Abstract
The brainstem nucleus hypoglossus contains motoneurons that provide the exclusive motor nerve supply to the tongue. In addition to voluntary tongue movements, tongue muscles rhythmically contract during a wide range of physiological activities, such as respiration, swallowing, chewing and sucking. Hypoglossal motoneurons are destroyed early in amyotrophic lateral sclerosis (ALS), a fatal neurodegenerative disease often associated with a deficit in the transport system of the neurotransmitter glutamate.
The present study shows how periodic electrical discharges of motoneurons are mainly produced by a neuronal network that drives them into bursting mode via glutamatergic excitatory synapses. Burst activity is, however, modulated by the intrinsic properties of motoneurons that collectively synchronize their discharges via gap junctions to create ‘group bursters’. When glial uptake of glutamate is blocked, a distinct form of pathological bursting spontaneously emerges and leads to motoneuron death. Conversely, H2O2-induced oxidative stress strongly increases motoneuron excitability without eliciting bursting. Riluzole (the only drug currently licensed for the treatment of ALS) suppresses bursting of hypoglossal motoneurons caused by blockage of glutamate uptake and limits motoneuron death. These findings highlight how different patterns of electrical oscillations of brainstem motoneurons underpin not only certain physiological activities, but also motoneuron death induced by glutamate transporter impairment.
Keywords: brainstem, amyotrophic lateral sclerosis, oscillation, glutamate uptake, oxidative stress, hydrogen peroxide
1. Rhythmic electrical discharges are necessary for stereotypic motor behaviour
Rhythmic electrical oscillations mediate information storage and transfer within neuronal networks (Sejnowski & Paulsen 2006). In addition, repetitive motor commands are required for behaviours based on rhythmic contractions of skeletal muscles, such as respiration (Suzue 1984; Onimaru et al. 1987; Ballanyi et al. 1999), mastication and swallowing (Jean 2001). These functions demand the coordinated discharges of a network of neurons that initiate and maintain the rhythmic output of motoneurons: hypoglossal motoneurons are driven by pre-motoneurons embedded in a functional ensemble termed ‘central pattern generator’ (Grillner 2006; Kiehn 2006; Rybak et al. 2007; Briggman & Kristan 2008) whose localization is believed to be distributed among several nuclei of the caudal brainstem. Important, yet unresolved, questions are the existence of multiple central pattern generators subserving analogous functions and the extent of their network overlap in anatomical or functional terms.
One major motor output of the brainstem is from the motoneurons of the nucleus hypoglossus that command tongue muscles to contract rhythmically during inspiratory movements, chewing, sucking, swallowing, and vocalization with different, function-related frequencies and properties (Lowe 1980). In neurodegenerative diseases such as the bulbar form of amyotrophic lateral sclerosis (ALS), the nucleus hypoglossus is among the most vulnerable (Krieger et al. 1994; Lips & Keller 1999; Laslo et al. 2001). In ALS, pathological clonic muscle contractions often precede muscle paralysis. Hence, studying the functional characteristics of hypoglossal motoneurons can provide useful information on the integrative properties of brainstem motor networks in health and disease.
Within this framework, the activity of hypoglossal motoneurons can be considered as the functional readout of information programmed and encoded in pre-motoneuronal networks. This notion is, perhaps, too simple as it does not include the possibility that motoneurons are endowed with intrinsic rhythmogenesis and/or that small local networks lacking distinct nuclear arrangement can potently drive rhythmic discharges of motoneurons. The present study intends to show the complexity of bursting modes generated by hypoglossal motoneurons through a spectrum of mechanisms that range from intrinsic rhythmicity to network-evoked activity via a blend of functional processes related to physiological or pathological conditions.
We have investigated these issues by using, as an experimental tool, a relatively thin (approx. 250 µm) coronal slice of the neonatal rat brainstem in which the nucleus hypoglossus can be readily identified and single living motoneurons can be viewed with infrared microscopy. In our studies, we have employed whole-cell patch clamp recording from hypoglossal motoneurons together with their histochemical analysis (Sharifullina et al. 2004, 2005; Lamanauskas & Nistri 2006, 2008). Bursts are defined as large, sustained changes in membrane potential (or holding current) caused by periodic up and down swings of the baseline, while oscillations are more rapid, low-amplitude fluctuations.
2. Heterogeneity of motoneuron rhythmic bursting evoked by neuromodulators
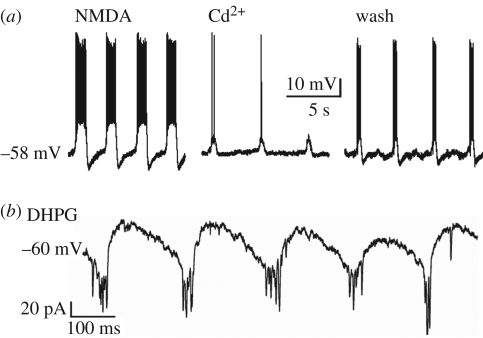
Can motoneurons display intrinsic bursting in the absence of a functional network? Membrane depolarization by intracellular current injection or application of high K+ solution consistently fails to trigger oscillatory activity (Sharifullina et al. 2005, 2008). Thus, unlike pre-motoneuronal networks that promptly generate rhythmic oscillations when depolarized with high K+, hypoglossal motoneurons do not share this property whether residing in an intact slice or isolated from their connections. Nevertheless, such motoneurons can produce persistent bursts (0.43 ± 0.07 Hz) when excited by the glutamate agonist N-methyl-d-aspartate (NMDA) (figure 1a). This activity is reversibly blocked by inorganic Ca2+ channel blockers like Cd2+ (Sharifullina et al. 2008; figure 1a). Conversely, the Na+ channel blocker tetrodotoxin can suppress bursting in a few motoneurons only.
