Abstract
The Hox genetic network plays a key role in the anteroposterior patterning of the rhombencephalon at pre- and early-segmental stages of development of the neural tube. In the mouse, it controls development of the entire brainstem respiratory neuronal network, including the pons, the parafacial respiratory group (pFRG) and the pre-Bötzinger complex (preBötC). Inactivation of Krox20/Egr2 eliminates the pFRG activity, thereby causing life-threatening neonatal apnoeas alternating with respiration at low frequency. Another respiratory abnormality, the complete absence of breathing, is induced when neuronal synchronization fails to develop in the preBötC. The present paper summarizes data on a third type of respiratory deficits induced by altering Hox function at pontine levels. Inactivation of Hoxa2, the most rostrally expressed Hox gene in the hindbrain, disturbs embryonic development of the pons and alters neonatal inspiratory shaping without affecting respiratory frequency and apnoeas. The same result is obtained by the Phox2a+/− mutation modifying the number of petrosal chemoafferent neurons, by eliminating acetylcholinesterase and by altering Hox-dependent development of the pons with retinoic acid administration at embryonic day 7.5. In addition, embryos treated with retinoic acid provide a mouse model for hyperpnoeic episodic breathing, widely reported in pre-term neonates, young girls with Rett's syndrome, patients with Joubert syndrome and adults with Cheyne–Stokes respiration. We conclude that specific respiratory deficits in vivo are assignable to anteroposterior segments of the brainstem, suggesting that the adult respiratory neuronal network is functionally organized according to the rhombomeric, Hox-dependent segmentation of the brainstem in embryos.
Keywords: Hox, Krox20/Egr2, retinoic acid, acetylcholinesterase, Joubert syndrome, Cheyne–Stokes
1. Introduction
Strong regulatory constraints couple the expression pattern in Hox gene clusters to the progression of embryogenesis in the brainstem (Lumsden & Krumlauf 1996). In vertebrates, the formation of rhombomeres (r) is accompanied by a sequential activation of these genes from 3′ to 5′ in the clusters. As a result, early structures are given an anterior identity with 3′ Hox genes as key determinants, while progressively later structures start expressing more 5′ Hox genes and acquire a more posterior identity. Thus, Hox genes play a key role in anteroposterior patterning of the rhombencephalon at pre- and early-segmental stages of development (embryonic days E 7.5–E 9.5 in mice, before the neurogenesis starts). Hoxa1 and Hoxb1 are expressed up to the rhombomeric r3/r4 boundary, and they are directly downregulated in r3 by the zinc-finger transcription factor Krox20 (Schneider-Maunoury et al. 1998), also named Egr2. This signalling pathway is required for the proper development of r3–r5 and boundaries between rhombomeres. Hoxa2, the most anterior Hox, is expressed up to the r1/r2 boundary, and it is upregulated by Krox20/Egr2 in r3. Other Hox are prevalent caudal to r5 in the so-called ‘post-otic’ rhombomeres (i.e. located caudal to the otic vesicles). Thus, in all vertebrates, the branchiomotor nuclei conform to this rhombomeric pattern with a two-segment periodicity. Trigeminal motoneurons originate from r2 and r3 and send their axons to an exit point in r2. In mammals, the facial branchial nucleus originates in r4, forming with adjacent rhombomeres the ‘parafacial’ level of the hindbrain. The ventral and dorsal motor nuclei of glossopharyngeal–vagal nerves are post-otic in origin (Lumsden & Krumlauf 1996).
