing to the suppression of respiratory muscle activity. These tonic excitatory and inhibitory drives to hypoglossal motoneurons are mediated by neurons intimately involved in states of brain arousal, i.e. neurons not classically involved in generating respiratory rhythm and pattern per se. The inhibitory effect on respiratory motor activity of tonic GABAergic inputs also has relevance to respiratory depression, especially during sleep when brainstem GABAergic neuronal activity is increased (Jones 2000; McGinty & Szymusiak 2000; Saper et al. 2005), and in the presence of sedative hypnotic drugs that can enhance the effect of GABA on respiratory motoneurons (Liu et al. 2003; Horner 2008a; Park et al. 2008). In a wider context, suppression of respiratory motor activity in sleep, in association with withdrawal of tonic excitatory inputs and increased inhibition, can explain the predisposition of some individuals to decreased functional residual capacity, hypoventilation and/or the sleep apnoea syndromes in a state-specific fashion.
Acknowledgements
R.L.H. holds a Tier 1 Canada Research Chair in Sleep and Respiratory Neurobiology. The author's research is supported by grants from the Canadian Institutes of Health Research.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Brainstem neural networks vital for life’.
References
- Anch A. M., Remmers J. E., Sauerland E. K., Degroot W. J.1981Oropharyngeal patency during walking and sleep in the Pickwickian syndrome: electromyographic activity of the tensor veli palatini. Electromyogr. Clin. Neurophysiol. 21, 317–330 [PubMed] [Google Scholar]
- Berger A. J.2000Determinants of respiratory motoneuron output. Respir. Physiol. 122, 259–269 (doi:10.1016/S0034-5687(00)00164-X) [DOI] [PubMed] [Google Scholar]
- Brooks P. L., Peever J. H.2008Glycinergic and GABAA-mediated inhibition of somatic motoneurons does not mediate REM sleep motor atonia. J. Neurosci. 28, 3535–3545 (doi:10.1523/JNEUROSCI.5023-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess C., Lai D., Siegel J., Peever J.2008An endogenous glutamatergic drive onto somatic motoneurons contributes to the stereotypical pattern of muscle tone across the sleep–wake cycle. J. Neurosci. 28, 4649–4660 (doi:10.1523/JNEUROSCI.0334-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E.2007Sir Charles Sherrington's The integrative action of the nervous system: a centenary appreciation. Brain 130, 887–894 (doi:10.1093/brain/awm022) [DOI] [PubMed] [Google Scholar]
- Chan E., Steenland H. W., Liu H., Horner R. L.2006Endogenous excitatory drive modulating respiratory muscle activity across sleep–wake states. Am. J. Respir. Crit. Care Med. 174, 1264–1273 (doi:10.1164/rccm.200605-597OC) [DOI] [PubMed] [Google Scholar]
- Chase M. H., Morales F. R.2000Control of motoneurons during sleep. In Principles and practice of sleep medicine (eds Kryger M. H., Roth T., Dement W. C.), pp. 155–168 Philadelphia, PA: W. B. Saunders [Google Scholar]
- Colten H. R., Altevogt B. M.2006Sleep disorders and sleep deprivation: an unmet public health problem, p. 386 Washington, DC: Institute of Medicine. Committee on Sleep Medicine and Research, Board on Health Sciences Policy, The National Academies Press; [PubMed] [Google Scholar]
- Donato R., Nistri A.2000Relative contribution by GABA or glycine to Cl−-mediated synaptic transmission on rat hypoglossal motoneurons in vitro. J. Neurophysiol. 84, 2715–2724 [DOI] [PubMed] [Google Scholar]