Figure 1.
Bursting of hypoglossal motoneurons induced by NMDA or DHPG. (a) Under current clamp condition, steady bursting is evoked by 25 µM NMDA. Each depolarization wave comprises a cluster of action potentials. In the presence of Cd2+ (400 µM), bursting is suppressed (middle panel) but reinstated during washout (right panel). (b) Theta frequency bursting evoked by DHPG (25 µM) applied to a motoneuron voltage clamped at −60 mV. Bursts are observed as inward current (large downward deflections) with superimposed fast oscillations; for further details, see Sharifullina et al. (2005, 2008).
In the majority of motoneurons recorded from a brainstem slice preparation with intact synaptic transmission, the NMDA-induced bursts tend to wane (despite sustained membrane depolarization), and are readily reinstated when the membrane potential is returned to baseline level by current injection. A significant minority of these neurons do, however, behave like persistent bursters at depolarized membrane potential. Curiously, NMDA-evoked bursts are resistant to elevated concentrations (5–12 mM) of extracellular Mg2+, probably because of the widespread expression of the NR2D receptor subunit that confers poor sensitivity of NMDA receptors to Mg2+ (Sharifullina et al. 2008). These properties are not generalized to all brainstem motoneurons. For instance, in trigeminal motoneurons, NMDA-evoked bursting develops after the first post-natal week, shows a different Mg2+ sensitivity and is powerfully facilitated by serotonin (Hsiao et al. 2002). Thus, it appears that the developmental maturation of motoneuron bursting patterns starts very early for hypoglossal motoneurons. It is tempting to speculate that such a bursting represents one mechanism for rhythmic tongue contractions typical of milk sucking (Kutsuwada et al. 1996) appearing immediately after birth.
To sum up, hypoglossal motoneurons can produce rhythmic bursting elicited by NMDA through a robust mechanism that is mainly intrinsic to these cells because it is unaffected by blockers of excitatory or inhibitory synaptic transmission (Sharifullina et al. 2008), and that is preserved despite fluctuations in extracellular Mg2+.
Activation of NMDA receptors is not the only glutamatergic stimulus to induce bursting. In fact, pharmacological stimulation by 3,5-dihydroxyphenylglycine (DHPG) of subtype 1 receptors of group I metabotropic glutamate receptors (mGluR; widely expressed in the brainstem; Del Negro & Chandler 1998) evokes a characteristic pattern of bursting (6.5 ± 0.6 Hz) that falls within the range of the so-called theta oscillations (Sharifullina et al. 2005; figure 1b). These bursts, which are preceded by a significant rise in cell input resistance and are usually accompanied by fast oscillations (18 ± 8 Hz; see rapid transients in figure 1b), are insensitive to NMDA receptor blockers and are due to a large enhancement of AMPA receptor-mediated responses at the network and motoneuron levels (Sharifullina et al. 2004). Bursting is apparently paced by the cyclic activation of KATP conductances, thus linking electrical discharges to the cell energy metabolism. Current clamp recording indicates that, during bursting, motoneuron spikes appear only at the peak of each burst, in contrast to the irregular firing emerging when the same cell is depolarized to a comparable level of membrane potential (Sharifullina et al. 2005). Hence, bursts strongly constrain the firing pattern of motoneurons and amplify AMPA receptor-mediated synaptic inputs. While the theta frequency of bursts induced by DHPG is too fast for the normal inspiratory rhythm of in vivo normoxic animals, it is likely that mGluR activation (even if not involved in eupnoea) contributes to the expression of the respiratory rhythm (Li & Nattie 1995).
Bursting emerging in the presence of DHPG is, therefore, an example of the mixed origin of such electrical events, namely coincidence of increased excitatory synaptic activity and enhanced sensitivity of motoneurons to these inputs (Nistri et al. 2006).
Our studies have indicated that theta frequency bursts can also be generated by an almost exclusively pre-synaptic mechanism of facilitation of glutamate release. Our former experiments have indicated that ambient acetylcholine facilitates glutamate release (Quitadamo et al. 2005). When nicotinic receptors are stimulated by nicotine (Lamanauskas & Nistri 2006), bursting (5.6 ± 0.3 Hz) appears with some delay after the initial inward current and is associated with a large increase in the frequency of glutamatergic synaptic events. Thus, oscillations emerge once network excitability is strongly heightened and abruptly disappear when the network excitability falls below threshold. Under these experimental conditions, bursting requires continuous activation of nicotinic receptors, and lasts for approximately 10 min, suggesting that nicotinic receptor desensitization develops slowly. As in the case of DHPG-elicited bursting, nicotine-evoked bursting transforms the irregular firing of motoneurons into a time-locked pattern in coincidence with the peak of each oscillatory event (Lamanauskas & Nistri 2006). Interestingly, nicotine-evoked bursting is suppressed by AMPA receptor blockers (though insensitive to NMDA receptor antagonism) or by group I mGluR antagonists. These observations suggest that nicotine-induced bursting requires a robust release of endogenous glutamate acting concurrently via AMPA as well as metabotropic receptors.