By investigating the respiratory behaviour of Krox20−/− and Hoxa1−/− mutants (Jacquin et al. 1996; Dominguez del Toro et al. 2001), we have established links between the transient rhombomere-nested expression of these genes and deletions, neoformations and reconfigurations of the respiratory rhythm generator (reviewed at post-natal stages by Bianchi et al. 1995; Feldman & Del Negro 2006). These mutations helped in the unveiling of a vital role of the rhombomeric parafacial domain for the specification of an adapted respiratory rhythm at birth (Borday et al. 2004, 2005; Chatonnet et al. 2006), which requires activity of the parafacial respiratory group (pFRG, Chatonnet et al. 2003b; Mellen et al. 2003; Onimaru & Homma 2003). Comparisons with mutations affecting adjacent embryonic territories now allow us to differentiate two additional regulatory levels controlling breathing, including: (i) caudally, at the vagal glossopharyngeal (post-otic) level, the inspiratory oscillator, named the pre-Bötzinger complex (pre-BötC, Smith et al. 1991; Thoby-Brisson et al. 2005; Thoby-Brisson & Greer 2008) necessary for breathing (Gray et al. 2001; McKay et al. 2005; Wallen-Mackenzie et al. 2006; Tan et al. 2008) and (ii) the rostral pons (close to the midbrain/hindbrain boundary, McCrimmon et al. 2004; Potts et al. 2005), altering the shape of inspiratory efforts, without affecting respiratory frequency (Chatonnet et al. 2007; Guimarães et al. 2007). The present paper concentrates on this pontine respiratory control and suggests interactions with the peripheral reflex and central cholinergic controls of breathing.
2. Parafacial control of respiratory rhythm frequency: induction requires Krox20/Egr2 and Hoxa1 but not kreisler/MafB
The rhombomere-related formation of the pFRG was first postulated from the observation of Hoxa1 (Dominguez del Toro et al. 2001) and Krox20/Egr2 (Jacquin et al. 1996) homozygous mice mutants that revealed the anti-apnoeic and respiratory frequency promoting functions of the pFRG at birth (figure 1a). Furthermore, the finding of an embryonic parafacial oscillator now clearly shows that pFRG derives from, and requires, Krox20-expressing progenitors (Thoby-Brisson et al. in press). In vivo/in vitro analysis also shows that the more caudal medulla including the preBötC is not affected by the Krox20−/− and Hoxa1−/− mutations (Jacquin et al. 1996; Dominguez del Toro et al. 2001). In fact, mutations disrupting preBötC function, e.g. the glutamatergic synchronization between preBötC, eliminate breathing behaviour (Wallen-Mackenzie et al. 2006). Thus, the rhombomeric organization supports the hypothesis of a dual control for respiratory frequency by distinct neuronal oscillators, pFRG and preBötC during foetal and early post-natal life (Chatonnet et al. 2003b; Mellen et al. 2003; Onimaru & Homma 2003).
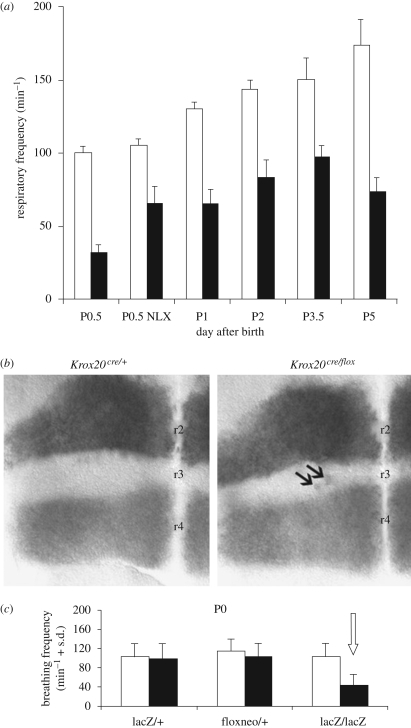
Figure 1.
