Abstract
Nicotine may link cigarette smoking during pregnancy with sudden infant death syndrome (SIDS). Pre-natal nicotine leads to diminished ventilatory responses to hypercarbia and reduced central chemoreception in mice at post-natal days 0–3. We studied how pre-natal nicotine exposure changes the cholinergic contribution to central respiratory chemoreception in neonatal isolated brainstem–spinal cord and slice preparations.
Osmotic minipumps, implanted subcutaneously into 5–7 days pregnant mice, delivered saline or nicotine ditartrate 60 mg kg−1 d−1 for up to 28 days. In control preparations, acidification of the superfusion medium from pH 7.4 to 7.3 increased the frequency and reduced the amplitude of fictive respiration. In nicotine-exposed neonatal mice, the reduction in amplitude induced by acidification was reduced. In control preparations, atropine suppressed respiratory responses to acidification, while hexamethonium did not. By contrast, in nicotine-exposed preparations, hexamethonium blocked chemosensory responses but atropine did not.
Our results indicate that pre-natal nicotine exposure switches cholinergic mechanisms of central chemosensory responses from muscarinic receptors to nicotinic receptors. Modification of the cholinergic contribution to central chemoreception may produce respiratory dysfunctions, as suggested by receptor-binding studies in victims of SIDS.
Keywords: central chemoreception, respiratory rhythm generator, sudden infant death syndrome, hypercarbia, muscarinic receptors, nicotinic receptors
1. Introduction
Cholinergic inputs provide excitatory drive to neurons of the respiratory pattern generator (Weinstock 1981; Murakoshi et al. 1985; Gillis et al. 1988; Nattie & Li 1990; Burton et al. 1994, 1995; Shao & Feldman 2000, 2001, 2005; Hatori et al. 2006) and play a part in central chemosensitivity to H+ and PCO2 (Dev & Loeschcke 1979a,b; Fukuda & Loeschcke 1979; Nattie et al. 1989; Monteau et al. 1990; Burton et al. 1997; Eugenin & Nicholls 1997). The sites of acetylcholine action overlap with CO2-chemosensitive regions, and the responses elicited by acetylcholine are similar to those elicited by low pH stimulation (Issa & Remmers 1992; Eugenin & Nicholls 1997). In addition, blockade of muscarinic receptors, which are expressed in various brainstem respiratory regions (Kinney et al. 1995a,b; Mallios et al. 1995), reduces respiratory responses to acidosis (Dev & Loeschcke 1979a,b; Monteau et al. 1990; Eugenin & Nicholls 1997).
If acetylcholine plays a neurotrophic role during brain development (Lauder & Schambra 1999; Gu 2002), treatment of a foetus with nicotine (a teratogen as well as an acetylcholine agonist) might give rise to changes in the properties of brainstem chemoreceptors in the neonatal animal (Mitchell et al. 1993; Kohlendorfer et al. 1998; Chong et al. 2004; Slotkin 2004). Indeed, there is evidence that links sudden infant death syndrome (SIDS), a cause of death in infants under 1 year old in developed countries, with smoking during pregnancy (Dwyer & Ponsonby 1995; Slotkin 1998). SIDS in turn could be due to abnormalities in the generation of the respiratory rhythm or its modulation by chemosensory input (Nattie & Kinney 2002; Eugenin et al. 2008). In fact, infants who died from SIDS had previously shown alterations of their breathing patterns during sleep (Schechtman et al. 1991; Kahn et al. 1992). Moreover, infant victims of SIDS or infants born of mothers who smoked showed a high incidence of central respiratory dysfunctions (Brady & McCann 1985), a major number of central apnoeas (Gennser et al. 1975; Kahn et al. 1988; Schechtman et al. 1991), diminished chemoreflexes (Shannon et al. 1977; Ueda et al. 1999) and decreased spontaneous and evoked arousability (Newman et al. 1989; Schechtman et al. 1992; Lewis & Bosque 1995; Tirosh et al. 1996; Kahn et al. 2002, 2003; Horne et al. 2004). Anatomical and pathological studies of children with SIDS suggest that there are alterations in brainstem respiratory chemosensory nuclei (Kinney et al. 2001; Nattie & Kinney 2002).
Neonatal rats and mice that were exposed to nicotine during pregnancy showed hypoventilation and increased frequency of apnoea (St John & Leiter 1999; Robinson et al. 2002; Huang et al. 2004; Eugenin et al. 2008). Pre-natal nicotine also impairs hypoxia-induced autoresuscitation from primary apnoea in neonatal rats (Fewell et al. 2001) and hypoxia- or hypercarbia-induced ventilatory reflexes in neonatal mice, awakening rats and sleeping lambs (St John & Leiter 1999; Hafstrom et al. 2002; Huang et al. 2004; Simakajornboon et al. 2004; Eugenin et al. 2008).
Recently, using brainstem–spinal cord preparations, we have shown that pre-natal nicotine reduces central respiratory chemosensitivity in neonatal mice (Eugenin et al. 2008). Since pre-natal nicotine can affect the expression of acetylcholine receptors (Slotkin 1998), a reduction in central chemoreception might be due to changes in cholinergic chemosensory drive. Previous results using the ‘en bloc’ brainstem–spinal cord preparation indicate a change in cholinergic contribution to central chemoreception (Eugenin et al. 2008). To explore this possibility in more detail, we studied the effects of pre-natal nicotine exposure upon the cholinergic tonic drive of fictive respiration and upon the muscarinic and nicotinic receptor contributions to the respiratory central chemoreception. We further compared the effects of nicotine on en bloc brainstem–spinal cord and slice preparations.
2. Methods
(a). Preparations
Fifteen adult CF1 mice, 5–7 days pregnant, were anaesthetized with ketamine/xylazine (80/20 mg kg−1 i.p.; Troy Laboratories, Smithfield, Australia and Alfasan International, Woerden, The Netherlands). Under strict aseptic conditions, subcutaneous implanting of 28-day osmotic minipumps (2004, Alzet, Cupertino, CA, USA) delivering saline (controls, n = 5) or nicotine bitartrate (60 mg kg−1 d−1, n = 10) at a rate of 0.25 µl h−1 was performed through an incision made between the scapulae. As described previously (Eugenin et al. 2008), osmotic minipumps allow one to distinguish between the effects of nicotine itself in the foetus from those caused either by the stress of daily injections (Suemaru et al. 1992; Houdi et al. 1995) or by possible foetal hypoxia–ischaemia owing to uterine vessel vasoconstriction caused by the peak of plasmatic nicotine achieved during injections. Mice were maintained in separate cages with water and food ad libitum at 22°C under a 12 L : 12 D cycle. At the end of the experiments, animals were sacrificed with an anaesthetic overdose.
