Abstract
Serotonin receptor (5-HTR) agonists that target 5-HT4(a)R and 5-HT1AR can reverse μ-opioid receptor (μ-OR)-evoked respiratory depression. Here, we have tested whether such rescuing by serotonin agonists also applies to the cardiovascular system. In working heart–brainstem preparations in situ, we have recorded phrenic nerve activity, thoracic sympathetic chain activity (SCA), vascular resistance and heart rate (HR) and in conscious rats, diaphragmatic electromyogram, arterial blood pressure (BP) and HR via radio-telemetry. In addition, the distribution of 5-HT4(a)R and 5-HT1AR in ponto-medullary cardiorespiratory networks was identified using histochemistry. Systemic administration of the μ-OR agonist fentanyl in situ decreased HR, vascular resistance, SCA and phrenic nerve activity. Subsequent application of the 5-HT1AR agonist 8-OH-DPAT further enhanced bradycardia, but partially compensated the decrease in vascular resistance, sympathetic activity and restored breathing. By contrast, the 5-HT4(a)R agonist RS67333 further decreased vascular resistance, HR and sympathetic activity, but partially rescued breathing. In conscious rats, administration of remifentanyl caused severe respiratory depression, a decrease in mean BP accompanied by pronounced bradyarrhythmia. 8-OH-DPAT restored breathing and prevented the bradyarrhythmia; however, BP and HR remained below baseline. In contrast, RS67333 further suppressed cardiovascular functions in vivo and only partially recovered breathing in some cases. The better recovery of μ-OR cardiorespiratory disturbance by 5-HT1AR than 5-HT4(a)R is supported by the finding that 5-HT1AR was more densely expressed in key brainstem nuclei for cardiorespiratory control compared with 5-HT4(a)R. We conclude that during treatment of severe pain, 5-HT1AR agonists may provide a useful tool to counteract opioid-mediated cardiorespiratory disturbances.
Keywords: cardiovascular control, neural control of breathing, apnoea, apneusis, cardio-sympathetic coupling, in vivo awake
1. Introduction
Serotonin (5-HT), a major neurotransmitter in the mammalian central and peripheral nervous system, is implicated in an impressive variety of physiological processes, ranging from visceral control to higher brain functions. At the cellular level, 5-HT binds to a variety of serotonin receptor (5-HTR) subtypes, most of which are metabotropic G protein-coupled receptors (Hoyer et al. 1994; Barnes & Sharp 1999; Richter et al. 2003). The signalling pathways of 5-HTR (except 5-HT2R and 5-HT3R) target adenylyl cyclase (AC) that regulates the formation of cellular cyclic adenosine 5′,3′-monophosphate (cAMP). In turn, the raised intracellular cAMP affects both the acute and long-term regulation of neuronal excitability.
The importance of 5-HT in numerous physiological processes has driven the design of selective agonists and antagonists to target a variety of 5-HTRs. Some of these have been used clinically and are of relevance to this report, for example, 5-HT4(a)R and 5-HT1AR agonists have been employed to treat central respiratory disturbances including apneusis or apnoeic periods (Lalley et al. 1994; Wilken et al. 1997; Sahibzada et al. 2000; El-Khatib et al. 2003; Manzke et al. 2003, 2009; Stettner et al. 2008; Yamauchi et al. 2008a,b). Although such neurological conditions causing central respiratory depression are relatively uncommon, these serotonergic agents may have a broader applicability in the treatment of opioid-mediated respiratory depression. Opioids are the most powerful analgesics available to treat pain in man, but their use is limited by side effects including the risk of fatal apnoea (Etches 1994; Goetz et al. 1994). The cellular mechanisms underlying the potentially beneficial effects of 5-HTR stimulation remain unexplained.
Recent evidence has provided insights into the cellular and network mechanisms involved in the 5-HT4(a)R- or 5-HT1AR-mediated recovery of μ-opioid receptor (μ-OR)-evoked respiratory depression (Manzke et al. 2003, 2009). Regarding 5-HT4(a)R, these are coexpressed with μ-OR on neurons in crucial regions of the respiratory network. While both receptor types share an AC-dependent intracellular signalling pathway, μ-OR activation decreases intracellular cAMP, causing reduced excitability of neurons in the respiratory network, while 5-HT4(a)Rs stimulate AC to elevate intracellular levels of cAMP, hence restoring respiratory neuronal function to reinstate breathing (Manzke et al. 2003). Importantly, 5-HT4(a)Rs were not expressed at the level of the spinal dorsal horn, which is the first relay for nociceptive afferents. Therefore, the beneficial analgesic effect of opioids can be maintained while breathing is protected by selective 5-HT receptor stimulation (Manzke et al. 2003). The mechanism underlying reactivation of breathing using 5-HT1AR agonists is described in detail by Manzke and co-workers in this issue (Manzke et al. 2009). In brief, both 5-HT1AR and μ-OR inhibit AC activity and thus a recovery of μ-OR-evoked respiratory depression using a 5-HT1AR agonist appears paradoxical. However, it now appears that 5-HT1AR is expressed predominantly on inhibitory glycinergic interneurons of the respiratory network, which have an essential function for respiratory rhythm generation and coordination of the pattern of cranial and spinal respiratory motor activity (Schmid et al. 1996; Pierrefiche et al. 1998; Büsselberg et al. 2001, 2003; Dutschmann & Paton 2002a,b; Ezure et al. 2003; Gomeza et al. 2003). Thus, inhibition of these respiratory glycinergic interneurons leads to network disinhibition, which reliably restores breathing after opioid-induced depression (Manzke et al. 2009). Since 5-HT1AR agonists per se have analgesic actions when applied at concentrations sufficient to compensate opioid-evoked respiratory depression (Guenther et al. 2009), opioid analgesia is maintained or even enhanced by 5-HT1AR agonists (Guenther et al. 2009; Manzke et al. 2009).
It is also clinically observed that systemic administration of opioids can cause cardiovascular depression typically featuring hypotension and bradycardia (e.g. Elliott et al. 2000). While 5-HT1AR- and 5-HT4(a)R-mediated recovery of opioid-mediated respiratory suppression is relatively well understood (Manzke et al. 2003, 2009), the actions on the cardiovascular system are not yet investigated. Therefore, the present study was designed to investigate the opioid depression of the cardiovascular system and whether 5-HTR activation is an effective rescue strategy. The studies reported herein have been performed in both the in situ-perfused working heart–brainstem preparation (WHBP) (Paton 1996) and conscious rats.
2. Material and methods
Two different preparations were used according to the methods described previously: the WHBP (Paton 1996) and radio-telemetry recordings of cardiorespiratory activity in awake animals (Waki et al. 2003, 2007).
(a). Working heart–brainstem preparation
Rats (n = 10) were deeply anaesthetized with halothane (AstraZeneca) such that the withdrawal responses to noxious pinching of the tail and paw were absent. The animals were transected caudal to the diaphragm, exsanguinated and submerged in a cooled Ringer solution. They were decerebrated at the precollicular level to make them insentient and skinned. The descending aorta was isolated and the lungs removed. Preparations were then transferred to a recording chamber. The descending aorta was cannulated and perfused retrogradely with a Ringer solution (in mM: NaCl, 125; NaHCO3, 24; KCl, 5; CaCl2, 2.5; MgSO4, 1.25; KH2PO4, 1.25; dextrose, 10) containing 1.25 per cent Ficoll (an oncotic agent; Sigma, St Louis, MO, USA) and a neuromuscular blocker (vecuronium bromide, 3–4 µg ml−1), using a roller pump (Watson-Marlow 502s) via a double-lumen cannula. The perfusion pressure (PP) was maintained in the range 50–70 mm Hg by adjusting the flow between 21 and 25 ml min−1 and by adding vasopressin (600–1200 pM, Sigma) to the perfusate, as described previously (Pickering & Paton 2006). The perfusate was gassed continuously with 5 per cent CO2 and 95 per cent O2, warmed to 31–32°C (temperature measured at the point of entry into the aorta) and filtered using a nylon mesh with 25 µm pore size.