Parafacial control of respiratory rhythm frequency. (a) Average (±s.d.) respiratory frequency measured by plethysmographic recording of wild-type (white, n = 17) and homozygous mice in which Krox20 (also called Egr2) is inactivated (black, n = 10; mutations by P. Charnay et al. Paris). Krox20 controls Hox gene expression in rhombomeres r3 and r5 and the development of r3 and r5, including the parafacial neuronal oscillator (pFRG) located ventral to the facial branchiomotor nucleus. In contrast to the silencing of the preBötC (Wallen-Mackenzie et al. 2006), elimination of the pFRG does not completely abolish breathing: respiration persists although at a very low frequency. During the first post-natal day (P0.5), mutants exhibit long apnoeas, so that survival requires naloxone administration (NLX; adapted from Jacquin et al. 1996; Chatonnet et al. 2003a, 2007). (b) Size of rhombomere r3 estimated at a segmental embryonic stage (E9.5) on flat-mounted hindbrains by labelling adjacent rhombomeres as follows: r4 by in situ hybridization with an Hoxb1 probe and r2 by detection of the alkaline phosphatase activity from an r2-specific transgene. The negative territory located in-between corresponds to the Krox20-expressing rhombomere r3: it is reduced by the hypomorphic mutation Krox20Cre/flox (on the right) compared with heterozygous Krox20Cre/+ embryos (on the left). A few mis-specified (Hoxb1-positive) cells are also observed within r3 in embryos (arrows, modified from Chatonnet et al. 2007). (c) The low breathing frequency of homozygous Krox20 mutants (lacZ/lacZ, right) is not seen in Krox20 heterozygous (lacZ/+) and hypomorphic (floxneo/+) neonates. These observations indicate that a subpopulation of Krox20-expressing progenitor cells, left active in hypomorphic and heterozygous mutants, is required for the development of a parafacial, anti-apnoeic and rhythm promoting control of respiration (adapted from Jacquin et al. 1996; Chatonnet et al. 2003a, 2007). Open squares, WT; filled squares, mutant.
Rhombomeres are polyclonal developmental compartments that include a large variety of cell types and lineages that are all affected in Hoxa1 and Krox20/Egr2 (Lumsden & Krumlauf 1996). To further investigate the effect of rhombomere reduction during the development of the parafacial system, we used kreisler homozyguous mutants with vestigial (almost absent) r5 (Manzanares et al. 1997; Sadl et al. 2003) and Krox20Cre/flox hypomorphic mutants (Voiculescu et al. 2000; Taillebourg et al. 2002) resulting in a reduced, though not absent, r3 territory. Krox20Cre/flox mutants were obtained by combining two previously developed Krox20 alleles, a Cre knock-in and a floxed allele. Compound heterozygous Krox20Cre/flox mutants express Krox20 only transiently, leading to a severe reduction in r3 (figure 1b). Analysis of the kreisler homozyguous (Chatonnet et al. 2002) and Krox20Cre/flox mutants (Chatonnet et al. 2007) revealed robustness of the dual rhythm generator in neonates, which is able to maintain a physiological respiratory frequency despite dramatic alterations of the brainstem anatomy. Both mutants exhibited a normal respiratory frequency (figure 1c) and normal durations of apnoeas during the first post-natal day. Therefore, a severe reduction of r3 or r5 is compatible with a normal control of the respiratory rhythm, suggesting that a subset of progenitors specifically generating the pFRG remains active in kreisler and Krox20 hypomorphic mutants. This lineage is now being identified and followed during migration in collaboration with Jean-François Brunet and Christo Goridis (Ecole Normale Supérieure, Paris): these neurons derive from Krox20-expressing progenitors and co-migrate caudally with the facial branchiomotor nucleus to reach a final position near the ventral surface of the brainstem; these neurons are characterized by intrinsic membrane pacemaker properties and expression of the developmental gene Phox2b (Thoby-Brisson et al. in press).