3. Factors responsible for motoneuron bursting
Throughout these investigations, it was apparent that bursting could be observed in the majority of hypoglossal motoneurons, though a significant number of them never generated bursts. For instance, 75 per cent of motoneurons typically exhibit NMDA-induced bursting (Sharifullina et al. 2008), while for DHPG- (Sharifullina et al. 2005) and nicotine-induced (Lamanauskas & Nistri 2006) bursting the percentage is 40–50%. Although one might assume that the stronger excitation elicited by NMDA versus DHPG or nicotine is an important factor to increase bursting occurrence, other processes are probably implicated as well.
One contributor is synaptic inhibition mediated by gamma-aminobutyric acid (GABA) and glycine (Marchetti et al. 2002). In all tests with different protocols to evoke bursts, pharmacological block of GABAA and glycine receptors consistently raises the likelihood of observing bursts without changing burst properties like frequency or duration. It is noteworthy that, unlike spinal networks (Bracci et al. 1996), pharmacological block of synaptic inhibition in the brainstem slice does not elicit bursting of hypoglossal motoneurons.
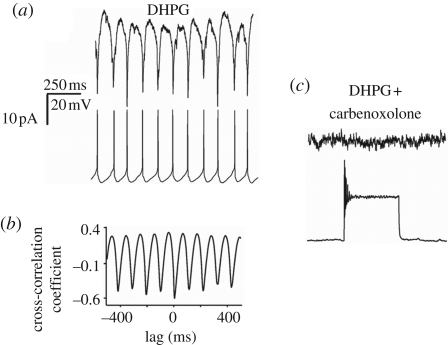
While diverse bursting patterns are induced with different protocols and show distinctive sensitivity to pharmacological agents, there is one common property consistently shared in every condition tested, namely the dependence on electrical coupling of motoneurons. Previous studies have identified electrical coupling between hypoglossal motoneurons under in vivo and in vitro conditions using dye coupling or electrophysiological recording (Mazza et al. 1992; Rekling et al. 2000). Figure 2a shows an example of strong, bidirectional coupling between adjacent motoneurons in the brainstem slice preparation (Sharifullina et al. 2005) in which, during bursting evoked by DHPG, two hypoglossal motoneurons are simultaneously recorded under voltage (upper record) and current (lower record) conditions. Signal coupling between burst currents and spikes is 1 : 1, with strong cross correlation as indicated by the plot in figure 2b. It seems likely that electrical coupling depends on gap junctions sensitive to carbenoxolone (100 µM) as demonstrated in figure 2c, in which this agent suppresses electrical coupling between the two motoneurons as well as their synchronous DHPG-induced bursting. The expression of various types of connexins in the neonatal and adult rat brainstem (Solomon et al. 2001; Solomon 2003) indicates that the neurons probably synchronize their bursting activity through these membrane proteins specialized in interneuronal communication. The electrical equivalent of this communication is the presence of ‘spikelets’ and ‘burstlets’, namely the action potentials and the depolarization waves spread via gap junction that recruit, excite and coordinate motoneuron discharge patterns (Sharifullina et al. 2005). Bursts are, therefore, the summated expression of synchronized depolarization and firing occurring in a group of motoneurons. This interpretation accords with the poor voltage sensitivity of bursts (Sharifullina et al. 2005, 2008) because their origin comes from functionally interconnected cells rather than from ligand and/or voltage-gated channels of single motoneurons.
Figure 2.
Bursting depends on electrical coupling between motoneurons. (a) Example of paired, patch clamp recording from two adjacent motoneurons in voltage (top row) and current (bottom row) conditions during application of DHPG (25 µM). Note the 1 : 1 coupling between burst currents and burst depolarizations. (b) Cross correlogram of data shown in A confirm tight coupling between events. (c) Carbenoxolone (100 µM) blocks bursting in both cells and prevents depolarization. A depolarizing current (0.6 nA) pulse injected into the current clamped cell evokes transient firing without any response in the voltage-clamped neuron, demonstrating loss of electrical coupling. All data are from the same pair of hypoglossal motoneurons; for further details, see Sharifullina et al. (2005).
To express and support bursting, the interplay among complex conductance mechanisms is necessary. For example, in the case of NMDA, it is proposed that the activity of persistent inward currents, fast Na+ current activation/inactivation and rhythmic activation and deactivation of K+ conductances pace the oscillation frequency (Sharifullina et al. 2008). Nevertheless, sustained NMDA-dependent bursting detected in the majority of motoneurons requires the drive by a few (electrically coupled) motoneurons that continuously burst (or fire) and, thus, provide strong excitation to the functionally coupled network of motoneurons. This notion is supported by the presence of hypoglossal nerve bursting even when concomitant recording from a single motoneuron shows burst fading (Sharifullina et al. 2008).
In networks of similar integrate-and-fire neurons, different network patterns depend on the strength of electrical coupling (Chow & Kopell 2000). Furthermore, this process may amplify the parameter range over which initially uncoupled neurons can start bursting (Soto-Trevinõ et al. 2005), especially because a neuromodulator (for example, DHPG or nicotine) can modify intrinsic properties, gap junctions and synaptic inputs, thereby changing the effective compartmental structure of the network (Soto-Trevinõ et al. 2005).