(b). Recording fictive respiration in en bloc brainstem–spinal cord preparations and brainstem slices
In order to evaluate whether pre-natal nicotine exposure affects chemosensory responses in a reduced preparation containing the preBötzinger complex (preBötC) (Smith et al. 1991), experiments were performed in slices and compared with en bloc brainstem–spinal cord preparations that also contained the pre-inspiratory parafacial respiratory group (Onimaru et al. 2006).
Experiments were carried out in 96 newborn animals (P0–P6), anaesthetized with methophane inhalation and cooled on ice. The central nervous system was removed, decerebrated through a ponto-bulbar transection and immersed in artificial cerebrospinal fluid (aCSF) containing (in mM): 125.0 NaCl, 5.0 KCl, 24.0 NaHCO3, 1.25 KH2PO4×H2O, 0.8 CaCl2, 1.25 MgSO4×7H2O (Sigma, St Louis, MO, USA), 30.0 d-glucose (Merck, Darmstadt, Germany) and equilibrated with O2 : CO2 = 95 per cent : 5 per cent (pH 7.40) at 4°C. For en bloc preparations, the isolated tissue constituted by the brainstem and the spinal cord was transferred to a recording chamber 2 ml in volume and superfused with aCSF at 25°C. A thin film partition, sealed with Vaseline at C1–C2, allowed us to superfuse the brainstem separately from the spinal cord with a continuous flow of aCSF (0.8–2.0 ml min−1). For the slice preparation, the brainstem was mounted on agar, and a 700 µm slice containing the preBötC was obtained using a vibratome as described previously (Pena & Ramirez 2004). Slices were transferred to a 0.5 ml recording chamber with a continuous flow of 1.0–2.0 ml min−1.
(c). Electrical recording
Spontaneous activities from C3–C5 ventral roots in en bloc preparations or from the ventral respiratory group (VRG) in slices were recorded using glass suction electrodes at 24–25°C. Under stereomicroscopic vision, the tip of the glass electrode driven by a three-axis micro-manipulator was placed in close contact with the caudal surface of the slice in the region of the VRG. Electrical signals were amplified by a low-noise differential amplifier (Grass, Model P55), integrated with a full-wave rectifier (time constant = 100 ms), displayed on an oscilloscope (VC 6041, Hitachi, Japan) and recorded and analysed with an Axoscope-Digipack 1320A AD acquisition system (Axon Instruments, Union City, CA, USA).
(d). Acidic stimulation
The pH of the brainstem superfusion medium (7.3 and 7.4) was obtained by equilibrating aCSF prepared with different final concentrations of sodium bicarbonate (19 and 24 mM, respectively) with 95 per cent O2/5 per cent CO2. Reduction in sodium bicarbonate from 24 to 19 mM was compensated by increasing the final concentration of NaCl to 130 mM to eliminate any osmotic effect. In previous work, we showed that, in the range of concentration used here, changes in [NaCl]o did not alter fictive respiration (Eugenin et al. 2006). In addition, changes in pH were obtained by switching the equilibrating gas mixture from 95 per cent O2 and 5 per cent CO2 (pH 7.4) to 90 per cent O2 and 10 per cent CO2 (pH 7.2). The tip of a micro-combination pH electrode (Model 9811, Orion, Beverly, MA, USA) was placed in the recording chamber and connected to a pH/ion amplifier (Model 2000, A-M Systems, Everett, WA, USA) to record the pH of the superfusion medium. Before the evaluation of the effects of acidification, ventral root activity had to be regular and stable for at least 3 min.
(e). Evaluation of cholinergic contribution to chemosensitivity and of cholinergic drive upon fictive respiration
Cholinergic contribution to central chemosensitivity was evaluated in en bloc and slice preparations. Fictive respiration was recorded while superfusion medium was switched from pH 7.4 to 7.3 in the presence or absence of aCSF containing muscarinic acetylcholine receptor blocker, atropine 100 µM (Sigma) or nicotinic acetylcholine receptor (nAChR) blocker, hexamethonium chloride 100 µM (Sigma). After pH 7.3 stimulation, the superfusion was returned to pH 7.4 and a recovery recording was performed.
Cholinergic drive of fictive respiration was evaluated in en bloc and slice preparations, by measuring the effects of muscarinic and nAChR blockers on the basal fictive respiration. Tonic actions of endogenous acetylcholine release were evaluated through the effects of an acetylcholinesterase blocker, neostigmine 100 µM, upon fictive respiration.
(f). Data analysis
Neonates in each experimental group were obtained from at least three to four litters. The amplitude of a burst of action potentials was estimated in vitro from the difference between the peak value of the integrated signal and the value of the integrated activity immediately before the onset of the burst, expressed in arbitrary units. Cycle duration was measured from the onset of one burst of action potentials to the onset of the next. Duration of the burst, which corresponded to the inspiratory duration for the respiratory-like rhythm, was measured from the onset to the offset of the burst. Instantaneous rhythm frequency was calculated from the reciprocal value of the cycle duration and expressed as bursts per minute. Values were expressed as mean ± s.e.m.
The statistical significance of differences induced by treatments (acidification and cholinergic drugs) was ascertained using a two-tailed p-level estimated through a Wilcoxon signed-rank test. Differences in the magnitude of responses between control and nicotine-exposed preparations were assessed with Student's t-test for independent samples. Comparison of multiple independent groups was performed using a two-tailed p-level estimated through ANOVA followed by Bonferroni post hoc test. Rejection of the null hypothesis was done if p < 0.05.
3. Results
(a). Basal activity and responses to acidification
In control neonatal mice, spontaneous rhythmic activity recorded from en bloc preparations consisted of bursts of action potentials appearing rhythmically at a frequency of 9.7 ± 0.7 bursts min−1 (ranging from 3 to 18 bursts min−1), with a duration of 0.91 ± 0.03 s (n = 15, figure 1a). In control slices, bursts of action potentials recorded from the surface of the VRG appeared rhythmically at a frequency of 11.3 ± 1.4 bursts min−1 and lasting 0.87 ± 0.03 s (n = 8, figure 1b). No significant differences in these parameters were found between en bloc and slice preparations.
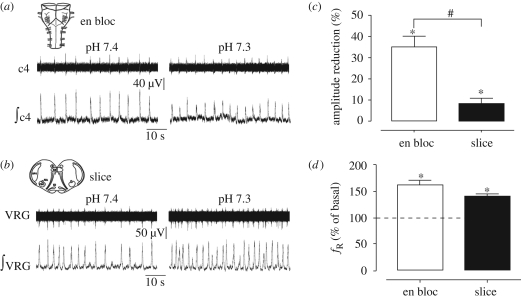
Figure 1.