- Duffin J.2004Functional organization of respiratory neurones: a brief review of current questions and speculations. Exp. Physiol. 89, 517–529 (doi:10.1113/expphysiol.2004.028027) [DOI] [PubMed] [Google Scholar]
- Duron B., Marlot D.1980Intercostal and diaphragmatic electrical activity during wakefulness and sleep in normal unrestrained adult cats. Sleep 3, 269–280 [DOI] [PubMed] [Google Scholar]
- Fenik V., Marchenko V., Janssen P., Davies R. O., Kubin L.2002A5 cells are silenced when REM sleep-like signs are elicited by pontine carbachol. J. Appl. Physiol. 93, 1448–1456 [DOI] [PubMed] [Google Scholar]
- Fenik V. B., Davies R. O., Kubin L.2005REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am. J. Respir. Crit. Care Med. 172, 1322–1330 (doi:10.1164/rccm.200412-1750OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink B. R.1961The stimulant effect of wakefulness on respiration: clinical aspects. Br. J. Anaesth. 33, 97–101 (doi:10.1093/bja/33.2.97) [DOI] [PubMed] [Google Scholar]
- Fuller P. M., Saper C. B., Lu J.2007The pontine REM switch: past and present. J. Physiol. 584, 735–741 (doi:10.1113/jphysiol.2007.140160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaut H., Duron B., Tassinari C. A., Lyagoubi S., Saier J.1969Mechanism of the respiratory pauses accompanying slumber in the Pickwickian syndrome. Activitas Nervosa Super. 11, 209–215 [PubMed] [Google Scholar]
- Goh A. S., Issa F. G., Sullivan C. E.1986Upper airway dilating forces during wakefulness and sleep in dogs. J. Appl. Physiol. 61, 2148–2155 [DOI] [PubMed] [Google Scholar]
- Goldstein R. S.1992Hypoventilation: neuromuscular and chest wall disorders. Clin. Chest Med. 13, 507–521 [PubMed] [Google Scholar]
- Henke K. G., Badr M. S., Skatrud J. B., Dempsey J. A.1992Load compensation and respiratory muscle function during sleep. J. Appl. Physiol. 72, 1221–1234 [DOI] [PubMed] [Google Scholar]
- Horner R. L.1996Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep 19, 827–853 [DOI] [PubMed] [Google Scholar]
- Horner R. L.2008aNeuromodulation of hypoglossal motoneurons during sleep. Respir. Physiol. Neurobiol. 164, 179–196 (doi:10.1016/j.resp.2008.06.012) [DOI] [PubMed] [Google Scholar]
- Horner R. L.2008bPathophysiology of obstructive sleep apnea. J. Cardiopulm. Rehab. Prev. 28, 289–298 [DOI] [PubMed] [Google Scholar]
- Horner R. L., Kozar L. F., Kimoff R. J., Phillipson E. A.1994aEffects of sleep on the tonic drive to respiratory muscle and the threshold for rhythm generation in the dog. J. Physiol. 474, 525–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner R. L., Kozar L. F., Phillipson E. A.1994bTonic respiratory drive in the absence of rhythm generation in the conscious dog. J. Appl. Physiol. 76, 671–680 [DOI] [PubMed] [Google Scholar]
- Hudgel D. W., Martin R. J., Johnson B., Hill P.1984Mechanics of the respiratory system and breathing pattern during sleep in normal humans. J. Appl. Physiol. 56, 133–137 [DOI] [PubMed] [Google Scholar]
- Jelev A., Sood S., Liu H., Nolan P., Horner R. L.2001Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep–wake states in rats. J. Physiol. 532, 467–481 (doi:10.1111/j.1469-7793.2001.0467f.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. E.2000Basic mechanisms of sleep–wake states. In Principles and practice of sleep medicine (eds Kryger M. H., Roth T., Dement W. C.), pp. 134–154 Philadelphia, PA: W. B. Saunders [Google Scholar]