4. Group bursters
Hypoglossal motoneurons are not endowed with spontaneous rhythmicity. To express synchronous, repeated discharges, several conditions must be met, among which, as shown earlier, the presence of electrical coupling or synchronous excitatory synaptic drive is of paramount importance. Hence, bursting is not due to the activity of pacemakers, but is an emergent property (Del Negro et al. 2002) of the pre-motoneuron/motoneuron network where the fundamental unit of oscillation is an ensemble of neurons (with similar intrinsic properties) that become rhythmically active through chemical and electrotonic excitatory synaptic interactions (Rekling & Feldman 1998). These concepts have been applied to brainstem respiratory neurons, leading to the proposal of ‘group pacemakers’ (Feldman & Del Negro 2006). This model is also useful for interpreting data concerning hypoglossal motoneurons that may be viewed as ‘group bursters’, bound together in a synchronous oscillatory activity by neuromodulators that raise motoneuron and pre-motoneuron excitability via facilitation of synaptic inputs and neuronal conductances.
Experimental and theoretical studies of cortical networks (Eytan & Marom 2006) have indicated that a threshold-governed, synchronized population event follows the logistics of neuronal recruitment in an effectively scale-free connected network in which synchronization time does not increase markedly with network size. It is likely that similar principles apply to the group bursters of hypoglossal motoneurons.
5. Pathophysiological bursting
Hypoglossal motoneurons are particularly sensitive to neurodegenerative diseases like ALS, whose early symptoms include slurred speech, dysphagia and tongue biting. A large number of patients show abnormally high concentrations of glutamate in their cerebrospinal fluid and impaired glutamate uptake by glia. For these reasons it has been argued that ALS may be caused by excitotoxicity of motoneurons due to their continuous over-stimulation by glutamate (Cleveland & Rothstein 2001; Rowland & Shneider 2001; Bruijn et al. 2004; Rao & Weiss 2004). Excitotoxicity is thought to involve production of free oxygen radicals and oxidative stress with depletion of cell energy metabolism (Rao & Weiss 2004). Rare cases of genetically transmitted ALS show a mutation of the enzyme superoxide dismutase 1 (SOD1) that normally scavenges these toxic compounds. Neonatal hypoglossal motoneurons from SOD1 mutant mice show enhanced excitability and synaptic inputs long before the onset of neurological symptoms (van Zundert et al. 2008).
Consistent with the view that glutamate can induce excitotoxicity of hypoglossal motoneurons is the demonstration that pharmacological inhibition by dl-threo-β-benzyloxyaspartate (TBOA; 50 µM) of the glutamate transporters elicits a deadly motoneuron bursting pattern that leads to patchy loss of motoneurons as demonstrated by Sharifullina & Nistri (2006). The TBOA-evoked bursting is clearly an emergent property of motoneurons that require efficient network activity to exhibit this pattern accompanied by strong enhancement in excitatory synaptic transmission, neuronal recruitment, massive depolarization and burst firing. Interestingly, TBOA bursts occur even in a minislice of the brainstem, which essentially excludes the reticular formation: this result is consistent with the view of scale-free group bursters synchronized independently from network size.
Unlike bursts observed following the application of neuromodulators, TBOA burst currents are very slow (occurring on average once every 2 min), very long (35 ± 2 s) and much larger than those produced by NMDA or DHPG. However, only approximately one-third of motoneurons show TBOA bursting, though block of synaptic inhibition significantly increases bursting occurrence. TBOA bursts possess certain characteristics similar to those of the less dramatic bursts induced by neuromodulators, namely they are synchronous between electrically coupled motoneurons and are fully suppressed by carbenoxolone and glutamate receptor antagonists.
The spatio-temporal evolution of TBOA bursts can be monitored with patch clamping and intracellular Ca2+ imaging (Sharifullina & Nistri 2006). Bursting motoneurons are scattered throughout the nucleus hypoglossus and show gradual elevation in their baseline Ca2+ with repeated transients. In a number of them, bursting is replaced by a massive, irreversible inward current and a persistently high level of intracellular Ca2+, suggesting severe cell damage. Histochemical analysis has confirmed significant loss of hypoglossal motoneurons, a phenomenon prevented by carbenoxolone or glutamate receptor blockers.
Our observations suggest that inhibition of glutamate uptake can have a deadly effect on motoneurons, that this is not a generalized phenomenon within the nucleus hypoglossus and that those motoneurons that display large bursts are probably those that later die.
As riluzole is the only drug licensed for delaying ALS disease progression in man, it may be useful to find out if riluzole can block TBOA bursts and motoneuron loss in the brainstem slice model. Furthermore, it is important, for ALS cellular pathophysiology, to understand whether the deleterious effect of TBOA is mediated by free oxygen radicals in analogy with the scenario proposed for SOD1-mutated ALS (Rao & Weiss 2004).