Effects of acidification on en bloc and slice preparations from control mice. (a) En bloc preparation, raw and integrated signals recorded from C4 ventral root at pH 7.4 and 7.3. (b) Slice preparation, raw and integrated signals recorded from the VRG at pH 7.4 and 7.3. Histograms correspond to the average of the amplitude reduction, expressed (c) in percentage, and (d) the frequency increase, expressed in percentage of basal values induced by acidification in 15 en bloc (open bars) and 8 slice (filled bars). #p < 0.01, unpaired t-test; *p < 0.05 respect to basal value (pH 7.4, Wilcoxon test).
Acidification of the superfusion medium induced changes in fictive respiration in control en bloc (P0–P3) and slice (P1–P6) preparations. As previously described (Infante et al. 2003; Eugenin et al. 2006), acidification of the brainstem superfusion medium from 7.4 to 7.3 reduced the amplitude of the integrated inspiratory burst and increased the frequency of the fictive respiration in en bloc preparations (figure 1a,c,d). In slices, a similar pattern of response was observed: an increase in respiratory frequency (figure 1b,d) and a decrease in the amplitude of the integrated burst (p < 0.01, Wilcoxon test). However, the reduction in amplitude was significantly lower than that observed in en bloc preparations (figure 1c, p < 0.01, unpaired t-test).
(b). Effects of pre-natal nicotine upon basal activity and acidification responses
Basal frequency of fictive respiration in en bloc preparations from nicotine-exposed mice was 7.0 ± 1.1 bursts min−1 (n = 10), which was lower than that found in controls (p < 0.01, unpaired t-test). The duration of the inspiratory burst was 0.97 ± 0.09 s (figure 2a) and not different from control. Contrary to en bloc preparations, slices from nicotine-exposed mice showed a higher basal frequency than that observed in control slices (14.6 ± 1.5 bursts min−1, n = 9, p < 0.01, unpaired t-test, figure 2b). The cycle duration in slices from nicotine-exposed mice was 0.85 ± 0.04 s.
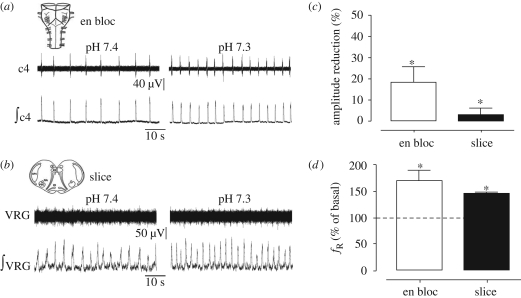
Figure 2.
Effects of acidification on en bloc and slice preparations from nicotine-exposed mice. (a) En bloc preparation, raw and integrated signals recorded from C4 ventral root at pH 7.4 and 7.3. (b) Slice preparation, raw and integrated signals recorded from the VRG at pH 7.4 and 7.3. Histograms average the amplitude reduction, expressed (c) in percentage, and the (d) frequency increase, expressed in percentage of basal values induced by acidification from 10 en bloc (open bars) and 8 slice (filled bars) preparations. *p < 0.05 respect to basal value (pH 7.4, Wilcoxon test).
As illustrated in figure 2c,d, in en bloc preparations from nicotine-exposed mice, acidification induced a decrease in the amplitude and an increase in the frequency (p < 0.01, Wilcoxon test). In slice preparations, acidification induced only a frequency increase but not an amplitude decrease (figure 2c,d). The increases in the basal frequency of fictive respiration induced by acidosis (figure 2a,b,d) in en bloc and slice preparations from nicotine-exposed mice were similar to those observed in controls. However, the reductions in amplitude were smaller in en bloc preparations obtained from nicotine-exposed mice than those from controls (p < 0.01, unpaired t-test).
(c). Cholinergic drive of fictive respiration in vitro
To evaluate the basal tonic cholinergic drive of fictive respiration, we studied the changes in amplitude and frequency induced by muscarinic (atropine) and nicotinic (hexamethonium) acetylcholine receptor blockers. In addition, we evaluated endogenous release of acetylcholine through the effects of neostigmine, an acetylcholinoesterase blocker.
(i). Muscarinic and nicotinic receptor blockade
Atropine, but not hexamethonium, reduced the amplitude and frequency of basal (pH 7.4) fictive respiration by approximately 30 per cent in en bloc preparations from control and nicotine-exposed mice (p < 0.05, Wilcoxon test). In contrast, neither atropine nor hexamethonium had a significant effect on fictive respiration recorded in slices from control or nicotine exposed mice (figure 3a,b).
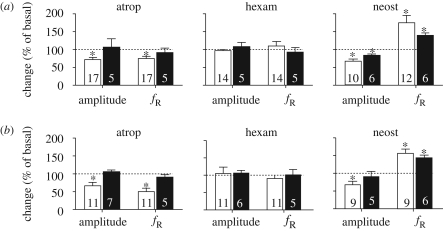
Figure 3.
Cholinergic drive of fictive respiration in preparations from (a) control and (b) nicotine-exposed mice. Changes in amplitude and frequency of fictive respiration (fR) after the application of 100 µM atropine (atrop), or 100 µM hexamethonium (hexam) or 100 µM neostigmine (neost) in en bloc (open bars) and slice (filled bars) preparations. *p < 0.05, when compared with the basal response (Wilcoxon test). Dotted lines indicate the basal amplitude and frequency (100%). The number of neonates is indicated inside bars.
(ii). Acetylcholinesterase blockade
Superfusion with neostigmine reduced the amplitude and increased the frequency of basal (pH 7.4) fictive respiration (p < 0.05, Wilcoxon test, figure 3a,b) in en bloc preparations from control and nicotine-exposed mice. This dual effect of neostigmine is similar to that observed with administration of carbachol, a synthetic acetylcholine agonist (data not shown). In control slices, neostigmine also increased the frequency and decreased the amplitude. But, in slices from nicotine-exposed mice, neostigmine increased only the frequency of fictive respiration (figure 3b).
(d). Cholinergic contribution to central respiratory chemosensitivity
In order to evaluate the cholinergic contribution to central respiratory chemoreceptory mechanisms, changes in fictive respiration induced by acidification of the superfusion medium (from pH 7.4 to 7.1) were recorded in en bloc and brainstem slice preparations from controls and nicotine-exposed mice in the absence and presence of muscarinic (atropine 100 µM) and nicotinic (hexamethonium 100 µM) acetylcholine receptor blockers.