- Kryger M. H.2000Restrictive lung disorders. In Principles and practice of sleep medicine (eds Kryger M. H., Roth T., Dement W. C.), pp. 976–983 Philadelphia, PA: W. B. Saunders [Google Scholar]
- Kubin L., Davies R. O., Pack L.1998Control of upper airway motoneurons during REM sleep. News Physiol. Sci. 13, 637–656 [DOI] [PubMed] [Google Scholar]
- Kubota K., Tanaka R.1966The fusimotor activity and natural sleep in the cat. Brain Res. 3, 198–201 (doi:10.1016/0006-8993(66)90078-3) [DOI] [PubMed] [Google Scholar]
- Kuna S., Remmers J. E.2000Anatomy and physiology of upper airway obstruction. In Principles and practice of sleep medicine (eds Kryger M. H., Roth T., Dement W. C.), pp. 840–858 Philadelphia, PA: W. B. Saunders [Google Scholar]
- Li A., Nattie E.2006Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J. Physiol. 570, 385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Sood S., Liu H., Nolan P., Morrison J. L., Horner R. L.2003Suppression of genioglossus muscle tone and activity during reflex hypercapnic stimulation by GABA-A mechanisms at the hypoglossal motor nucleus in-vivo. Neuroscience 116, 249–259 (doi:10.1016/S0306-4522(02)00564-X) [DOI] [PubMed] [Google Scholar]
- Luppi P. H., Gervasoni D., Verret L., Goutagny R., Peyron C., Salvert D., Leger L., Fort P.2006Paradoxical (REM) sleep genesis: the switch from an aminergic-cholinergic to a GABAergic-glutamatergic hypothesis. J. Physiol. Paris 100, 271–283 (doi:10.1016/j.jphysparis.2007.05.006) [DOI] [PubMed] [Google Scholar]
- Lydic R., Baghdoyan H. A.2005Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology 103, 1268–1295 (doi:10.1097/00000542-200512000-00024) [DOI] [PubMed] [Google Scholar]
- Lydic R., Orem J.1979Respiratory neurons of the pneumotaxic center during sleep and wakefulness. Neurosci. Lett. 15, 187–192 (doi:10.1016/0304-3940(79)96111-1) [DOI] [PubMed] [Google Scholar]
- McGinty D., Szymusiak R.2000The sleep–wake switch: a neuronal alarm clock. Nat. Med. 6, 510–511 (doi:10.1038/74988) [DOI] [PubMed] [Google Scholar]
- Mendelson W. B.2000Hypnotics: basic mechanisms and pharmacology. In Principles and practice of sleep medicine (eds Kryger M. H., Roth T., Dement W. C.), pp. 407–413 Philadelphia, PA: W. B. Saunders [Google Scholar]
- Morales F. R., Engelhardt J. K., Soja P. J., Pereda A. E., Chase M. H.1987Motoneuron properties during motor inhibition produced by microinjection of carbachol into the pontine reticular formation of the decerebrate cat. J. Neurophysiol. 57, 1118–1129 [DOI] [PubMed] [Google Scholar]
- Morrison J. L., Sood S., Liu X., Liu H., Park E., Nolan P., Horner R. L.2002Glycine at the hypoglossal motor nucleus: genioglossus activity, CO2 responses and the additive effects of GABA. J. Appl. Physiol. 93, 1786–1796 [DOI] [PubMed] [Google Scholar]
- Morrison J. L., Sood S., Liu H., Park E., Nolan P., Horner R. L.2003aGABA-A receptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. J. Physiol. 548, 569–583 (doi:10.1113/jphysiol.2002.033696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. L., Sood S., Liu H., Park E., Nolan P., Horner R. L.2003bRole of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J. Physiol. 552, 975–991 (doi:10.1113/jphysiol.2003.052357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J. A., Berger A. J.1999Cotransmission of GABA and glycine to brain stem motoneurons. J.Neurophysiol. 82, 1638–1641 [DOI] [PubMed] [Google Scholar]
- Orem J.1990The nature of the wakefulness stimulus for breathing. Prog. Clin. Biol. Res. 345, 23–30; discussion 31 [PubMed] [Google Scholar]