6. A model to predict if drugs can block the excitotoxic damage of hypoglossal motoneurons
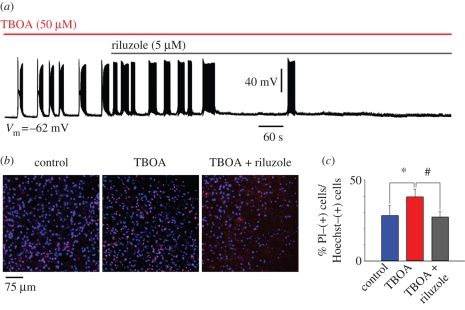
Riluzole has complex pharmacological effects on brainstem motoneurons. At low concentrations (5–10 µM), the main targets of its action are pre-synaptic inhibition of the release of glutamate and suppression of voltage-gated persistent inward currents mediated by Na+ and Ca2+ (Lamanauskas & Nistri 2008). It seems likely that these combined effects contribute to limit motoneuron excitability and, perhaps, to retard the relentless progression of ALS. Although riluzole can block nicotine-evoked bursting (Lamanauskas & Nistri 2008), it seems useful to explore whether riluzole can suppress the much stronger TBOA-evoked bursts. Figure 3a shows that, under current clamp conditions, riluzole (5 µM), applied after the onset of bursting, suppresses this phenomenon. Bursts are blocked within 319 ± 138 s without significant change in their period or duration, or in baseline membrane potential. Similarly, motoneuron damage (monitored on the basis of propidium iodide staining) following TBOA application is also limited by applying riluzole after TBOA (figure 3b,c).
Figure 3.
Riluzole inhibits TBOA-evoked bursting. (a) Sample trace of current clamp recording from hypoglossal motoneuron exposed to TBOA (50 µM) that, on average, produces 3.0 ± 0.7 mV depolarization (n = 8; data not shown). In this example (recorded in the presence of 0.4 µM strychnine and 10 µM bicuculline), bursts emerge with a latency of 60 s. Note that the burst structure is made up of a depolarization envelope with superimposed fast spikes followed by transient inactivation and subsequent return of firing. This pattern confers a ‘butterfly’ shape to each burst event. Riluzole (5 µM) added after 4.30 min from the start of TBOA application slowly inhibits bursting, which, in this example, is suppressed after 10 min. (b) Histochemical demonstration of motoneuron damage produced by 60 min application of TBOA and its antagonism by riluzole (applied 14 min from the start of TBOA application). Cell damage is assessed with propidium iodide (a DNA dye that labels membrane-damaged cells; Sharifullina & Nistri 2006) staining (shown in red) versus staining due to Hoechst 33342 (shown in blue; a cell-permeable DNA dye). (c) Histograms quantify increased death of motoneurons after exposure to TBOA (n = 13) and its prevention by riluzole (n = 23), which preserves the same number of surviving cells found in control conditions (n = 19). Data are expressed as percentage of propidium iodide-positive cells versus Hoechst 33342-positive cells and quantified with ImageJ software. *p = 0.038 for the difference between control and TBOA, and #p = 0.025 for the difference between TBOA and TBOA plus riluzole. Slices were collected from 10 rats (A. Cifra 2008, unpublished data).
These data suggest that TBOA bursting is a useful in vitro model to test the efficiency of neuroprotective drugs to inhibit excitotoxicity and pathological discharges of brainstem motoneurons.
7. Are excitotoxicity and oxidative stress two closely knitted pathways to motoneuron damage?
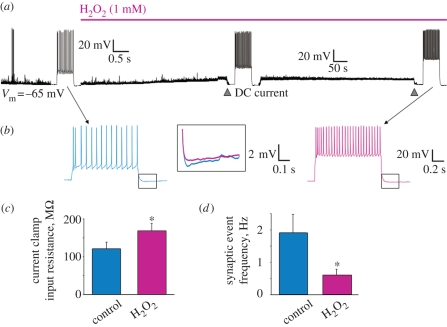
The aetiology and pathogenesis of sporadic ALS remain poorly understood in terms of known environmental toxic agents. Current theories favour ‘convergence’ of excitotoxicity and oxidative stress as the two cell processes concurring to produce motoneuron death in ALS (Cleveland & Rothstein 2001; Bruijn et al. 2004; Rao & Weiss 2004). Hence, if a strong excitotoxic stimulus prompts damage via oxidative stress, one may test whether oxidative stress implies excitotoxicity or, at least, changes in motoneuron excitability similar to those evoked by excitotoxicity. Hydrogen peroxide (H2O2) is a natural neuromodulator (at low µM concentrations; Kamsler & Segal 2004) as well as a robust donor of free oxygen radicals to evoke severe oxidative stress (at mM concentrations) downstream of SOD1 activity (Kato et al. 2004; Nicholls 2004). Figure 4a shows that 1 mM H2O2 evokes complex effects on hypoglossal motoneurons, which, in most cases, are actually opposite to those elicited by TBOA.
Figure 4.
Changes in motoneuron excitability induced by H2O2. (a) Examples of responses of hypoglossal motoneuron to bath-applied H2O2. Trace is a continuous, slow record (in current clamp configuration) of a cell with initial membrane potential of −65 mV (for methods, see Sharifullina et al. 2005; Lamanauskas & Nistri 2008). Segments of this trace are also shown on a faster time base to depict spike firing evoked by intracellular injection of 300 pA (500 ms). Note that, following the application of H2O2, there is gradual membrane depolarization (8 mV) associated with reduction in global synaptic events (decrease in baseline thickness and noise; see (d) for quantification of event frequency) and enhanced spike firing (when the membrane potential is repolarized back to rest). (b) Fast time base example of spike activity in control (blue) and during H2O2 application (pink). Note the larger number of spikes and the larger amplitude of the membrane voltage response to the same current injection pulse (300 pA). The box section shows (at higher gain) superimposed spike afterhyperpolarizations recorded in control or during H2O2 application. Despite the higher number of spikes, the afterhyperpolarization is decreased. (c) Change in cell input resistance (measured from voltage response to a hyperpolarizing current pulse of −50 pA) in the presence of H2O2. Data are averages from five motoneurons (*p < 0.01). (d) Reduction in global synaptic event frequency elicited by H2O2 (data are from five motoneurons voltage clamped at −70 mV; *p < 0.04) (F. Nani 2008, unpublished data).