3. Pontine inspiratory control: distinct Hoxa2- and Krox20/Egr2-related respiratory phenotypes
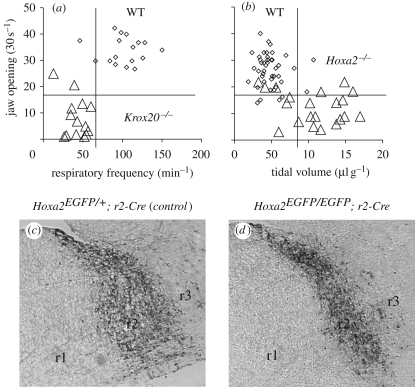
We compared the phenotypes of mice carrying targeted inactivations of Krox20/Egr2 (affecting the pFRG development, as discussed earlier) and Hoxa2, the only Hox gene expressed up to r2 and controlling the development of the rostral pons (Chatonnet et al. 2007). In addition to breathing, we investigated the impact of such mutations on the neural circuits controlling jaw opening, compatible with Hoxa2-dependent trigeminal defects (r2r3 derived) and direct upregulation of Hoxa2 by Krox20 in r3. We found that Hoxa2 mutants displayed an impaired oro-buccal reflex similar to Krox20 mutants (jaw opening in figure 2a), but that the respiratory phenotypes were different. Inactivation of Hoxa2 did not affect the breathing frequency, but, instead, led to a larger-than-normal inspiratory amplitude (tidal volume in figure 2b). We analysed the distribution of EGFP+ cells in knock-in mice in which EGFP expression was selectively induced in r2-derived Hoxa2-expressing cells upon Cre-mediated recombination (in collaboration with F. Rijli, see Oury et al. 2006). This was done by mating the Hoxa2EGFP(lox-neo-lox) knock-in allele with an r2-specific Cre transgenic line (Chatonnet et al. 2007). This mating scheme resulted in full Hoxa2 targeted inactivation and, concomitantly, allowed selective tracing of the Hoxa2-expressing EGFP+ cells in the r2-derived progeny in controls (figure 2c) and homozygous mutants (figure 2d). Tracing of r2-specific progenies of Hoxa2-expressing cells indicated that the control of inspiratory activity resided in rostral pontine areas (Chatonnet et al. 2007). Inspiratory deficit was ascribed to the expansion of structures deriving from r1 (the catecholaminergic Locus Coeruleus, the cholinergic pediculo-pontine tegmentum, the pontine respiratory groups parabrachial medial and Kölliker–Fuse nuclei), at the expense of r2 (see reduction of r2 in figure 2d).
Figure 2.
Pontine control of the tidal volume at birth (adapted from Jacquin et al. 1996; Chatonnet et al. 2003a, 2007). Top: impaired oro-buccal reflex and respiratory parameters in the Hoxa2 homozyguous mutant (a), compared with Krox20 mutants (b; each symbol represents a neonate; triangles, mutant; diamonds, wild-type), during the first hours following birth. Ordinates show the number of jaw openings within 30 s, induced by a gentle mechanical stimulation of the mouth: the observed deficit in both mutants (less than 20 openings per 30 s in a series) is compatible with Hoxa2-dependent trigeminal defects (r2 and r3 derived) and direct upregulation of Hoxa2 by Krox20 in r3. Regarding breathing (abscissas: frequency in (a); tidal volume in (b)), Hoxa2−/− mutants show large inspiratory amplitudes (b), but none of them show the low respiratory frequency that results from the elimination of the pFRG in Krox20−/− (a). Bottom: increase in rhombomere r1 at the expense of r2 by homozygous inactivation of Hoxa2 (d) compared with heterozygous (normal, c). Parasagittal sections (rostral is on the left and dorsal on the top) showing the r2-derived territory by immunodetection of EGFP in mice produced by mating Hoxa2EGFP(lox-neo-lox)/− mutants with an r2::Cre transgenic line (mutations by P. Rijli, Strasbourg). The homozygous mutants exhibit irreversible abnormality of the pons linked to the hypertrophy of the r1-derived territory (on the left; position of the r2/r3 boundary, on the right, is unchanged). These observations identify in vivo the respiratory-related function of pontine circuits deriving from r1r2.