- Orem J.1994Respiratory neurons and sleep. In Principles and practice of sleep medicine (eds Kryger M. H., Roth T., Dement W. C.), pp. 177–193 Philadelphia, PA: W. B. Saunders [Google Scholar]
- Orem J., Anderson C. A.1996Diaphragmatic activity during REM sleep in the adult cat. J. Appl. Physiol. 81, 751–760 [DOI] [PubMed] [Google Scholar]
- Orem J., Osorio I., Brooks E., Dick T.1985Activity of respiratory neurons during NREM sleep. J. Neurophysiol. 54, 1144–1156 [DOI] [PubMed] [Google Scholar]
- Orem J., Lovering A. T., Dunin-Barkowski W., Vidruk E. H.2000Endogenous excitatory drive to the respiratory system in rapid eye movement sleep in cats. J. Physiol. 527, 365–376 (doi:10.1111/j.1469-7793.2000.00365.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Younes M., Horner R. L.2008Systemic vs. central administration of common hypnotics reveals opposing effects on genioglossus muscle activity in rats. Sleep 31, 355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peever J. H., Mateika J. H., Duffin J.2001Respiratory control of hypoglossal motoneurones in the rat. Pflugers Arch. 442, 78–86 (doi:10.1007/s004240000502) [DOI] [PubMed] [Google Scholar]
- Peever J. H., Shen L., Duffin J.2002Respiratory pre-motor control of hypoglossal motoneurons in the rat. Neuroscience 110, 711–722 (doi:10.1016/S0306-4522(01)00594-2) [DOI] [PubMed] [Google Scholar]
- Phillipson E. A., Bowes G.1986Control of breathing during sleep. In Handbook of physiology, section 3, the respiratory system, Vol. II, control of breathing, part 2 (eds Cherniack N. S., Widdicombe J. G.), pp. 649–689 Bethesda, MD: American Physiological Society [Google Scholar]
- Rekling J. C., Funk G. D., Bayliss D. A., Dong X. W., Feldman J. L.2000Synaptic control of motoneuronal excitability. Physiol. Rev. 80, 767–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers J. E., deGroot W. J., Sauerland E. K., Anch A. M.1978Pathogenesis of upper airway occlusion during sleep. J. Appl. Physiol. 44, 931–938 [DOI] [PubMed] [Google Scholar]
- Robinson R. W., Zwillich C. W.2000Medications, sleep and breathing. In Principles and practice of sleep medicine (eds Kryger M. H., Roth T., Dement W. C.), pp. 797–812 Philadelphia, PA: W. B. Saunders [Google Scholar]
- Saper C. B., Scammell T. E., Lu J.2005Hypothalamic regulation of sleep and circadian rhythms. Nature 437, 1257–1263 (doi:10.1038/nature04284) [DOI] [PubMed] [Google Scholar]
- Sears T. A., Berger A. J., Phillipson E. A.1982Reciprocal tonic activation of inspiratory and expiratory motoneurones by chemical drives. Nature 299, 728–730 (doi:10.1038/299728a0) [DOI] [PubMed] [Google Scholar]
- Shao X. M., Feldman J. L.1997Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Botzinger complex: differential roles of glycinergic and GABAergic neural transmission. J. Neurophysiol. 77, 1853–1860 [DOI] [PubMed] [Google Scholar]
- Sherrington C. S.1906The integrative action of the nervous system. New Haven, CT: Yale University Press [Google Scholar]
- Siegel J. M.2000Brainstem mechanisms generating REM sleep. In Principles and practice of sleep medicine (eds Kryger M. H., Roth T., Dement W. C.), pp. 112–133 Philadelphia, PA: W. B. Saunders [Google Scholar]
- Singer J. H., Talley E. M., Bayliss D. A., Berger A. J.1998Development of glycinergic synaptic transmission to rat brain stem motoneurons. J. Neurophysiol. 80, 2608–2620 [DOI] [PubMed] [Google Scholar]