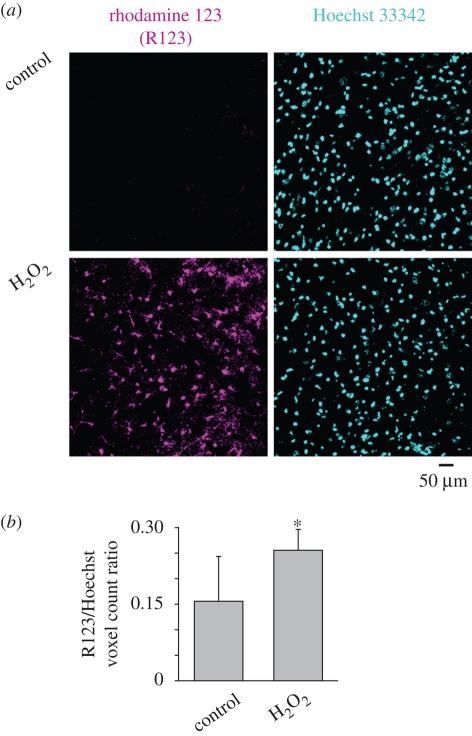
Thus, H2O2 induces a slow depolarization (8 mV) accompanied by increased firing in response to current injection from similar baseline membrane potential, probably due to a depression of the spike afterhyperpolarization that normally contributes to spike frequency accommodation (figure 4b). In contrast to TBOA bursting, H2O2 induces a significant rise in motoneuron input resistance (figure 4c) and large depression of all synaptic events without the emergence of any oscillatory activity (figure 4d). The effects elicited by H2O2 are insensitive to blockers of excitatory or inhibitory synaptic transmission. Hence, oxidative stress paradoxically makes motoneurons more isolated from their network and yet more intrinsically excitable. This condition is indeed associated with oxidative stress damage of motoneurons as shown in figure 5a,b, where this process is assessed on the basis of the rhodamine 123 marker.
Figure 5.
Oxidative stress of hypoglossal motoneurons induced by H2O2. (a) Histological example of rhodamine 123 staining of cells in hypoglossal slices incubated for 20 min in control solution or in the presence of H2O2 (1 mM). After rinsing, cells were stained with rhodamine 123 (5 µM; magenta pseudocolour) to reveal intracellular oxidative processes (Gomes et al. 2005; Jiang et al. 2006) and Hoechst 33342 (cyan pseudocolour) to show cell nuclei (Sharifullina & Nistri 2006). Note that, notwithstanding the analogous number of Hoechst-positive cells, there is a larger number of rhodamine 123-positive elements following H2O2. (b) Histograms show the ratio of rhodamine-positive cells over Hoechst-positive cells, indicating a significant rise after H2O2. Data are from 11 slices in control conditions and 14 slices after H2O2 and are quantified with ImageJ software (*p < 0.03). (F. Nani 2008, unpublished data).
It is interesting to compare the acute action of H2O2 with the motoneuron phenotype of the mutated SOD1 mice with aberrant handling of reactive oxygen species (van Zundert et al. 2008). In either case, hypoglossal motoneurons fire more intensely: however, in the mutant, the persistent Na+ current is enhanced without change in input resistance or afterhyperpolarization. The consequence of acute application of H2O2 to wild-type motoneurons is, on the other hand, depression of the persistent inward current and increased input resistance. Because data from SOD1 mutant motoneurons have been collected in the pre-symptomatic phase, it seems likely that they indicate compensatory mechanisms to cope with the results of gene mutation before the disease flares up.
8. Conclusions
In vitro studies of hypoglossal motoneurons have disclosed new properties that, rather than being simple operators of motor programmes generated by central pattern generators, appear to contribute to a variety of repetitive motor patterns essential for physiological functions. Nevertheless, the appearance of deranged motor patterns may indicate impending motoneuron damage and might represent a predictive model to assess potential pharmacological agents against neurodegenerative diseases like ALS.
Acknowledgements
This work is supported by a PRIN grant from MIUR and by a research grant from the Friuli Venezia Giulia regional government for the project SPINAL. We thank Dr Micaela Grandolfo for her help with histochemical experiments.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Brainstem neural networks vital for life’.