(b). Phrenic and sympathetic chain recordings
Activity in the central end of the phrenic nerve (left) was recorded using a bipolar suction electrode. Its rhythmic ramping activity gave a continuous physiological index of preparation viability. The electrocardiogram was visible on the phrenic recording, and using a window discriminator, the R-wave was captured and the inter-R wave interval displayed as heart rate (HR). The efferent activity of the left thoracic sympathetic chain (tSNA) was recorded at the spinal level of T8–T12 via a bipolar suction electrode. All signals were amplified, bandpass filtered (0.5–5 kHz) and acquired in an A/D converter (CED 1401, Cambridge Electronic Design, CED, Cambridge, UK; 6 kHz sampling frequency) to a computer using Spike 2 software (CED). The frequency of phrenic discharge was determined by the time interval between consecutive phrenic bursts, and analysis of the thoracic sympathetic activity was carried out on the rectified and integrated signals (time constant of 100 ms). All the analyses were performed offline using Spike 2 software with custom-written scripts.
(c). Experimental protocols
Cardiorespiratory activity of the WHBP was recorded for at least 5 min during control and during the subsequent administration (to perfusate) of fentanyl (5–10 nM; μ-OR agonist) and either 8-OH-DPAT (1–5 µM; 5HT1aR agonist) or RS67333 (5–10 µM; 5HT4(a)R agonist). All preparations received naloxone (20–30 nM; μ-OR antagonist).
(d). Telemetry recordings in awake rats
We used a radio-telemetry system (Data Sciences International, USA) for recording blood pressure (BP). The system consists of three basic elements: (i) a transmitter for monitoring BP (TA11PA-C40); (ii) a receiver (RPC-1); and (iii) an adapter (R11CPA) with an ambient pressure monitor (APR-1) that produces analogue output signals of pulsatile BP. The system is calibrated relative to atmospheric pressure. Telemetered data were acquired by Hey-Presto (Mizuno Software, Miyagi, Japan) through an analogue input box (IP-810A, GigaTex Co., Ltd, Miyagi, Japan) and analogue I/O PC card (AD12-8(PM), CONTEC Co. Ltd, Japan), displayed on the computer screen and stored on a hard drive.
(e). Implantation of transmitter
Transmitters were implanted at least 7 days before any experimental protocol began. Rats (n = 8) were anaesthetized with an intramuscular injection of ketamine (60 mg kg−1) and medetomidine (250 µg kg−1). The level of anaesthesia was checked frequently by assessing limb withdrawal reflexes to a noxious paw pinch. A midline incision of the abdominal wall was made in the supine position, and the intestines were retracted to allow visualization of the abdominal aorta. The tip of the catheter (diameter 0.7 mm, thin-walled thermoplastic membrane, filled with anticoagulant gel) from the transmitter (TA11PA-C40; diameter 15 mm × 20 mm) was inserted into the abdominal aorta caudal to the root of the left renal artery. The catheter was held in the aorta with tissue adhesive (Vetbond, 3 M). The transmitter was secured by suturing it to the ventral wall of the abdominal cavity. Penicillin (1000 U) was injected intramuscularly. After the surgery, the anaesthesia was reversed with a subcutaneous injection of atipamezole (1 mg kg−1), and the rat returned to its home cage for recovery.
(f). Implantation of venous cannula
Forty-eight hours before any experimental protocol began, a double lumen catheter (inside diameter 0.28 mm, outside diameter 0.61 mm; Portex Limited, UK) was inserted into the right jugular vein under ketamine (60 mg kg−1) and medetomidine (250 µg kg−1) as described earlier. The opposite end was tunnelled subcutaneously and protruded through a small hole in the skin of the neck on the dorsal side.
(g). Recording of diaphragm electromyography (EMG)
After implantation of the venous cannula, the electrode for diaphragm EMG was implanted. The electrode consisted of two fine insulated stainless steel wires (diameter 0.25 mm, Unique Medical, Japan). The tips of the wire were bared of insulation (approx. 1 cm), and a small loop formed in one to avoid tissue damage. A midline incision (approx. 1 cm) of the abdominal wall was made just below the xyphloid process, and one wire inserted through this incision until the loop of the wire touched the diaphragm (approx. 3 cm). The wire was secured by suturing to the ventral abdominal wall. The tip of the other wire was placed subcutaneously and secured at the level of the sternum. The opposite ends of these two wires were tunnelled subcutaneously and protruded through a small hole in the skin of the neck on the dorsal side. After the surgery, the anaesthesia was reversed with a subcutaneous injection of atipamezole (1 mg kg−1) and the rat returned to its home cage for recovery. The electrical activity was amplified and filtered (500 to 5 kHz; Neurolog, Digitimer, UK). The EMG was acquired by Hey-Presto through an analogue input box and analogue I/O PC card as described earlier. Data were displayed on the computer screen and stored on the hard drive.
(h). Experimental protocols
After recording cardiorespiratory parameters during control (quiet) conditions, remifentanyl was infused intravenously (IV) using an infusion pump for all animals. Remifentanyl infusion commenced at 0.5 µg kg−1 min−1, and at intervals, the perfusion rate was increased in 0.5 µg kg−1 min−1 steps until the animals showed substantial suppression of cardiorespiratory function. This was accompanied by the development of an abnormal posture and opioid-induced rigidity. Disturbed cardiorespiratory functions were recorded for 5 min, followed by a single bolus injection (IV via other lumen of double lumen cannula) of either 1 mg kg−1 8-OH-DPAT or 5 mg kg−1 RS67333. In the rare case that the first injection of serotonergic agonists was ineffective in altering cardiorespiratory activity, a second bolus injection of the same dose was performed 5 min later. After recording the effects of 5-HT4(a)R and 5-HT1AR agonists, the infusion of remifentanyl was stopped. One to three minutes after stopping the remifentanyl infusion, animals regained normal posture and locomotor function. After 20–30 min, all animals showed full recovery of cardiorespiratory function and behaviour.
(i). Data analysis
HR and BP variability was analysed using the fast Fourier transform function of the Hey-Presto software (Waki et al. 2003). For each 5 min recording period, the systolic BP and beat-to-beat pulse intervals were converted into data points every 100 ms using a spline interpolation. According to Murasato et al. (1998), the magnitude of power was integrated in both the low-frequency (LF) band between 0.27 and 0.75 Hz and the high-frequency (HF) band (0.75–3.3 Hz). In addition, the respiratory frequency and inspiratory duration were analysed from the diaphragm-EMG signal. The variability of the instantaneous pulse pressure and heart beat interval was analysed during control and pharmacological manipulations.
(j). Histochemistry
Juvenile rats (P28–P32) were deeply anaesthetized with isoflurane (1-chloro-2,2,2-trifluoroethyl-difluoromethylether; Abbott, Wiesbaden, Germany). The animals were transcardially perfused with 50 ml 0.9 per cent sodium chloride, followed by 200 ml 4 per cent phosphate-buffered formaldehyde. The brainstem and spinal cord were removed and post-fixed for 4 h with the same fixative at 4°C including 30 per cent sucrose. Later, series of 40 µm thick transverse brainstem sections were cut using a cryostat (Frigocut, Reichert-Jung, Germany).
(k). Applied antisera
The polyclonal anti-5-HT1AR antibody derived from guinea pigs was obtained from Chemicon (Catalogue no. AB5406, Temecula, USA). Specificity of the 5-HT4(a)R home-made affinity-purified polyclonal rabbit antibody was published previously (Manzke et al. 2003).
(l). Peroxidase–anti-peroxidase (PAP) staining
The intrinsic peroxidase activity was blocked with hydrogen peroxide–methanol (1 : 100) for 45 min at room temperature (RT) in the dark. After washing, sections were permeabilized with 0.2 per cent Triton X-100 for 30 min and directly transferred into phosphate buffered saline (PBS) containing 5 per cent bovine serum albumin for 1 h at RT to block non-specific binding sites. Sections were incubated overnight at 4°C in primary antibody solution at 1 : 1000 dilution. Secondary antibodies (diluted 1 : 100; DAKO, Denmark) were applied for 1 h at RT. After incubation in PAP solution (1 : 100; RT; DAKO) for 1 h, sections were pre-incubated with freshly prepared filtered diaminobenzidine (DAB) solution (120 µl DAB (75 mg DAB dissolved in 1.5 ml 0.1 M phosphate buffer), 240 µl 1.25% CoCl2 and 240 µl 5% Ni(NH4)2SO4 diluted in 29.4 ml PBS) for 10 min at RT. The enzymatic reaction was started by adding 10 µl of 35 per cent H2O2 to 10 ml DAB solution and stopped with PBS after 1–5 min. DAB-stained sections were mounted onto gelatin-coated slides, dehydrated (2 × 50% ethanol, 2 × 80% ethanol and 2 × 99.9% ethanol, 5 min each) and coverslipped with mounting medium (DePeX from Serva, Heidelberg, Germany). Analysis was performed with the digital microscope Coolscope (Nikon, Melville, NY, USA). Images were taken at 2048 × 2048 dpi, imported into Adobe Photoshop CS3 and were digitally adjusted for brightness and contrast. The distribution of the labelled cells was plotted using a Neurolucida system (MBF Bioscience, Williston, VT, USA).