Essentially, the contribution of muscarinic acetylcholine receptors was diminished, while that of nAChRs was increased. In control en bloc preparations, atropine, but not hexamethonium, blocked the acidification-induced reductions in amplitude and increases in frequency of fictive respiration (figures 4a and 5a, p < 0.05, Wilcoxon test). In contrast, in en bloc preparations from nicotine-exposed mice, hexamethonium but not atropine blocked both the acidification-induced reductions in amplitude and the increases in frequency of fictive respiration (figures 4b and 5b).
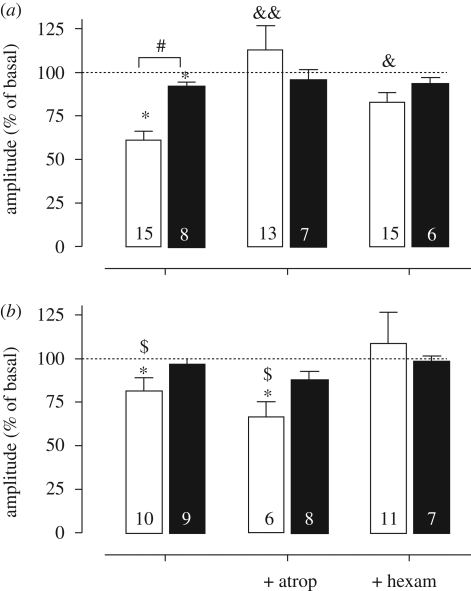
Figure 4.
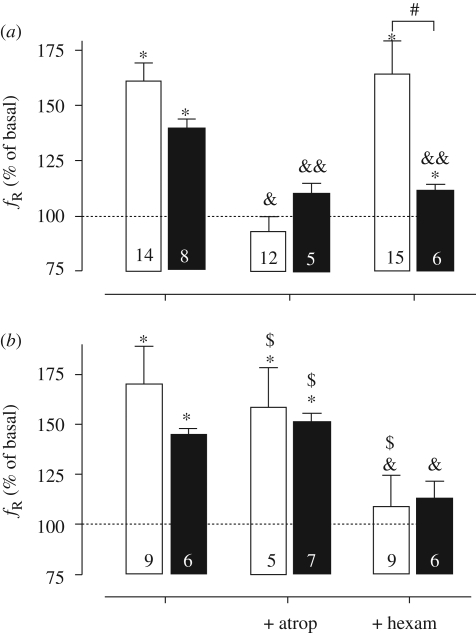
Changes in amplitude induced by acidification in en bloc (open bars) and slice (filled bars) preparations from (a) control and (b) nicotine-exposed mice. Preparations from P0 to P6 neonates were acidified in the absence or presence of atropine (100 µM) or hexamethonium (100 µM). *p < 0.05 respect to basal value (pH 7.4, Wilcoxon test); #p < 0.01 between en bloc and slice preparations, ANOVA and Bonferroni post-test; &p < 0.05 and &&p < 0.01 when compared with the basal condition (without antagonists, ANOVA); $p < 0.05 respect to control animals, unpaired t-test. Changes are expressed as percentage of basal values. Bars and vertical lines represent mean and s.e.m., respectively. Dotted lines indicate the basal amplitude without acidification (100%). Number of neonates is indicated inside bars.
Figure 5.
Changes in frequency induced by acidification in en bloc (open bars) and slice (filled bars) preparations from (a) control and (b) nicotine-exposed mice. Preparations from P0 to P6 neonates were acidified in the absence or presence of atropine (100 µM) or hexamethonium (100 µM). *p < 0.05 respect to basal value (pH 7.4, Wilcoxon test); #p < 0.05 between en bloc and slice preparations (ANOVA and Bonferroni post-test); &p < 0.05 and &&p < 0.01 respect to the basal condition (without antagonists, ANOVA); $p < 0.05 respect to control animals, unpaired t-test. Changes are expressed as percentage of basal values. Bars and vertical lines represent mean and s.e.m., respectively. Dotted lines indicate the basal frequency without acidification (100%). Number of neonates is indicated inside bars.
In control slices, atropine or hexamethonium blocked the acidification-induced reduction in amplitude and the increase in frequency of fictive respiration (figures 4a and 5a, respectively, p < 0.05, Wilcoxon test). On the other hand, in slices from nicotine-exposed mice, no significant change in amplitude was observed in the absence or presence of atropine or hexamethonium. However, hexamethonium, but not atropine, blocked the acidification-induced increase in frequency observed in slice preparations from nicotine-exposed mice.
In summary, in general terms, in control preparations, atropine, but not hexamethonium, suppressed the respiratory responses to acidification of the superfusion medium from pH 7.4 to 7.1 (figures 4a and 5a). However, in preparations from nicotine-exposed mice, atropine did not block the chemosensory responses. In contrast, hexamethonium, which does not modify the chemosensory responses in control preparations, abolished the expected increase in respiratory frequency induced by acidification (figures 4a and 5a).
4. Discussion
Our findings show that pre-natal nicotine exposure modifies the cholinergic contribution to central respiratory chemosensitivity. This switch from muscarinic to nicotinic receptor-based mechanisms is compatible with the known effect of pre-natal nicotine exposure of inducing downregulation of muscarinic acetylcholine receptors and upregulation of nAChRs in rat and mouse brains (Slotkin 1998). Pre-natal nicotine reduces the binding of M2 muscarinic receptors in the rat brainstem at early post-natal periods (Slotkin et al. 1999). In other systems, pre-natal nicotine can alter muscarinic receptor actions, either by uncoupling G-protein-dependent mechanisms in rat striatum and hippocampus (Zahalka et al. 1993) or by reducing mRNA of the muscarinic receptor in basal ganglia (Frank et al. 2001). Chronic nicotine exposure may lead to desensitization of nAChRs (Wang & Sun 2005) or their upregulation (Gaimarri et al. 2007). Chronic nicotine can affect selectively the number, stoichiometry, subunit composition and functionality of specific nAchRs (Van De Kamp & Collins 1994; Nguyen et al. 2003; Gaimarri et al. 2007; Walsh et al. 2008), especially the α4β2 subtype (Perry et al. 1999; Gentry & Lukas 2002).
Although en bloc and slice preparations differ in their responses in controls, both kinds of preparations showed modification of the cholinergic contribution to chemosensitivity after pre-natal nicotine exposure. The most obvious difference in the pattern of responses in controls is the magnitude of the reduction in amplitude induced by acidification. This is minimal in slices, despite the similar increases in frequency observed in both preparations. Differential distribution of cholinergic receptors along respiratory brainstem nuclei may account for differential effects upon amplitude and frequency. Since the changes in frequency must rely on respiratory rhythm generators such as the preBötC, the changes in amplitude must be mostly related to other brainstem regions that are present in the en bloc preparation, but absent in slices. In agreement with this, neostigmine decreased the amplitude in en bloc but not in slice preparations.