References
- Ballanyi K., Onimaru H., Homma I.1999Respiratory network function in the isolated brainstem–spinal cord of newborn rats. Prog. Neurobiol. 59, 583–634 (doi:10.1016/S0301-0082(99)00009-X) [DOI] [PubMed] [Google Scholar]
- Bracci E., Ballerini L., Nistri A.1996Spontaneous rhythmic bursts induced by pharmacological block of inhibition in lumbar motoneurons of the neonatal rat spinal cord. J. Neurophysiol. 75, 640–647 [DOI] [PubMed] [Google Scholar]
- Briggman K. L., Kristan W. B.2008Multifunctional pattern-generating circuits. Annu. Rev. Neurosci 31, 271–294 (doi:10.1146/annurev.neuro.31.060407.125552) [DOI] [PubMed] [Google Scholar]
- Bruijn L. I., Miller T. M., Cleveland D. W.2004Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 27, 723–749 (doi:10.1146/annurev.neuro.27.070203.144244) [DOI] [PubMed] [Google Scholar]
- Chow C. C., Kopell N.2000Dynamics of spiking neurons with electrical coupling. Neural Comput. 12, 1643–1678 (doi:10.1162/089976600300015295) [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Rothstein J. D.2001From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2, 806–819 (doi:10.1038/35097565) [DOI] [PubMed] [Google Scholar]
- Del Negro C. A., Chandler S. H.1998Regulation of intrinsic and synaptic properties of neonatal rat trigeminal motoneurons by metabotropic glutamate receptors. J. Neurosci. 18, 9216–9226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro C. A., Morgado-Valle C., Feldman J. L.2002Respiratory rhythm: an emergent network property? Neuron 34, 821–830 (doi:10.1016/S0896-6273(02)00712-2) [DOI] [PubMed] [Google Scholar]
- Eytan D., Marom S.2006Dynamics and effective topology underlying synchronization in networks of cortical neurons. J. Neurosci. 26, 8465–8476 (doi:10.1523/JNEUROSCI.1627-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. L., Del Negro C. A.2006Looking for inspiration: new perspectives on respiratory rhythm. Nat. Rev. Neurosci. 7, 232–242 (doi:10.1038/nrn1871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A., Fernandes E., Lima J. L. F. C.2005Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 65, 45–80 (doi:10.1016/j.jbbm.2005.10.003) [DOI] [PubMed] [Google Scholar]
- Grillner S.2006Biological pattern generation: the cellular and computational logic of network in motion. Neuron 52, 751–766 (doi:10.1016/j.neuron.2006.11.008) [DOI] [PubMed] [Google Scholar]
- Hsiao C. F., Wu N., Levine M. S., Chandler S. H.2002Development and serotonergic modulation of NMDA bursting in rat trigeminal motoneurons. J. Neurophysiol. 87, 1318–1328 [DOI] [PubMed] [Google Scholar]
- Jean A.2001Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol. Rev. 81, 929–969 [DOI] [PubMed] [Google Scholar]
- Jiang Z. G., et al. 2006A multifunctional cytoprotective agent that reduces neurodegeneration after ischemia. PNAS 103, 1581–1586 (doi:10.1073/pnas.0510573103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamsler A., Segal M.2004Hydrogen peroxide as a diffusible signal molecule in synaptic plasticity. Mol. Neurobiol. 29, 167–178 (doi:10.1385/MN:29:2:167) [DOI] [PubMed] [Google Scholar]
- Kato S., et al. 2004Histological evidence of redox system breakdown caused by superoxide dismutase 1 (SOD1) aggregation is common to SOD1-mutated motor neurons in humans and animal models. Acta Neuropathol. 107, 149–158 (doi:10.1007/s00401-003-0791-1) [DOI] [PubMed] [Google Scholar]
- Kiehn O.2006Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 29, 279–306 (doi:10.1146/annurev.neuro.29.051605.112910) [DOI] [PubMed] [Google Scholar]
- Krieger C., Jones K., Kim S. U., Eisen A. A.1994The role of intracellular free calcium in motor neuron disease. J. Neurol. Sci. 124, 27–32 (doi:10.1016/0022-510X(94)90173-2) [DOI] [PubMed] [Google Scholar]
- Kutsuwada T., et al. 1996Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron 16, 333–344 (doi:10.1016/S0896-6273(00)80051-3) [DOI] [PubMed] [Google Scholar]
- Lamanauskas N., Nistri A.2006Persistent rhythmic oscillations induced by nicotine on neonatal rat hypoglossal motoneurons in vitro. Eur. J. Neurosci. 24, 2543–2556 (doi:10.1111/j.1460-9568.2006.05137.x) [DOI] [PubMed] [Google Scholar]
- Lamanauskas N., Nistri A.2008Riluzole blocks persistent Na+ and Ca2+ currents and modulates release of glutamate via presynaptic NMDA receptors on neonatal rat hypoglossal motoneurons in vitro. Eur. J. Neurosci. 27, 2501–2514 (doi:10.1111/j.1460-9568.2008.06211.x) [DOI] [PubMed] [Google Scholar]
- Laslo P., Lipski J., Nicholson L. F., Miles G. B., Funk G. D.2001GluR2 AMPA receptor subunit expression in motoneurons at low and high risk for degeneration in amyotrophic lateral sclerosis. Exp. Neurol. 169, 461–471 (doi:10.1006/exnr.2001.7653) [DOI] [PubMed] [Google Scholar]
- Li A., Nattie E. E.1995Prolonged stimulation of respiration by brain stem metabotropic glutamate receptors. J. Appl. Physiol. 79, 1650–1656 [DOI] [PubMed] [Google Scholar]
- Lips M. B., Keller B. U.1999Activity-related calcium dynamics in motoneurons of the nucleus hypoglossus from mouse. J. Neurophysiol. 82, 2936–2946 [DOI] [PubMed] [Google Scholar]
- Lowe A. A.1980The neural regulation of tongue movements. Prog. Neurobiol. 15, 975–983 (doi:org/10.1016/0301-0082(80)90008-8) [DOI] [PubMed] [Google Scholar]