3. Results
The effects of the 5-HT1AR and 5-HT4(a)R agonists, 8-OH-DPAT and RS67333, respectively, on the fentanyl-evoked cardiorespiratory disturbances were investigated in a total of 18 rats (n = 10 WHBP and n = 8 in vivo experiments).
(a). Compensation of opioid-evoked cardiorespiratory disturbances in the working heart–brainstem preparation in situ
(i). 5-HT1A receptor agonist 8-OH-DPAT (n = 5)
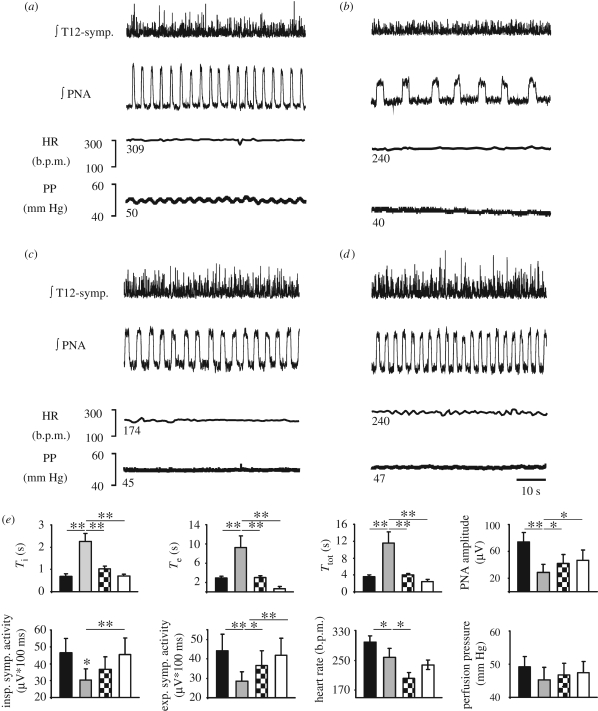
Systemic application of fentanyl (10 nM; figure 1a,b,e and table 1) caused significant prolongation of the duration of the inspiratory phase (Ti; p < 0.01), expiratory phase (Te; p < 0.01) and consequently of the respiratory cycle length (Ttot; p < 0.01), while PNA amplitude was reduced (p < 0.01). These changes were accompanied by bradycardia (figure 1e and table 1), and reductions in vascular resistance reflected by small falls in PP. Furthermore, fentanyl reduced sympathetic chain activity (SCA) (figure 1a,b,e and table 1) during both the inspiratory (SCAi; p < 0.05) and expiratory (SCAe; p < 0.01) intervals. Subsequent application of 8-OH-DPAT (figure 1c,e) significantly recovered Ti (p < 0.01), Te (p < 0.01), Ttot (p < 0.01), PNA amplitude (p < 0.05) and SCAe (p < 0.05). However, PP and SCAi showed trends in recovery only (table 1). By contrast, HR was further depressed after 8-OH-DPAT application (p < 0.05). Finally, subsequent application of naloxone to prove that the 8-OH-DPAT effects were not due to a time-dependent weakening action of fentanyl restored, at least partially, most of the recorded cardiovascular parameters to baseline values and increased breathing activity to levels above baseline (figure 1d,e and table 1).
Figure 1.
Cardiorespiratory and sympathetic activity recorded in a WHBP during control (a) and subsequent application of fentanyl (10 nM) (b). The traces illustrate that fentanyl (μ-OR agonist) caused severe respiratory depression accompanied by prolongation of the inspiratory phrenic burst (PNA), decreased tSNA activity (T12-symp.), fall in heart rate (HR) and decreased vascular resistance indicated by the drop in PP. The subsequent application of 8-OH-DPAT (5 µM) (5-HT1AR agonist) in the presence of fentanyl significantly increased all recorded cardiorespiratory parameters with the exception of HR (c). Application of the μ-OR antagonist naloxone (30 nM) (d), increased PNA and T12-symp. above baseline levels and further recovered HR and PP. (e) Group data and statistical significance (*p < 0.05, **p < 0.01, repeated-measures ANOVA, followed by Fisher's LSD post hoc) of subsequent drug applications. Ti, time of inspiration; Te, time of expiration; Ttot, total respiratory cycle length. Black bar, control; grey bar, remifentanyl; black and white checked bar, RS67333; white bar, naloxone.
Table 1.
Summary of numerical data of the in situ experiments and of 8-OH-DPAT effects in conscious rat. *p < 0.05; **p < 0.01; ***p < 0.001. Ttot, total respiratory cycle length; Ti, time of inspiration; Te, time of expiration; PNA ampl., phrenic nerve discharge amplitude; SCAi, sympathetic chain activity during inspiration; SCAe, sympathetic chain activity during expiration; PP, perfusion pressure; HR, heart rate; RR, respiratory rate; MABP, mean arterial blood pressure; sBRG, spontaneous baroreceptor reflex gain; HF, high-frequency pulse interval variations of BP, high–low pulse interval variations of BP; Var. HBI, variability of instantaneous heart beat interval; Var. PPF, variability of instantaneous pulse pressure fluctuation of BP.
| control | fentanyl | 8-OH-DPAT | naloxone | |
|---|---|---|---|---|
|
in situ experiments (mean ± s.d.) | ||||
| Ttot (s) | 3.6 ± 0.4 | 11.5 ± 2.6** | 4 ± 0.3** | 2.5 ± 0.4** |
| Ti (s) | 0.7 ± 0.1 | 2.3 ± 0.4** | 1 ± 0.1** | 0.7 ± 0.08** |
| Te (s) | 2.9 ± 0.3 | 9.3 ± 2.4** | 3 ± 0.4** | 1.8 ± 0.4** |
| PNA ampl. (µV) | 74.1 ± 14 | 28.7 ± 12.3** | 42 ± 13.7** | 46.9 ± 15* |
| SCAi (µV s) | 46. 5 ± 8.6 | 30.4 ± 6.5* | 36.8 ± 7.4a | 45.4 ± 9.8** |
| SCAe (µV s) | 44.3±8.4 | 28.4 ± 4.9** | 36.5 ± 7.8* | 42.0 ± 8.8** |
| PP (mm Hg) | 49.1 ± 3.1 | 45.2 ± 3.8a | 46.7 ± 3.5a | 47.4 ± 3.4a |
| HR (b.p.m.) | 299 ± 16.5 | 258 ± 24a | 201 ± 15.6* | 238 ± 12a |
| control | fentanyl | RS67333 | naloxone | |
| Ttot (s) | 3.8 ± 0.2 | 9.2 ± 0.7** | 5 ± 0.8** | 3.2 ± 0.7** |
| Ti (s) | 0.8 ± 0.1 | 1.9 ± 0.1** | 1.6 ± 0.2* | 0.8 ± 0.1** |
| Te (s) | 3 ± 0.2 | 7.3 ± 0.6** | 3.5 ± 0.6* | 2.4 ± 0.6** |
| PNA ampl. (µV) | 54.1 ± 3.4 | 38.6 ± 3.5** | 37 ± 5.2a | 50.3 ± 10* |
| SCAi (µV s) | 48.3 ± 12.5 | 36.6 ± 8.5a | 34.8 ± 9.5a | 45.0 ± 11.2a |
| SCAe (µV s) | 42.1 ± 11 | 37.2 ± 8.5* | 33.3 ± 9.5a | 40.1 ± 9.6a |
| PP (mm Hg) | 68 ± 8.8 | 58 ± 6.7a | 50 ± 6.7a | 58.8 ± 9.6a |
| HR (b.p.m.) | 305 ± 7.3 | 290 ± 16a | 248 ± 13* | 247 ± 25a |
|
in vivo experiments (mean ± s.d.) | ||||
| control | remifentanyl | 8-OH-DPAT | remifent. off | |
| RR (breaths min−1) | 70.2 ± 8.1 | 34.8±13.7** | 52 ± 16.7* | 60.5 ± 2.8** |
| Ti (s) | 0.12 ± 0.06 | 0.26 ± 0.08** | 0.15 ± 0.06** | 0.1 ± 0.08** |
| MABP (mm Hg) | 93.5 ± 3.2 | 78.1 ± 12** | 67 ± 10.7a | 86.1 ± 6.8** |
| HR (b.p.m.) | 353.8 ± 13.7 | 280 ± 88* | 277 ± 58a | 311 ± 22.8** |
| sBRG (ms mm Hg−1) | 1.28 ± 0.22 | 2.7 ± 0.5** | 3.5 ± 0.6a | 1.2 ± 0.3** |
| HF (ms2) | 12.9 ± 2.1 | 80 ± 11.6*** | 58 ± 16.3a | 22.4 ± 10.5** |
| HF–LF (ms2) | 0.46 ± 0.09 | 1.08 ± 0.07* | 0.84 ± 0.2a | 0.27 ± 0.06** |
| Var. HBI (ms) | 2.18 ± 0.2 | 103.8 ± 7.2*** | 55.8 ± 16.9* | 10.5 ± 5*** |
| Var. PPF (mm Hg) | 5.3 ± 0.4 | 139.5 ± 14.3*** | 53.5 ± 19.1** | 9.1 ± 3.2** |
aNot significant.