Among the chemosensitive nuclei included in the slices, a possible target for nicotine actions is the preBötC. This chemosensitive nucleus (Solomon 2003) is crucial for generating the rhythm (Smith et al. 1991), and their neurons express M3 mAchR and α4β2 nAchR, receptors whose activation increases the respiratory frequency (Shao & Feldman 2002; Shao et al. 2008). Whether these receptor subtypes are involved in central chemosensitivity has not been defined. The greatest amount of mAchR binding in the respiratory network is found in the lateral and medial parabrachial nuclei and the lateral nucleus of the solitary tract (Mallios et al. 1995). Interestingly, mAChRs were also found in the nuclei of the VRG (nucleus ambiguus and retrofacial nucleus) and ventral medulla (retrotrapezoid nucleus and ventrolateral medulla), which could participate in the cholinergic drive of central chemosensitivity (Nattie et al. 1989; Guyenet et al. 2008). In addition, pre-natal nicotine exposure reduces α7 nAChRs in the forebrain and upregulates these in the brainstem and cerebellum of rats (Slotkin et al. 2004). It has been proposed that dysfunction of the alpha7 nAChRs can lead to impairment in the modulation of the pre-synaptic release of GABA and glutamate, which, in turn, could lead to changes in the density and/or function of post-synaptic receptors (Fregosi & Pilarski 2008). In fact, pre-natal nicotine exposure increases the inhibitory effects of GABA and glycine on preBötzinger neurons (Luo et al. 2004, 2007).
Our results indicate that the tonic basal cholinergic drive of fictive respiration was not affected by pre-natal nicotine. Thus, the magnitude and pattern of changes in fictive respiration induced by neostigmine and acetylcholine receptor blockers were similar in control and nicotine-exposed preparations. At a first glance, this result appears puzzling since the central chemosensory response, which in part is mediated by cholinergic mechanisms, is decreased by pre-natal nicotine. Such disparity may reflect that cholinergic contribution to the central chemosensitivity is a low fraction of cholinergic mechanisms involved in respiratory neural control. In addition, our results show complex interactions between different cholinergic mechanisms. The prediction from neostigmine effects is that the cholinergic blockade during basal conditions should increase the amplitude and decrease the frequency of fictive respiration. Indeed, cholinergic blockade decreased both the amplitude and the frequency of fictive respiration. Access of acetylcholine to extra-synaptic receptors might account for this discrepancy. Whether adaptive mechanisms are triggered at the brainstem to counterbalance an increased nicotinic contribution with a decay in the cholinergic drive supported by muscarinic receptors is an open question.
Finally, it should be remarked that nicotine infusion alone is not a model for tobacco smoking, because nicotine is one of several chemicals in cigarette smoke that have addictive and potentially teratogenic effects (Rose 2006). In addition, minipumps do not produce intermittent infusion of nicotine as occurs in smokers. However, they allow us to study the nicotine effects on brain development, mimicking the steady-state plasma levels of nicotine (15–45 ng ml−1) observed in pregnant women considered moderate smokers (Benowitz & Jacob 1984) but without confounding hypoxia and stress-derived factors associated with multiple subcutaneous injections. As well as exposure to tobacco smoke, nicotine infusion results in a similar upregulation of nAChRs in the cortex and brainstem (Slotkin et al. 2002). The nicotine infusion used in mice is 10 times greater than that used in rats and produces reliable levels of plasmatic nicotine around 250 ng ml−1 (Robinson et al. 2002; Eugenin et al. 2008). Such doses induce similar levels of nicotinic receptor upregulation in hypothalamus, hippocampus and cortex (Van De Kamp & Collins 1994) and in respiratory-related regions of the brainstem in mice (Pauly et al. 1991; Robinson et al. 2002). In addition, these doses do not affect litter size, birth weight or post-natal growth curve in mice (Robinson et al. 2002; Eugenin et al. 2008).
In conclusion, we show that pre-natal nicotine exposure decreases the central chemosensory responses and modifies the cholinergic contribution to central chemosensitivity, reducing muscarinic and increasing nicotinic commands. Dysfunction in central chemoreception and its cholinergic drive may play a role in disorders of respiratory control such as SIDS. In fact, infant victims of SIDS show a decrease in the muscarinic binding in the arcuate nucleus, which would contribute to the chemosensory drive of respiration in humans (Kinney et al. 1995a,b).
Experiments were performed according to the Institute for Laboratory Animal Research (ILAR) Guide for the Care and Use of Laboratory Animals and approved by the Bioethics Committee of the Universidad de Santiago de Chile.
Acknowledgements
This work was supported by grant FONDECYT no. 1060110. C.C. is a postdoctoral fellow VRID, Universidad de Santiago de Chile, USACH. E.B. is a graduate student in the Biotechnology PhD program, Universidad de Santiago de Chile, USACH.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Brainstem neural networks vital for life’.