- Smith P. L., Schwartz A. R.2002Biomechanics of the upper airway during sleep. In Sleep apnea: pathogenesis, diagnosis and treatment (ed. Pack A. I.), pp. 31–56 New York, NY: Dekker [Google Scholar]
- Smith J. C., Abdala A. P., Koizumi H., Rybak I. A., Paton J. F.2007Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J. Neurophysiol. 98, 3370–3387 (doi:10.1152/jn.00985.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood S., Liu X., Liu H., Nolan P., Horner R. L.20035-HT at hypoglossal motor nucleus and respiratory control of genioglossus muscle in anesthetized rats. Respir. Physiol. Neurobiol. 138, 205–221 (doi:10.1016/j.resp.2003.07.001) [DOI] [PubMed] [Google Scholar]
- Sood S., Morrison J. L., Liu H., Horner R. L.2005Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am. J. Respir. Crit. Care Med. 172, 1338–1347 (doi:10.1164/rccm.200502-258OC) [DOI] [PubMed] [Google Scholar]
- Sood S., Raddatz E., Liu X., Liu H., Horner R. L.2006Inhibition of serotonergic medullary raphe obscurus neurons suppresses genioglossus and diaphragm activities in anesthetized but not conscious rats. J. Appl. Physiol. 100, 1807–1821 (doi:10.1152/japplphysiol.01508.2005) [DOI] [PubMed] [Google Scholar]
- Sood S., Liu X., Liu H., Horner R. L.2007Genioglossus muscle activity and serotonergic modulation of hypoglossal motor output in obese Zucker rats. J. Appl. Physiol. 102, 2240–2250 (doi:10.1152/japplphysiol.01229.2006) [DOI] [PubMed] [Google Scholar]
- Steenland H. W., Liu H., Horner R. L.2008Endogenous glutamatergic control of rhythmically active mammalian respiratory motoneurons in vivo. J. Neurosci. 28, 6826–6835 (doi:10.1523/JNEUROSCI.1019-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John W. M., Paton J. F.2004Role of pontile mechanisms in the neurogenesis of eupnea. Respir. Physiol. Neurobiol. 143, 321–332 (doi:10.1016/j.resp.2004.05.010) [DOI] [PubMed] [Google Scholar]
- Takakusaki K., Kohyama J., Matsuyama K., Mori S.2001Medullary reticulospinal tract mediating the generalized motor inhibition in cats: parallel inhibitory mechanisms acting on motoneurons and on interneuronal transmission in reflex pathways. Neuroscience 103, 511–527 (doi:10.1016/S0306-4522(00)00586-8) [DOI] [PubMed] [Google Scholar]
- Tangel D. J., Mezzanotte W. S., White D. P.1991Influence of sleep on tensor palatini EMG and upper airway resistance in normal men. J. Appl. Physiol. 70, 2574–2581 [DOI] [PubMed] [Google Scholar]
- Withington-Wray D. J., Mifflin S. W., Spyer K. M.1988Intracellular analysis of respiratory-modulated hypoglossal motoneurons in the cat. Neuroscience. 25, 1041–1051 (doi:10.1016/0306-4522(88)90057-7) [DOI] [PubMed] [Google Scholar]
- Woch G., Kubin L.1995Non-reciprocal control of rhythmic activity in respiratory-modulated XII motoneurons. Neuroreport 6, 2085–2088 (doi:10.1097/00001756-199510010-00031) [DOI] [PubMed] [Google Scholar]
- Younes M.2008Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J. Appl. Physiol. 105, 1389–1405 (doi:10.1152/japplphysiol.90408.2008) [DOI] [PubMed] [Google Scholar]
- Young T., Palta M., Dempsey J., Skatrud J., Weber S., Badr S.1993The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 328, 1230–1235 (doi:10.1056/NEJM199304293281704) [DOI] [PubMed] [Google Scholar]
- Zuperku E. J., McCrimmon D. R.2002Gain modulation of respiratory neurons. Respir. Physiol. Neurobiol. 131, 121–133 (doi:10.1016/S1569-9048(02)00042-3) [DOI] [PubMed] [Google Scholar]