(ii). Partial 5-HT4(a) receptor agonist RS67333 (n = 5)
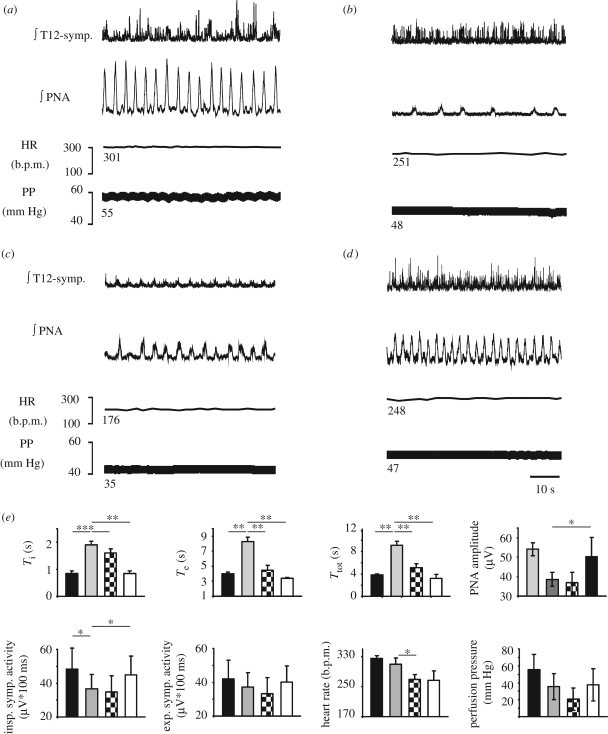
As mentioned earlier, systemic application of fentanyl (10 nM) caused significant prolongation of Ti (p < 0.01), Te (p < 0.01) and consequently of Ttot (p < 0.01), while PNA amplitude was reduced (p < 0.01; figure 2a,b,e; table 1). Accompanying cardiovascular changes (figure 2 and table 1) included bradycardia, and falls in vascular resistance and SCA (figure 2a, b,e and table 1). The subsequent application of the 5-HT4(a)R agonist partially recovered Ti (p < 0.05), Te (p < 0.01) and Ttot (p < 0.01). However, RS67333 had no effects on PNA amplitude, PP and inspiratory or expiratory SCA (table 1). In addition, HR was further suppressed (p < 0.05). Application of naloxone partially restored most of the recorded cardiovascular parameters to baseline values and increased breathing activity to levels above baseline (figure 2d,e and table 1).
Figure 2.
Illustration of the cardiorespiratory and sympathetic activity recorded in a WHBP during control (a) and during subsequent application of fentanyl (10 nM) (b). Comparable to figure 1, fentanyl caused severe cardiorespiratory depression. The subsequent application of RS67333 (10 µM) (5-HT4(a)R agonist) in the presence of fentanyl restored PNA, but further significantly decreased T12-symp, HR and PP (c). Application of naloxone (30 nM) (d), increased PNA and T12-symp. activity above baseline and partially recovered HR and PP. For abbreviations, see figure 1. Importantly, the HR and PP clearly remained below baseline after blocking fentanyl effects with naloxone. This indicates negative peripheral effects of RS67333 in contrast to 8-OH-DPAT (cf. figure 1). (e) Group data and statistical significance of subsequent drug applications (*p < 0.05, **p < 0.01, repeated-measures ANOVA, followed by Fisher's LSD post hoc). Black bar, control; grey bar, remifentanyl; black and white checked bar, RS67333; white bar, naloxone.
(b). Compensation of opioid-evoked cardiorespiratory disturbances in awake rats
(i). 5-HT1A receptor agonist 8-OH-DPAT (n = 4)
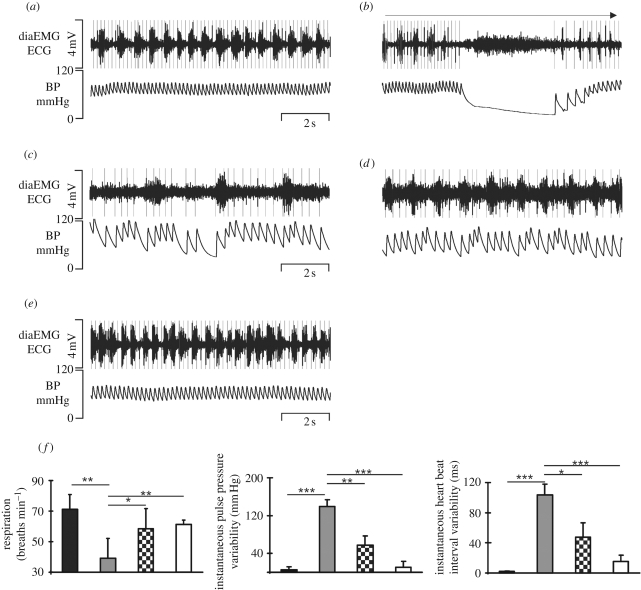
Continuous IV infusion of remifentanyl (0.5–3 µg kg−1 min−1) led to both a drop in respiratory rate (p < 0.01) and an increase in Ti (p < 0.01; figure 3 and table 1). The mean arterial blood pressure (MABP) showed a downward trend and HR dropped significantly (p < 0.05). Remifentanyl also evoked bradyarrhythmia and large spontaneous fluctuations in MABP (figure 3a–c). Spontaneous baroreceptor reflex gain (sBRG) increased (p < 0.01), while both HF and HF–LF pulse interval variations, which are indicative of parasympathetic and sympathetic activity, respectively, increased after remifentanyl (p < 0.001; p < 0.05). Subsequent IV bolus injection of 8-OH-DPAT (1 mg kg−1) with continuous remifentanyl infusion immediately compensated the remifentanyl-evoked bradyarrhythmia (figure 3—compare c,d) and stabilized the fluctuations in MABP as well as restoring breathing (figure 3 and table 1). Breathing frequency (p < 0.05) and Ti (p < 0.05) significantly recovered. Although HR, MABP, HF and HF–LF pulse interval variations showed no significant effects (figure S1, electronic supplementary material; table 1), a significant recovery of fentanyl-induced variability of the instantaneous mean pulse pressure and heart beat interval was observed after 8-OH-DPAT application (figure 3f and table 1: both p < 0.05). Termination of the remifentanyl infusion restored all cardiorespiratory parameters back to baseline levels (figure 3 and table 1; figure S1, electronic supplementary material).
Figure 3.
Radio-telemetry recordings of electrocardiogram (ECG), diaphragm EMG (diaEMG) and arterial BP in a conscious rat during control (a). Initial response to remifentanyl infusion (b) and cardiorespiratory activity 3 min after the start of remifentanyl infusion (c). Cardiorespiratory activity 2 min after a bolus injection of 8-OH-DPAT during continuous remifentanyl infusion (d). Note the recovery of diaEMG activity and the decrease in the severity of the bradyarrhythmia illustrated in the BP trace (cf. c,d). Full recovery of cardiorespiratory activity 5 min after remifentanyl infusion (e). Group data of beneficial effects of 8-OH-DPAT and breathing frequency and variability of instantaneous mean pulse pressure and heart beat interval are illustrated in (f). *p < 0.05, **p < 0.01, ***p < 0.001, repeated-measures ANOVA, followed by Fisher's LSD post hoc. Black bar, control; grey bar, 2 min after remifentanyl; black and white checked bar, 2 min after 8-OH-DPAT; white bar, 5 min after remifentanyl stop.