References
- Benowitz N. L., Jacob P., III1984Daily intake of nicotine during cigarette smoking. Clin. Pharmacol. Ther. 35, 499–504 [DOI] [PubMed] [Google Scholar]
- Brady J. P., McCann E. M.1985Control of ventilation in subsequent siblings of victims of sudden infant death syndrome. J. Pediatr. 106, 212–217 (doi:10.1016/S0022-3476(85)80289-4) [DOI] [PubMed] [Google Scholar]
- Burton M. D., Nouri K., Baichoo S., Samuels-Toyloy N., Kazemi H.1994Ventilatory output and acetylcholine: perturbations in release and muscarinic receptor activation. J. Appl. Physiol. 77, 2275–2284 [DOI] [PubMed] [Google Scholar]
- Burton M. D., Nouri M., Kazemi H.1995Acetylcholine and central respiratory control: perturbations of acetylcholine synthesis in the isolated brainstem of the neonatal rat. Brain Res. 670, 39–47 (doi:10.1016/0006-8993(94)01249-H) [DOI] [PubMed] [Google Scholar]
- Burton M. D., Johnson D. C., Kazemi H.1997The central respiratory effects of acetylcholine vary with CSF pH. J. Auton. Nerv. Syst. 62, 27–32 (doi:10.1016/S0165-1838(96)00104-X) [DOI] [PubMed] [Google Scholar]
- Chong D. S., Yip P. S., Karlberg J.2004Maternal smoking: an increasing unique risk factor for sudden infant death syndrome in Sweden. Acta Paediatr. 93, 471–478 (doi:10.1080/08035250310023495) [DOI] [PubMed] [Google Scholar]
- Dev N. B., Loeschcke H. H.1979aTopography of the respiratory and circulatory responses to acetylcholine and nicotine on the ventral surface of the medulla oblongata. Pflugers Arch. 379, 19–27 (doi:10.1007/BF00622900) [DOI] [PubMed] [Google Scholar]
- Dev N. B., Loeschcke H. H.1979bA cholinergic mechanism involved in the respiratory chemosensitivity of the medulla oblongata in the cat. Pflugers Arch. 379, 29–36 (doi:10.1007/BF00622901) [DOI] [PubMed] [Google Scholar]
- Dwyer T., Ponsonby A. L.1995SIDS epidemiology and incidence. Pediatr. Ann. 24, 350–352 [DOI] [PubMed] [Google Scholar]
- Eugenin J., Nicholls J. G.1997Chemosensory and cholinergic stimulation of fictive respiration in isolated CNS of neonatal opossum. J. Physiol. (Lond.) 501, 425–437 (doi:10.1111/j.1469-7793.1997.425bn.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin J., Von Bernhardi R., Muller K. J., Llona I.2006Development and pH sensitivity of the respiratory rhythm of fetal mice in vitro. Neuroscience 141, 223–231 (doi:10.1016/j.neuroscience.2006.03.046) [DOI] [PubMed] [Google Scholar]
- Eugenin J., Otarola M., Bravo E., Coddou C., Cerpa V., Reyes-Parada M., Llona I., Von Bernhardi R.2008Prenatal to early postnatal nicotine exposure impairs central chemoreception and modifies breathing pattern in mouse neonates: a probable link to sudden infant death syndrome. J. Neurosci. 28, 13 907–13 917 (doi:10.1523/JNEUROSCI.4441-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell J. E., Smith F. G., Ng V. K.2001Prenatal exposure to nicotine impairs protective responses of rat pups to hypoxia in an age-dependent manner. Respir. Physiol. 127, 61–73 (doi:10.1016/S0034-5687(01)00232-8) [DOI] [PubMed] [Google Scholar]
- Frank M. G., Srere H., Ledezma C., O'Hara B., Heller H. C.2001Prenatal nicotine alters vigilance states and AchR gene expression in the neonatal rat: implications for SIDS. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1134–R1140 [DOI] [PubMed] [Google Scholar]
- Fregosi R. F., Pilarski J. Q.2008Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing. Respir. Physiol. Neurobiol 164, 80–86 (doi:10.1016/j.resp.2008.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y., Loeschcke H. H.1979A cholinergic mechanism involved in the neuronal excitation by H+ in the respiratory chemosensitive structures on the ventral medulla oblongata of rats in vitro. Pflugers Arch. 379, 125–135 (doi:10.1007/BF00586938) [DOI] [PubMed] [Google Scholar]
- Gaimarri A., Moretti M., Riganti L., Zanardi A., Clementi F., Gotti C.2007Regulation of neuronal nicotinic receptor traffic and expression. Brain Res. Rev. 55, 134–143 (doi:10.1016/j.brainresrev.2007.02.005) [DOI] [PubMed] [Google Scholar]
- Gennser G., Marsal K., Brantmark B.1975Maternal smoking and fetal breathing movements. Am. J. Obstet. Gynecol. 123, 861–867 [DOI] [PubMed] [Google Scholar]
- Gentry C. L., Lukas R. J.2002Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr. Drug Target CNS Neurol. Disord. 1, 359–385 (doi:10.2174/1568007023339184) [DOI] [PubMed] [Google Scholar]
- Gillis R. A., Walton D. P., Quest J. A., Namath I. J., Hamosh P., Dretchen K. L.1988Cardiorespiratory effects produced by activation of cholinergic muscarinic receptors on the ventral surface of the medulla. J. Pharmacol. Exp. Ther. 247, 765–773 [PubMed] [Google Scholar]
- Gu Q.2002Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 111, 815–835 (doi:10.1016/S0306-4522(02)00026-X) [DOI] [PubMed] [Google Scholar]
- Guyenet P. G., Stornetta R. L., Bayliss D. A.2008Retrotrapezoid nucleus and central chemoreception. J. Physiol. 586, 2043–2048 (doi:10.1113/jphysiol.2008.150870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafstrom O., Milerad J., Sundell H. W.2002Prenatal nicotine exposure blunts the cardiorespiratory response to hypoxia in lambs. Am. J. Respir. Crit. Care Med. 166, 1544–1549 (doi:10.1164/rccm.200204-289OC) [DOI] [PubMed] [Google Scholar]
- Hatori E., Sakuraba S., Kashiwagi M., Kuribayashi J., Tsujita M., Hosokawa Y., Takeda J., Kuwana S.2006Association of nicotinic acetylcholine receptors with central respiratory control in isolated brainstem–spinal cord preparation of neonatal rats. Biol. Res. 39, 321–330 [DOI] [PubMed] [Google Scholar]
- Horne R. S., Franco P., Adamson T. M., Groswasser J., Kahn A.2004Influences of maternal cigarette smoking on infant arousability. Early Hum. Dev. 79, 49–58 (doi:10.1016/j.earlhumdev.2004.04.005) [DOI] [PubMed] [Google Scholar]
- Houdi A. A., Dowell R. T., Diana J. N.1995Cardiovascular responses to cigarette smoke exposure in restrained conscious rats. J. Pharmacol. Exp. Ther. 275, 646–653 [PubMed] [Google Scholar]
- Huang Y. H., Brown A. R., Costy-Bennett S., Luo Z., Fregosi R. F.2004Influence of prenatal nicotine exposure on postnatal development of breathing pattern. Respir. Physiol. Neurobiol. 143, 1–8 (doi:10.1016/j.resp.2004.07.002) [DOI] [PubMed] [Google Scholar]