- Marchetti C., Pagnotta S., Donato R., Nistri A.2002Inhibition of spinal or hypoglossal motoneurons of the newborn rat by glycine or GABA. Eur. J. Neurosci. 15, 975–983 (doi:10.1046/j.1460-9568.2002.01927.x) [DOI] [PubMed] [Google Scholar]
- Mazza E., Nunez-Abades P. A., Spielmann J. M., Cameron W. E.1992Anatomical and electrotonic coupling in developing genioglossal motoneurons of the rat. Brain Res. 598, 127–137 (doi:10.1016/0006-8993(92)90176-A) [DOI] [PubMed] [Google Scholar]
- Nicholls D. G.2004Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Curr. Mol. Med. 4, 149–177 (doi:10.2174/1566524043479239) [DOI] [PubMed] [Google Scholar]
- Nistri A., Ostroumov K., Sharifullina E., Taccola G.2006Tuning and playing a motor rhythm: how metabotropic glutamate receptors orchestrate generation of motor patterns in the mammalian central nervous system. J. Physiol. 572, 323–334 (doi:10.1113/jphysiol.2005.100610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H., Arata A., Homma I.1987Localization of respiratory rhythm-generating neurons in the medulla of brainstem–spinal cord preparations from newborn rats. Neurosci. Lett. 78, 151–155 (doi:10.1016/0304-3940(87)90624-0) [DOI] [PubMed] [Google Scholar]
- Quitadamo C., Fabbretti E., Lamanauskas N., Nistri A.2005Activation and desensitization of neuronal nicotinic receptors modulate glutamatergic transmission on neonatal rat hypoglossal motoneurons. Eur. J. Neurosci. 22, 2723–2734 (doi:10.1111/j.1460-9568.2005.04460.x) [DOI] [PubMed] [Google Scholar]
- Rao S. D., Weiss J. H.2004Excitotoxic and oxidative cross-talk between motor neurons and glia in ALS pathogenesis. Trends Neurosci. 27, 17–23 (doi:10.1016/j.tins.2003.11.001) [DOI] [PubMed] [Google Scholar]
- Rekling J. C., Feldman J. L.1998PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu. Rev. Physiol. 60, 385–405 (doi:10.1146/annurev.physiol.60.1.385) [DOI] [PubMed] [Google Scholar]
- Rekling J. C., Shao X. M., Feldman J. L.2000Electrical coupling and excitatory synaptic transmission between rhythmogenic respiratory neurons in the preBotzinger complex. J. Neurosci. 20, RC113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland L. P., Shneider N. A.2001Amyotrophic lateral sclerosis. N. Engl. J. Med. 344, 1688–1700 (doi:10.1056/NEJM200105313442207) [DOI] [PubMed] [Google Scholar]
- Rybak I. A., Abdala A. P., Markin S. N., Paton J. F., Smith J. C.2007Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog. Brain. Res. 165, 201–220 (doi:10.1016/S0079-6123(06)65013-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejnowski T. J., Paulsen O.2006Network oscillations: emerging computational principles. J. Neurosci. 26, 1673–1676 (doi:10.1523/JNEUROSCI.3737-05d.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifullina E., Nistri A.2006Glutamate uptake block triggers deadly rhythmic bursting of neonatal rat hypoglossal motoneurons. J. Physiol. 572, 407–423 (doi:10.1113/jphysiol.2005.100412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifullina E., Ostroumov K., Nistri A.2004Activation of group I metabotropic glutamate receptors enhances efficacy of glutamatergic inputs to neonatal rat hypoglossal motoneurons in vitro. Eur. J. Neurosci. 20, 1245–1254 (doi:10.1111/j.1460-9568.2004.03590.x) [DOI] [PubMed] [Google Scholar]
- Sharifullina E., Ostroumov K., Nistri A.2005Metabotropic glutamate receptor activity induces a novel oscillatory pattern in neonatal rat hypoglossal motoneurones. J. Physiol. 563, 139–159 (doi:10.1113/jphysiol.2004.079509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifullina E., Ostroumov K., Grandolfo M., Nistri A.2008N-methyl-d-aspartate triggers neonatal rat hypoglossal motoneurons in vitro to express rhythmic bursting with unusual Mg2+ sensitivity. Neuroscience 154, 804–820 (doi:10.1016/j.neuroscience.2008.03.010) [DOI] [PubMed] [Google Scholar]
- Solomon I. C.2003Connexin36 distribution in putative CO2-chemosensitive brainstem regions in rat. Respir. Physiol. Neurobiol. 139, 1–20 (doi:10.1016/j.resp.2003.09.004) [DOI] [PubMed] [Google Scholar]
- Solomon I. C., Halat T. J., El-Maghrabi M. R., O'Neal M. H.2001Localization of connexin26 and connexin32 in putative CO2-chemosensitive brainstem regions in rat. Respir. Physiol. 129, 101–121 (doi:10.1016/S0034-5687(01)00299-7) [DOI] [PubMed] [Google Scholar]
- Soto-Trevinõ C., Rabbah P., Marder E., Nadim F.2005Computational model of electrically coupled, intrinsically distinct pacemaker neurons. J. Neurophysiol. 94, 590–604 (doi:10.1152/jn.00013.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzue T.1984Respiratory rhythm generation in the in vitro brain stem–spinal cord preparation of the neonatal rat. J. Physiol. 354, 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zundert B., Peuscher M. H., Hynynen M., Chen A., Neve R. L., Brown R. H., Jr, Constantine-Paton M., Bellingham M. C.2008Neonatal neuronal circuitry shows hyperexcitable disturbance in a mouse model of the adult-onset neurodegenerative disease yotrophic lateral sclerosis. J. Neurosci. 28, 10 864–10 874 (doi:10.1523/JNEUROSCI.1340-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]