(ii). Partial 5-HT4(a) receptor agonist RS67333 (n = 4)
In this series, remifentanyl dropped respiratory frequency from 61 ± 5.4 to 40 ± 7.6 breaths min−1 (p < 0.05). MABP decreased from 94 ± 4 to 63 ± 5 mm Hg (p < 0.01) and HR fell from 364 ± 25 to 231 ± 86 b.p.m. (p < 0.05). However, sBRG increased from 0.86 ± 0.4 to 4.3 ± 0.6 ms mm Hg−1 (p < 0.01). HF and HF–LF pulse variation also increased after remifentanyl infusion (HF: from 16.1 ± 9 to 82 ± 30 ms2, p < 0.05; HF–LF: from 0.49 ± 0.07 to 1.08 ± 0.49 ms2, p < 0.05). Subsequent IV bolus injection of RS67333 (1 mg kg−1) with continuous remifentanyl infusion had no positive effect on any of the cardiorespiratory parameters analysed (figure S1, electronic supplementary material). As in the previous experiments, termination of the remifentanyl infusion restored cardiorespiratory activity partially (figure S1, electronic supplementary material).
(c). Control experiments
We tested the effects of either 8-OH-DPAT (n = 4, 1 mg kg−1) or RS67333 (n = 4, 5 mg kg−1) alone on cardiorespiratory activity in the absence of remifentanyl in conscious rats. 8-OH-DPAT had no significant effect on breathing rate (74 ± 12 versus 65 ± 3.6 breaths min−1), HR (388 ± 29 versus 329 ± 27 b.p.m.), HF of pulse interval (20.3 ± 11.9 versus 16.5 ± 2.6 ms2) and HF–LF of pulse interval (0.56 ± 0.2 versus 0.49 ± 0.1 ms2), but decreased MABP (from 95 ± 3 to 85 ± 7 mm Hg, p < 0.05) and increased sBRG (from 0.72 ± 0.4 to 1.2 ± 0.4 ms mm Hg−1, p < 0.01). In stark contrast, RS67333 injections produced no measurable effects on any of the cardiorespiratory variables measured.
(d). Distribution of 5-HT1A and 5-HT4(a) receptors within the pontomedullary brainstem
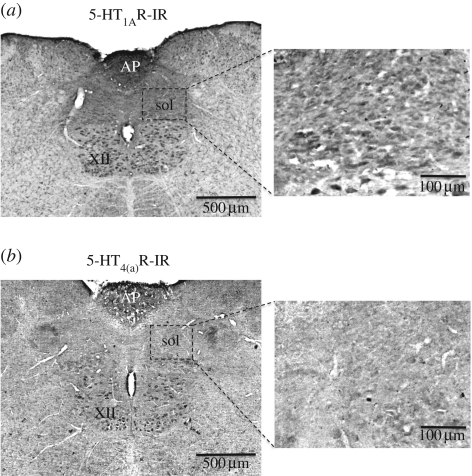
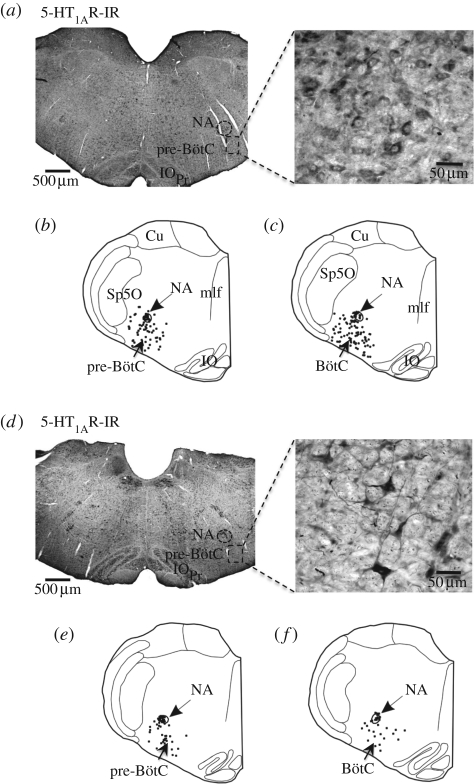
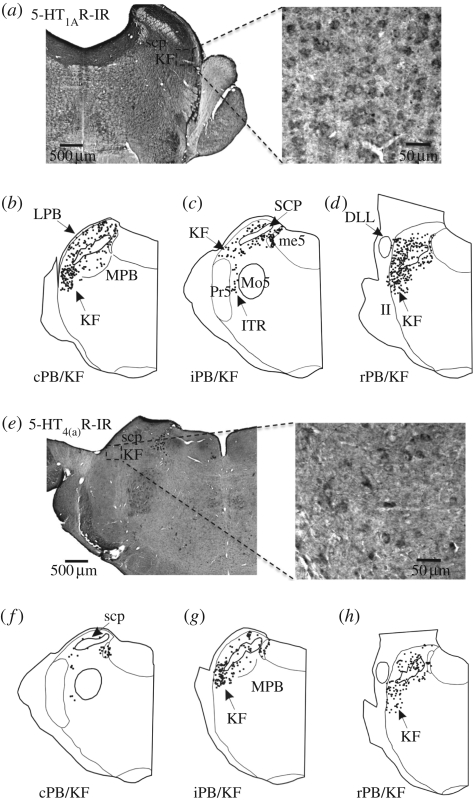
The expression and distribution of 5-HT1AR and 5-HT4(a)R at the level of nucleus tractus solitarii (NTS), pre-Bötzinger complex (pre-BötC), Bötzinger complex (BötC) including the C1 region, the pontine parabrachial complex (PB) and Kölliker–Fuse nucleus (KF) are summarized in figures 4–6. A comparative analysis of the expression profile revealed a denser expression of 5-HT1AR in all key cardiorespiratory control nuclei compared with 5-HT4(a)R. For example, at the level of the medial NTS (figure 4), a crucial relay for baro- and chemoreceptor afferents, 5-HT4(a)R expression is sparse while 5-HT1AR was abundant. In the pre-BötC and BötC including the C1 region, 5-HT4(a)R expression was strong (figure 5), but 5-HT1AR was more widely expressed (figure 6). Finally, in the pontine PB and, in particular, the KF region, 5-HT1ARs were relatively dense compared with those of 5-HT4(a)R.
Figure 4.
Expression pattern of (a) 5-HT1AR and (b) 5-HT4(a)R in the nucleus of the solitary tract (Sol) at the level of the area postrema (AP). The insets in (a) and (b) show labelled neurons at a higher magnification. XII, hypoglossal motor nucleus; IR, immunoreactivity.
Figure 5.
(a) 5-HT1AR expression pattern in the pre-BötC. The schematics (b,c) represent 5-HT1AR immunoreactivity (-IR) within the pre-BötC (b) and BötC (c). (d) The corresponding expression pattern for the 5-HT4(a)R in the pre-BötC. The schematics (e–f) illustrate 5-HT4(a)R-IR within the pre-BötC (e) and BötC (f). The insets in (a) and (d) show labelled neurons at a higher magnification. BötC, Bötzinger complex; NA, nucleus ambiguus; pre-BötC, pre-Bötzinger complex; IOPr, principal nucleus of the inferior olive; Sp5l, interpolar spinal trigeminal nucleus.
Figure 6.
(a,e) Both 5-HT1AR and 5-HT4(a)R are expressed in neurons of the parabrachial complex. Within the nucleus KF, the 5-HT4(a)R expression is weak compared with 5-HT1ARs. The plots (b–d) represent 5-HT1ARs immunoreactivity (IR) within the caudal, intermediate or rostral PB/KF complex (cPB/KF, iPB/KF or rPB/KF). (f–h) Corresponding expression pattern for the 5-HT4(a)Rs. KF, Kölliker–Fuse nucleus; Mo5, motor trigeminal nucleus; me5, mesencephalic trigeminal tract; Pr5, principal sensory trigeminal nucleus; scp, superior cerebellar peduncle; DLL, dorsal nucleus of the lateral lemniscus; ITR, intertrigeminal region; MPB, medial parabrachial nucleus; LPB, lateral parabrachial nucleus; II, intermediate interstitial nucleus of the medial longitudinal fasciculus.
4. Discussion
Our findings indicate that activation of serotonergic receptor subtypes can rescue the cardiovascular and respiratory depression induced by μ-opioid activation. Our data indicate that 5-HT1AR appears more efficacious than 5-HT4(a)R in restoring central respiratory activity and disturbances in HR and BP. This may relate to the finding that 5-HT1AR was more densely expressed than 5-HT4(a)R in brainstem regions known to control cardiorespiratory function.