- Infante C. D., Von Bernhardi R., Rovegno M., Llona I., Eugenin J. L.2003Respiratory responses to pH in the absence of pontine and dorsal medullary areas in the newborn mouse in vitro. Brain Res. 984, 198–205 (doi:10.1016/S0006-8993(03)03155-X) [DOI] [PubMed] [Google Scholar]
- Issa F. G., Remmers J. E.1992Identification of a subsurface area in the ventral medulla sensitive to local changes in PCO2. J. Appl. Physiol. 72, 439–446 [DOI] [PubMed] [Google Scholar]
- Kahn A., Blum D., Rebuffat E., Sottiaux M., Levitt J., Bochner A., Alexander M., Grosswasser J., Muller M. F.1988Polysomnographic studies of infants who subsequently died of sudden infant death syndrome. Pediatrics 82, 721–727 [PubMed] [Google Scholar]
- Kahn A., Groswasser J., Rebuffat E., Sottiaux M., Blum D., Foerster M., Franco P., Bochner A., Alexander M., Bachy A.1992Sleep and cardiorespiratory characteristics of infant victims of sudden death: a prospective case–control study. Sleep 15, 287–292 [DOI] [PubMed] [Google Scholar]
- Kahn A., Groswasser J., Franco P., Scaillet S., Sawaguchi T., Kelmanson I., Bernanrd D.2002Sudden infant deaths: arousal as a survival mechanism. Sleep Med. 3(Suppl. 2), S11–S14 (doi:10.1016/S1389-9457(02)00157-0) [DOI] [PubMed] [Google Scholar]
- Kahn A., Groswasser J., Franco P., Scaillet S., Sawaguchi T., Kelmanson I., Dan B.2003Sudden infant deaths: stress, arousal and SIDS. Early Hum. Dev. 75(Suppl.), S147–S166 (doi:10.1016/j.earlhumdev.2003.08.018) [DOI] [PubMed] [Google Scholar]
- Kinney H. C., Filiano J. J., Sleeper L. A., Mandell F., Valdes-Dapena M., White W. F.1995aDecreased muscarinic receptor binding in the arcuate nucleus in sudden infant death syndrome. Science 269, 1446–1450 (doi:10.1126/science.7660131) [DOI] [PubMed] [Google Scholar]
- Kinney H. C., Panigrahy A., Rava L. A., White W. F.1995bThree-dimensional distribution of [3H]quinuclidinyl benzilate binding to muscarinic cholinergic receptors in the developing human brainstem. J. Comp. Neurol. 362, 350–367 (doi:10.1002/cne.903620305) [DOI] [PubMed] [Google Scholar]
- Kinney H. C., Filiano J. J., White W. F.2001Medullary serotonergic network deficiency in the sudden infant death syndrome: review of a 15-year study of a single dataset. J. Neuropathol. Exp. Neurol. 60, 228–247 [DOI] [PubMed] [Google Scholar]
- Kohlendorfer U., Kiechl S., Sperl W.1998Sudden infant death syndrome: risk factor profiles for distinct subgroups [see comments]. Am. J. Epidemiol. 147, 960–968 [DOI] [PubMed] [Google Scholar]
- Lauder J. M., Schambra U. B.1999Morphogenetic roles of acetylcholine. Environ. Health Perspect. 107(Suppl. 1), 65–69 (doi:10.2307/3434473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K. W., Bosque E. M.1995Deficient hypoxia awakening response in infants of smoking mothers: possible relationship to sudden infant death syndrome. J. Pediatr. 127, 691–699 (doi:10.1016/S0022-3476(95)70155-9) [DOI] [PubMed] [Google Scholar]
- Luo Z., Costy-Bennett S., Fregosi R. F.2004Prenatal nicotine exposure increases the strength of GABAA receptor-mediated inhibition of respiratory rhythm in neonatal rats. J. Physiol. 561, 387–393 (doi:10.1113/jphysiol.2004.062927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z., McMullen N. T., Costy-Bennett S., Fregosi R. F.2007Prenatal nicotine exposure alters glycinergic and GABAergic control of respiratory frequency in the neonatal rat brainstem–spinal cord preparation. Respir. Physiol. Neurobiol 157, 226–234 (doi:10.1016/j.resp.2007.01.005) [DOI] [PubMed] [Google Scholar]
- Mallios V. J., Lydic R., Baghdoyan H. A.1995Muscarinic receptor subtypes are differentially distributed across brain stem respiratory nuclei. Am. J. Physiol. 268, L941–L949 [DOI] [PubMed] [Google Scholar]
- Mitchell E. A., et al. 1993Smoking and the sudden infant death syndrome. Pediatrics 91, 893–896 [PubMed] [Google Scholar]
- Monteau R., Morin D., Hilaire G.1990Acetylcholine and central chemosensitivity: in vitro study in the newborn rat. Respir. Physiol. 81, 241–254 (doi:10.1016/0034-5687(90)90049-5) [DOI] [PubMed] [Google Scholar]
- Murakoshi T., Suzue T., Tamai S.1985A pharmacological study on respiratory rhythm in the isolated brainstem–spinal cord preparation of the newborn rat. Br. J. Pharmacol. 86, 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E., Kinney H.2002Nicotine, serotonin, and sudden infant death syndrome. Am. J. Respir. Crit. Care Med. 166, 1530–1531 (doi:10.1164/rccm.2210001) [DOI] [PubMed] [Google Scholar]
- Nattie E. E., Li A. H.1990Ventral medulla sites of muscarinic receptor subtypes involved in cardiorespiratory control. J. Appl. Physiol. 69, 33–41 [DOI] [PubMed] [Google Scholar]
- Nattie E. E., Wood J., Mega A., Goritski W.1989Rostral ventrolateral medulla muscarinic receptor involvement in central ventilatory chemosensitivity. J. Appl. Physiol. 66, 1462–1470 [DOI] [PubMed] [Google Scholar]
- Newman N. M., Trinder J. A., Phillips K. A., Jordan K., Cruickshank J.1989Arousal deficit: mechanism of the sudden infant death syndrome? Aust. Paediatr. J. 25, 196–201 [PubMed] [Google Scholar]
- Nguyen H. N., Rasmussen B. A., Perry D. C.2003Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J. Pharmacol. Exp. Ther. 307, 1090–1097 (doi:10.1124/jpet.103.056408) [DOI] [PubMed] [Google Scholar]
- Onimaru H., Kumagawa Y., Homma I.2006Respiration-related rhythmic activity in the rostral medulla of newborn rats. J. Neurophysiol. 96, 55–61 (doi:10.1152/jn.01175.2005) [DOI] [PubMed] [Google Scholar]
- Pauly J. R., Marks M. J., Gross S. D., Collins A. C.1991An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J. Pharmacol. Exp. Ther. 258, 1127–1136 [PubMed] [Google Scholar]
- Pena F., Ramirez J. M.2004Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J. Neurosci. 24, 7549–7556 (doi:10.1523/JNEUROSCI.1871-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D. C., Davila-Garcia M. I., Stockmeier C. A., Kellar K. J.1999Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J. Pharmacol. Exp. Ther. 289, 1545–1552 [PubMed] [Google Scholar]