The potency of 5-HT1AR and 5-HT4(a)R agonists in recovery from opioid-induced respiratory depression was demonstrated previously in anaesthetized rodents in vivo (Sahibzada et al. 2000; Manzke et al. 2003). The present study (in vivo and in situ) confirms these findings for the 5-HT1A agonist, but not for the 5-HT4(a)R agonist used here. The discrepancy in the present data with that reported earlier for 5-HT4(a)R may relate to the fact that we used a different agonist. Previously, the 5-HT4(a)R agonist, BIMU-8, stabilized breathing in the anaesthetized rat (Manzke et al. 2003), whereas RS67333 used in the present study failed to recover breathing following opioid-induced depression. These differences may be owing to different pharmacological profiles of the agonists potentially targeting cell-specific splice variants of 5-HT4(a)R expressed in different tissues (Mine et al. 1997; De Maeyer et al. 2008). Additionally, RS67333 may not penetrate the blood–brain barrier optimally under in vivo conditions. Finally, the preparations used in the present study were both unanaesthetized, whereas those used previously were anaesthetized (Manzke et al. 2003). Interestingly, in humans, both the 5-HT1AR agonist, buspirone, and the 5-HT4(a)R agonist, mosapride, were found to be ineffective in compensating opioid-evoked respiratory depression (Lötsch et al. 2005; Oertel et al. 2007). The ineffectiveness of buspirone in humans is surprising and disappointing: first, in humans, buspirone has been used to reverse major respiratory arrhythmias including apneusis (Wilken et al. 1997; Saito et al. 1999; El-Khatib et al. 2003; Richter et al. 2003; O'Sullivan et al. 2008). Second, in the rat, both recent evidence (Sahibzada et al. 2000; Guenther et al. 2009; Manzke et al. 2009) and data from the present study show a potent recovery from opioid-evoked respiratory and, in part, cardiovascular suppression with the administration of 8-OH-DPAT. The reason(s) for this remains unresolved, but may be species related in terms of receptor subunit differences and/or their distribution on cardiorespiratory neurons. Finally, BIMU-8, which stabilized breathing in anaesthetized rats (Manzke et al. 2003), has not been tried in humans.
(a). Cardiovascular effects following 5-HT1A and 5-HT4(a) receptor activation
The serotonergic system exerts powerful influences on both the central (Jordan 2005) and peripheral cardiovascular systems (Kaumann & Levy 2006). Thus, a major concern for the use of serotonergic agonists in the treatment of centrally mediated respiratory dysfunction is the possible augmentation of the deleterious effects on cardiovascular activity. Here, we showed that the 5-HT1AR agonist administered alone lowered arterial pressure and greatly enhanced baroreceptor reflex gain (suggesting increased excitability of cardiac vagal motoneurons, Gilbey et al. 1984), while 5-HT4(a)R activation failed to alter baseline parameters. Indeed, the application of RS67333 showed additional deleterious effects on the opioid-evoked cardiovascular disturbance both in situ and in vivo. Also, the administration of 8-OH-DPAT enhanced the opioid-evoked bradycardia, which is consistent with previous data indicating activation of cardiac vagal motoneurons by this serotonin receptor subtype (for review, see Jordan 2005). However, in conscious animals, 8-OH-DPAT helped to reduce the occurrence of the opioid-evoked bradyarrhythmia and arterial pressure instability, although HR and BP levels remained below control (figure 4). In line with previous reports (Jordan 2005), we observed that injection of 8-OH-DPAT caused a mild bradycardia, increased baroreceptor gain and decreased BP, indicating that centrally acting 8-OH-DPAT can increase tonic cardiac vagal tone. In turn, increased tonic vagal tone and improved HR variability prevent cardiac arrhythmias and sudden cardiac death (La Rovere et al. 1998; Villareal et al. 2002). Moreover, as this agonist rescued breathing, this may have re-enforced respiratory–autonomic coupling to stabilize HR; the latter is supported from our in situ experiments in which phrenic-triggered coupling to sympathetic activity was increased.
(b). Differences in the expression profile of 5-HT1A and 5-HT4(a) receptors within cardiovascular-respiratory brainstem regions
The μ-OR is abundantly expressed in the entire ponto-medullary respiratory network (Mansour et al. 1994; Haji et al. 2003; Lonergan et al. 2003; Manzke et al. 2003). Therefore, the respiratory depression that accompanies opioid administration (which includes μ-OR activation) in the treatment of severe pain can involve actions within the brainstem respiratory network. Our comparative immunohistochemical analyses of the expression pattern of 5-HT1AR and 5-HT4(a)R revealed obvious differences. In all parts of the ponto-medullary respiratory network investigated, which included the afferent integration nucleus (NTS) and the pontine PB/KF complex (figures 5 and 6), the 5-HT1ARs showed more dense expression. Both NTS and PB/KF have essential functions in the control of inspiratory duration (for review, see Kubin et al. 2006; Dutschmann et al. 2008). This is relevant since in both in vivo and in situ experiments, μ-OR activation depressed respiratory rate and caused apneusis, a pathological prolongation of the inspiratory phase (figures 1–3). From the lack of direct opioid-mediated effects on medullary respiratory neurons, it was concluded that suppressive actions of opioids essentially include neurons upstream to medullary respiratory neurons (Lalley 2006). We suggest that these upstream respiratory neurons involve those in the PB/KF. Consistent with this proposal is that blockade or lesion of PB/KF triggers apneusis, including the loss of post-inspiratory activity (Dutschmann & Herbert 2006; Smith et al. 2007). Indeed, the proposed mechanism of 8-OH-DPAT-mediated recovery from opioid-evoked respiratory depression involves the re-instatement of post-inspiratory activity (Manzke et al. 2009), which is known to be present not only in medullary respiratory groups (Büsselberg et al. 2001, 2003; Dutschmann & Paton 2002a,b; Ezure et al. 2003; Smith et al. 2007) but also in the PB/KF (Dick et al. 1994; Mörschel & Dutschmann 2009). Furthermore, a recent publication suggested that the pons, including the PB/KF, is crucial for the coupling of both cardiac vagal and sympathetic activity to centrally generated respiratory drive (Baekey et al. 2008). Taken together, these data could explain why activation of 5-HT1ARs stabilizes the μ-OR-evoked cardiorespiratory disturbance. Thus, we propose that activation of 5-HT1AR, particularly those in the pons, reverses the μ-OR-mediated apneusis and cardiovascular dysfunction (bradyarrhythmia and arterial pressure instability) by reinstating post-inspiratory activity that underlies much of the respiratory-related coupling to autonomic motor outflows (figures 1–3; Bainton et al. 1985; Baekey et al. 2008; Zoccal et al. 2008; Simms et al. 2009).
Based on the greater density of 5-HT1AR relative to 5-HT4(a)R in brainstem cardiovascular and respiratory control regions, we suggest that this explains the more efficacious recovery of the opioid-induced cardiorespiratory depression by 8-OH-DPAT than RS67333. The abundant expression of the 5-HT1ARs in the ponto-medullary brainstem enables 8-OH-DPAT to produce a global network disinhibition, which was shown to be the underlying mechanism to recover breathing after opioid poisoning (for details, see Manzke et al. 2009). Indeed, in respiratory nuclei of the medulla oblongata, 5-HT1ARs are predominantly expressed on small-sized glycinergic interneurons (Manzke et al. 2009). However, 5-HT4(a)R expression is confined to larger neurons (figure 4). As shown previously, 5-HT4(a)R-mediated recovery of opioid-induced respiratory depression is based on a mechanism different from that of 5-HT1ARs. 5-HT4(a)Rs antagonize the μ-OR-mediated decrease in intracellular cAMP through activation of AC (Lalley et al. 1997; Manzke et al. 2003). The lower expression profile of 5-HT4(a)R in the brainstem cardiorespiratory network can limit the efficacy of 5-HT4(a)R, particularly if partial agonists such as RS67333 are used, as is the case herein.
(c). Summary and clinical implications
The present study revealed that the 5-HT1AR agonist 8-OH-DPAT evoked a potent recovery of breathing, following opioid-induced depression in the unanaesthetized decerebrated in situ rat preparation and in conscious animals. In addition, stabilization of cardiovascular activity was observed, which we propose relates, in part, to a re-inforcement of pontine respiratory-cardiovascular autonomic coupling. Despite the finding that 8-OH-DPAT has undesired effects on both central and peripheral cardiovascular activity, the beneficial effects of 5-HT1AR activation may be a useful strategy to overcome opioid-evoked cardiorespiratory disturbances. Clinically, the 5-HT1AR agonist buspirone is available and broadly used as an anti-anxiolytic drug. Results from investigations in the rat demonstrate identical respiratory responses to 8-OH-DPAT and buspirone (Sahibzada et al. 2000; Manzke et al. 2009). Further, buspirone has been used effectively in the treatment of apneustic disturbances in man (Wilken et al. 1997; Saito et al. 1999; El-Khatib et al. 2003; Richter et al. 2003; O'Sullivan et al. 2008). We suggest that clinically the 5-HT1AR agonist such as buspirone could serve as a pharmacological intervention to avoid and/or recover μ-OR-evoked cardiorespiratory disturbances.
Acknowledgements
All procedures conformed to the UK Animals (Scientific Procedures) Act 1986 and were approved by the University of Bristol Ethical Review Committee.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Brainstem neural networks vital for life’.
References
- Baekey D. M., Dick T. E., Paton J. F.2008Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp. Physiol. 93, 803–816 (doi:10.1113/expphysiol.2007.041400) [DOI] [PubMed] [Google Scholar]
- Bainton C. R., Richter D. W., Seller H., Ballantyne D., Klein J. P.1985Respiratory modulation of sympathetic activity. J. Auton. Nerv. Syst. 12, 77–90 (doi:10.1016/0165-1838(85)90041-4) [DOI] [PubMed] [Google Scholar]
- Barnes N. M., Sharp T.1999A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152 (doi:10.1016/S0028-3908(99)00010-6) [DOI] [PubMed] [Google Scholar]
- Büsselberg D., Bischoff A. M., Paton J. F., Richter D. W.2001Reorganisation of respiratory network activity after loss of glycinergic inhibition. Pflugers Arch. 441, 444–449 (doi:10.1007/s004240000453) [DOI] [PubMed] [Google Scholar]
- Büsselberg D., Bischoff A. M., Richter D. W.2003A combined blockade of glycine and calcium-dependent potassium channels abolishes the respiratory rhythm. Neuroscience 122, 831–841 (doi:10.1016/j.neuroscience.2003.07.014) [DOI] [PubMed] [Google Scholar]
- Chamberlin N. L., Mansour A., Watson S. J., Saper C. B.1999Localization of μ-opioid receptors on amygdaloid projection neurons in the parabrachial nucleus of the rat. Brain Res. 827, 198–204 (doi:10.1016/S0006-8993(99)01168-3) [DOI] [PubMed] [Google Scholar]
- De Maeyer J. H., Lefebvre R. A., Schuurkes J. A.20085-HT4 receptor agonists: similar but not the same. Neurogastroenterol. Motil. 20, 99–112 [DOI] [PubMed] [Google Scholar]
- Dick T. E., Bellingham M. C., Richter D. W.1994Pontine respiratory neurons in anesthetized cats. Brain Res. 636, 259–269 (doi:10.1016/0006-8993(94)91025-1) [DOI] [PubMed] [Google Scholar]
- Dutschmann M., Herbert H.2006The Kölliker–Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur. J. Neurosci. 24, 1071–1084 (doi:10.1111/j.1460-9568.2006.04981.x) [DOI] [PubMed] [Google Scholar]
- Dutschmann M., Paton J. F.2002aGlycinergic inhibition is essential for co-ordinating cranial and spinal respiratory motor outputs in the neonatal rat. J. Physiol. 543, 643–653 (doi:10.1113/jphysiol.2001.013466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M., Paton J. F.2002bInhibitory synaptic mechanisms regulating upper airway patency. Respir. Physiol. Neurobiol. 131, 57–63 (doi:10.1016/S1569-9048(02)00037-X) [DOI] [PubMed] [Google Scholar]
- Dutschmann M., Mörschel M., Reuter J., Zhang W., Gestreau C., Stettner G. M., Kron M.2008Postnatal emergence of synaptic plasticity associated with dynamic adaptation of the respiratory motor pattern. Respir. Physiol. Neurobiol. 164, 72–79 (doi:10.1016/j.resp.2008.06.013) [DOI] [PubMed] [Google Scholar]
- El-Khatib M. F., Kiwan R. A., Jamaleddine G. W.2003Buspirone treatment for apneustic breathing in brain stem infarct. Respir. Care 48, 956–958 [PubMed] [Google Scholar]
- Elliott P., O’Hare R., Bill K. M., Phillips A. S., Gibson F. M., Mirakhur R. K.2000Severe cardiovascular depression with remifentanil. Anesth. Analg. 91, 58–61 (doi:10.1097/00000539-200007000-00011) [DOI] [PubMed] [Google Scholar]
- Etches R. C.1994Respiratory depression associated with patient-controlled analgesia: a review of eight cases. Can. J. Anaesth. 41, 125–132 (doi:10.1007/BF03009805) [DOI] [PubMed] [Google Scholar]
- Ezure K., Tanaka I., Kondo M.2003Glycine is used as a transmitter by decrementing expiratory neurons of the ventrolateral medulla in the rat. J. Neurosci. 23, 8941–8948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbey M. P., Jordan D., Richter D. W., Spyer K. M.1984Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in the cat. J. Physiol. 356, 65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz A. M., Rogers P. L., Schlichtig R., Muder R. R., Diven W. F., Prior R. B.1994Adult respiratory distress syndrome associated with epidural fentanyl infusion. Crit. Care Med. 22, 1579–1583 [PubMed] [Google Scholar]
- Gomeza J., Ohno K., Hulsmann S., Armsen W., Eulenburg V., Richter D. W., Laube B., Betz H.2003Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron 40, 797–806 (doi:10.1016/S0896-6273(03)00673-1) [DOI] [PubMed] [Google Scholar]
- Guenther U., Manzke T., Wrigge H., Dutschmann M., Zinserling J., Putensen C., Hoeft A.2009Counteraction of opioid-induced ventilatory depression by serotonin 1A-agonist 8-OH-DPAT does not antagonize antinociception in rat in situ and in vivo. Anesth. Analg. 108, 1169–1176 (doi:10.1213/ane.0b013e318198f828) [DOI] [PubMed] [Google Scholar]
- Haji A., Yamazaki H., Ohi Y., Takeda R.2003Distribution of mu receptors in the ventral respiratory group neurons; immunohistochemical and pharmacological studies in decerebrate cats. Neurosci. Lett. 351, 37–40 (doi:10.1016/S0304-3940(03)00951-0) [DOI] [PubMed] [Google Scholar]
- Hoyer D., Clarke D. E., Fozard J. R., Hartig P. R., Martin G. R., Mylecharane E. J., Saxena P. R., Humphrey P. P.1994International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol. Rev. 46, 157–203 [PubMed] [Google Scholar]
- Jordan D.2005Vagal control of the heart: central serotonergic (5-HT) mechanisms. Exp. Physiol. 90, 175–181 (doi:10.1113/expphysiol.2004.029058) [DOI] [PubMed] [Google Scholar]
- Kaumann A. J., Levy F. O.20065-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol. Ther. 111, 674–706 (doi:10.1016/j.pharmthera.2005.12.004) [DOI] [PubMed] [Google Scholar]
- Kubin L., Alheid G. F., Zuperku E. J., McCrimmon D. R.2006Central pathways of pulmonary and lower airway vagal afferents. J. Appl. Physiol. 101, 618–627 (doi:10.1152/japplphysiol.00252.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley P. M.2006Opiate slowing of feline respiratory rhythm and effects on putative medullary phase-regulating neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1387–R1396 (doi:10.1152/ajpregu.00530.2005) [DOI] [PubMed] [Google Scholar]
- Lalley P. M., Bischoff A. M., Richter D. W.1994Serotonin 1A-receptor activation suppresses respiratory apneusis in the cat. Neurosci. Lett. 172, 59–62 (doi:10.1016/0304-3940(94)90662-9) [DOI] [PubMed] [Google Scholar]