- Robinson D. M., Peebles K. C., Kwok H., Adams B. M., Clarke L. L., Woollard G. A., Funk G. D.2002Prenatal nicotine exposure increases apnoea and reduces nicotinic potentiation of hypoglossal inspiratory output in mice. J. Physiol. 538, 957–973 (doi:10.1113/jphysiol.2001.012705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. E.2006Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl.) 184, 274–285 (doi:10.1007/s00213-005-0250-x) [DOI] [PubMed] [Google Scholar]
- Schechtman V. L., Harper R. M., Wilson A. J., Southall D. P.1991Sleep apnea in infants who succumb to the sudden infant death syndrome. Pediatrics 87, 841–846 [PubMed] [Google Scholar]
- Schechtman V. L., Harper R. M., Wilson A. J., Southall D. P.1992Sleep state organization in normal infants and victims of the sudden infant death syndrome. Pediatrics 89, 865–870 [PubMed] [Google Scholar]
- Shannon D. C., Kelly D. H., O'Connell K.1977Abnormal regulation of ventilation in infants at risk for sudden-infant-death syndrome. N. Engl. J. Med. 297, 747–750 [DOI] [PubMed] [Google Scholar]
- Shao X. M., Feldman J. L.2000Acetylcholine modulates respiratory pattern: effects mediated by M3-like receptors in preBötzinger complex inspiratory neurons. J. Neurophysiol. 83, 1243–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X. M., Feldman J. L.2001Mechanisms underlying regulation of respiratory pattern by nicotine in preBötzinger complex. J. Neurophysiol. 85, 2461–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X. M., Feldman J. L.2002Pharmacology of nicotinic receptors in preBötzinger complex that mediate modulation of respiratory pattern. J. Neurophysiol. 88, 1851–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X. M., Feldman J. L.2005Cholinergic neurotransmission in the preBötzinger complex modulates excitability of inspiratory neurons and regulates respiratory rhythm. Neuroscience 130, 1069–1081 (doi:10.1016/j.neuroscience.2004.10.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X. M., Tan W., Xiu J., Puskar N., Fonck C., Lester H. A., Feldman J. L.2008α4* nicotinic receptors in preBötzinger complex mediate cholinergic/nicotinic modulation of respiratory rhythm. J. Neurosci. 28, 519–528 (doi:10.1523/JNEUROSCI.3666-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simakajornboon N., Vlasic V., Li H., Sawnani H.2004Effect of prenatal nicotine exposure on biphasic hypoxic ventilatory response and protein kinase C expression in caudal brain stem of developing rats. J. Appl. Physiol. 96, 2213–2219 (doi:10.1152/japplphysiol.00935.2003) [DOI] [PubMed] [Google Scholar]
- Slotkin T. A.1998Fetal nicotine or cocaine exposure: which one is worse? J. Pharmacol. Exp. Ther. 285, 931–945 [PubMed] [Google Scholar]
- Slotkin T. A.2004Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 198, 132–151 (doi:10.1016/j.taap.2003.06.001) [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Epps T. A., Stenger M. L., Sawyer K. J., Seidler F. J.1999Cholinergic receptors in heart and brainstem of rats exposed to nicotine during development: implications for hypoxia tolerance and perinatal mortality. Brain Res. Dev. Brain Res. 113, 1–12 (doi:10.1016/S0165-3806(98)00173-4) [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Pinkerton K. E., Auman J. T., Qiao D., Seidler F. J.2002Perinatal exposure to environmental tobacco smoke upregulates nicotinic cholinergic receptors in monkey brain. Brain Res. Dev. Brain Res. 133, 175–179 [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Southard M. C., Adam S. J., Cousins M. M., Seidler F. J.2004α7 nicotinic acetylcholine receptors targeted by cholinergic developmental neurotoxicants: nicotine and chlorpyrifos. Brain Res. Bull. 64, 227–235 (doi:10.1016/j.brainresbull.2004.07.005) [DOI] [PubMed] [Google Scholar]
- Smith J. C., Ellenberger H. H., Ballanyi K., Richter D. W., Feldman J. L.1991Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254, 726–729 (doi:10.1126/science.1683005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon I. C.2003Focal CO2/H+ alters phrenic motor output response to chemical stimulation of cat pre-Bötzinger complex in vivo. J. Appl. Physiol. 94, 2151–2157 [DOI] [PubMed] [Google Scholar]
- St John W. M., Leiter J. C.1999Maternal nicotine depresses eupneic ventilation of neonatal rats. Neurosci. Lett. 267, 206–208 (doi:10.1016/S0304-3940(99)00364-X) [DOI] [PubMed] [Google Scholar]
- Suemaru K., Oishi R., Gomita Y., Saeki K., Araki Y.1992Effect of long-term cigarette smoke exposure on locomotor activity and brain monoamine levels in rats. Pharmacol. Biochem. Behav. 41, 655–658 (doi:10.1016/0091-3057(92)90388-V) [DOI] [PubMed] [Google Scholar]
- Tirosh E., Libon D., Bader D.1996The effect of maternal smoking during pregnancy on sleep respiratory and arousal patterns in neonates. J. Perinatol. 16, 435–438 [PubMed] [Google Scholar]
- Ueda Y., Stick S. M., Hall G., Sly P. D.1999Control of breathing in infants born to smoking mothers. J. Pediatr. 135, 226–232 (doi:10.1016/S0022-3476(99)70026-0) [DOI] [PubMed] [Google Scholar]
- Van De Kamp J. L., Collins A. C.1994Prenatal nicotine alters nicotinic receptor development in the mouse brain. Pharmacol. Biochem. Behav. 47, 889–900 (doi:10.1016/0091-3057(94)90293-3) [DOI] [PubMed] [Google Scholar]
- Walsh H., Govind A. P., Mastro R., Hoda J. C., Bertrand D., Vallejo Y., Green W. N.2008Up-regulation of nicotinic receptors by nicotine varies with receptor subtype. J. Biol. Chem. 283, 6022–6032 (doi:10.1074/jbc.M703432200) [DOI] [PubMed] [Google Scholar]
- Wang H., Sun X.2005Desensitized nicotinic receptors in brain. Brain Res. Rev. 48, 420–437 (doi:10.1016/j.brainresrev.2004.09.003) [DOI] [PubMed] [Google Scholar]
- Weinstock M.1981Activation of central muscarinic receptors causes respiratory stimulation in conscious animals. Br. J. Pharmacol. 74, 587–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahalka E. A., Seidler F. J., Yanai J., Slotkin T. A.1993Fetal nicotine exposure alters ontogeny of M1-receptors and their link to G-proteins. Neurotoxicol. Teratol. 15, 107–115 (doi:10.1016/0892-0362(93)90069-Z) [DOI] [PubMed] [Google Scholar]