- Lalley P. M., Pierrefiche O., Bischoff A. M., Richter D. W.1997cAMP-dependent protein kinase modulates expiratory neurons in vivo. J. Neurophysiol. 77, 1119–1131 [DOI] [PubMed] [Google Scholar]
- La Rovere M. T., Bigger J. T., Jr, Marcus F. I., Mortara A., Schwartz P. J.1998Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) investigators. Lancet 351, 478–484 (doi:10.1016/S0140-6736(97)11144-8) [DOI] [PubMed] [Google Scholar]
- Lonergan T., Goodchild A. K., Christie M. J., Pilowsky P. M.2003Mu opioid receptors in rat ventral medulla: effects of endomorphin-1 on phrenic nerve activity. Respir. Physiol. Neurobiol. 138, 165–178 (doi:10.1016/S1569-9048(03)00173-3) [DOI] [PubMed] [Google Scholar]
- Lötsch J., Skarke C., Schneider A., Hummel T., Geisslinger G.2005The 5-hydroxytryptamine 4 receptor agonist mosapride does not antagonize morphine-induced respiratory depression. Clin. Pharmacol. Ther. 78, 278–287 (doi:10.1016/j.clpt.2005.05.010) [DOI] [PubMed] [Google Scholar]
- Mansour A., Fox C. A., Thompson R. C., Akil H., Watson S. J.1994mu-Opioid receptor mRNA expression in the rat CNS: comparison to mu-receptor binding. Brain Res. 643, 245–265 (doi:10.1016/0006-8993(94)90031-0) [DOI] [PubMed] [Google Scholar]
- Manzke T., Guenther U., Ponimaskin E. G., Haller M., Dutschmann M., Schwarzacher S., Richter D. W.20035-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science 301, 226–229 (doi:10.1126/science.1084674) [DOI] [PubMed] [Google Scholar]
- Manzke T., Dutschmann M., Schlaf G., Mörschel M., Koch U. R., Ponimaskin E., Bidon O., Lalley P. M., Richter D. W.2009Serotonin targets inhibitory synapses to induce modulation of network functions. Phil. Trans. R. Soc. B 364, 2589–2602 (doi:10.1098/rstb.2009.0068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine Y., Yoshikawa T., Oku S., Nagai R., Yoshida N., Hosoki K.1997Comparison of effect of mosapride citrate and existing 5-HT4 receptor agonists on gastrointestinal motility in vivo and in vitro. J. Pharmacol. Exp. Ther. 283, 1000–1008 [PubMed] [Google Scholar]
- Mörschel M., Dutschmann M.2009Pontine respiratory activity involved in inspiratory/expiratory phase transition. Phil. Trans. R. Soc. B 364, 2517–2526 (doi:10.1098/rstb.2009.0074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murasato Y., Hirakawa H., Harada Y., Nakamura T., Hayashida Y.1998Effects of systemic hypoxia on R–R interval and blood pressure variabilities in conscious rats. Am. J. Physiol. 275, H797–H804 [DOI] [PubMed] [Google Scholar]
- Oertel B. G., Schneider A., Rohrbacher M., Schmidt H., Tegeder I., Geisslinger G., Lötsch J.2007The partial 5-hydroxytryptamine1A receptor agonist buspirone does not antagonize morphine-induced respiratory depression in humans. Clin. Pharmacol. Ther. 81, 59–68 (doi:10.1038/sj.clpt.6100018) [DOI] [PubMed] [Google Scholar]
- O'Sullivan R. J., Brown I. G., Pender M. P.2008Apneusis responding to buspirone in multiple sclerosis. Mult. Scler. 14, 705–707 (doi:10.1177/1352458507085802) [DOI] [PubMed] [Google Scholar]
- Paton J. F. R.1996A working heart-brainstem preparation of the mouse. J. Neurosci. Meth. 65, 63–68 (doi:10.1016/0165-0270(95)00147-6) [DOI] [PubMed] [Google Scholar]
- Pickering A. E., Paton J. F. R.2006A decerebrate, artificially-perfused in situ preparation of rat: utility for the study of autonomic and nociceptive processing. J. Neurosci. Meth. 155, 260–271 (doi:10.1016/j.jneumeth.2006.01.011) [DOI] [PubMed] [Google Scholar]
- Pierrefiche O., Schwarzacher S. W., Bischoff A. M., Richter D. W.1998Blockade of synaptic inhibition within the pre-Bötzinger complex in the cat suppresses respiratory rhythm generation in vivo. J. Physiol. 509, 245–254 (doi:10.1111/j.1469-7793.1998.245bo.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D. W., Manzke T., Wilken B., Ponimaskin E.2003Serotonin receptors: guardians of stable breathing. Trends Mol. Med. 9, 542–548 (doi:10.1016/j.molmed.2003.10.010) [DOI] [PubMed] [Google Scholar]
- Sahibzada N., Ferreira M., Wasserman A. M., Taveira-DaSilva A. M., Gillis R. A.2000Reversal of morphine-induced apnea in the anesthetized rat by drugs that activate 5-hydroxytryptamine(1A) receptors. J. Pharmacol. Exp. Ther. 292, 704–713 [PubMed] [Google Scholar]
- Saito Y., Hashimoto T., Iwata H., Takahashi K., Fukumizu M., Sasaki M., Hanaoka S., Sugai K.1999Apneustic breathing in children with brainstem damage due to hypoxic–ischemic encephalopathy. Dev. Med. Child Neurol. 41, 560–567 (doi:10.1017/S0012162299001188) [DOI] [PubMed] [Google Scholar]
- Schmid K., Foutz A. S., Denavit-Saubie M.1996Inhibitions mediated by glycine and GABAA receptors shape the discharge pattern of bulbar respiratory neurons. Brain Res. 710, 150–160 (doi:10.1016/0006-8993(95)01380-6) [DOI] [PubMed] [Google Scholar]
- Simms A. E., Paton J. F. R., Pickering A. E., Allen A. M.2009Amplified respiratory–sympathetic coupling in neonatal and juvenile spontaneously hypertensive rats: does it contribute to hypertension? J. Physiol. 587, 597–610 (doi:10.1113/jphysiol.2008.165902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Abdala A. P., Koizumi H., Rybak I. A., Paton J. F. R.2007Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J. Neurophysiol. 98, 3370–3387 (doi:10.1152/jn.00985.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner G. M., Zanella S., Hilaire G., Dutschmann M.20088-OH-DPAT suppresses spontaneous central apneas in the C57BL/6J mouse strain. Respir. Physiol. Neurobiol. 161, 10–15 (doi:10.1016/j.resp.2007.11.001) [DOI] [PubMed] [Google Scholar]
- Villareal R. P., Liu B. C., Massumi A.2002Heart rate variability and cardiovascular mortality. Curr. Atheroscler. Rep. 4, 120–127 (doi:10.1007/s11883-002-0035-1) [DOI] [PubMed] [Google Scholar]
- Waki H., Kasparov S., Wong L. F., Murphy D., Shimizu T., Paton J. F. R.2003Chronic inhibition of endothelial nitric oxide synthase activity in nucleus tractus solitarii enhances baroreceptor reflex in conscious rats. J. Physiol. 546, 233–242 (doi:10.1113/jphysiol.2002.030270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki H., Liu B., Miyake M., Katahira K., Murphy D., Kasparov S., Paton J. F. R.2007Junctional adhesion molecule-1 is upregulated in spontaneously hypertensive rats: evidence for a prohypertensive role within the brain stem. Hypertension 49, 1321–1327 (doi:10.1161/HYPERTENSIONAHA.106.085589) [DOI] [PubMed] [Google Scholar]
- Wilken B., Lalley P., Bischoff A. M., Christen H. J., Behnke J., Hanefeld F., Richter D. W.1997Treatment of apneustic respiratory disturbance with a serotonin-receptor agonist. J. Pediatr. 130, 89–94 (doi:10.1016/S0022-3476(97)70315-9) [DOI] [PubMed] [Google Scholar]
- Yamauchi M., Ocak H., Dostal J., Jacono F. J., Loparo K. A., Strohl K. P.2008aPost-sigh breathing behavior and spontaneous pauses in the C57BL/6J (B6) mouse. Respir. Physiol. Neurobiol. 162, 117–125 (doi:10.1016/j.resp.2008.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M., Dostal J., Kimura H., Strohl K. P.2008bEffects of buspirone on posthypoxic ventilatory behavior in the C57BL/6J and A/J mouse strains. J. Appl. Physiol. 105, 518–526 (doi:10.1152/japplphysiol.00069.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal D. B., Simms A. E., Bonagamba L. G., Braga V. A., Pickering A. E., Paton J. F. R., Machado B. H.2008Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J. Physiol. 586, 3253–3265 (doi:10.1113/jphysiol.2008.154187) [DOI] [PMC free article] [PubMed] [Google Scholar]