Our data point to the importance of rhombomere-specific genetic control in the development of modular neural networks in the mammalian hindbrain: we show that inspiratory shaping and respiratory frequency are under the control of distinct Hox-dependent segmental cues and pontine (Hoxa2-dependent), parafacial (Hoxa1-dependent) and post-otic segments of the neural tube.
4. Loss of acetylcholinesterase or muscarinic receptors increases inspiratory depth of breathing
We have studied impairment of the cholinergic system within the brainstem by the (r1 derived) pediculo-pontine tegmentum in adults after homozygous inactivation of the gene encoding the enzyme acetylcholinesterase (Chatonnet et al. 2003a; Boudinot et al. 2004a,b, 2005). In these mice, inspirations were deeper than normal, while rhythm frequency appeared normal and no apnoeas were seen. It has long been known that blockade of acetylcholinesterase by organo-phosphorus compounds produces rapid death by respiratory failure (reviewed in Brimblecombe 1981). We have shown in vitro that one of the mechanisms by which respiratory failure is prevented is the decreased sensitivity of preBötC neurons to nicotinic and muscarinic agonists in the preBötC (Chatonnet et al. 2003a). Indeed, muscarinic receptors are involved. We found that when muscarinic M1 receptors are eliminated, mice show unaffected respiratory frequency but elevated tidal volume at rest and unaffected ventilatory responses to hypoxia and hypercapnia in vivo. Mice knock-out for the M3 receptors had elevated tidal volume at rest and a slightly decreased tidal volume response slope to hypercapnia and responses to hypoxia (Boudinot et al. 2008). The results suggest that M1–M3 muscarinic receptors play significant roles in the regulation of the depth of inspiration at rest and that M3 receptors are activated in the afferent pathway originating from peripheral chemoreceptors, thus linking pontine inspiratory shaping and sensory afferent synaptic integration (Boudinot et al. 2008). These results suggest that cholinergic neurons of the pediculo-pontine tegmentum contribute with the pontine respiratory group (parabrachial medial and Kölliker–Fuse nuclei) to alter inspiratory depth after the abnormal Hox control of the pontine development.
5. Pre-segmental retinoic treatment reduces inspirations and causes episodic breathing
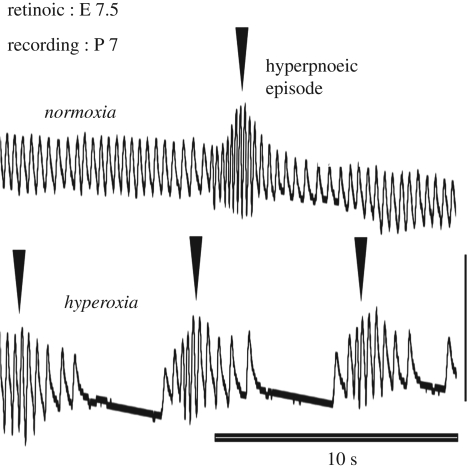
The experiments on the Krox20/Hox signalling discussed earlier suggest that segmental signalling at end-segmental stages (E 9.5) is sufficient to induce the development of a parafacial rhythm generator. However, segmentation of the hindbrain starts earlier at E 7.5 in mice. Retinoic acid treatment at E 7.5 with low doses (0.5 mg kg−1, one to two orders of magnitude lower than the teratogenic dose) was found to be convenient in mice to further investigate the importance of this early developmental stage for the breathing behaviour (Guimarães et al. 2007). Retinoic acid at E 7.5 is known to affect the spread of Hox and Gbx2 expression domains into the anterior hindbrain, followed by a retraction, leaving behind a posteriorized expression pattern (Li & Joyner 2001). In keeping with these data on embryo, we found rostral pontine abnormalities that persisted until adulthood and were opposite to those observed in Hoxa2 mutants: reduced tidal volume (with unchanged respiratory frequency) and reduced size of the pontine parabrachial respiratory area. The treatment also changed the shape of the pediculo-pontine tegmentum and induced a displacement of the superior cerebellar peduncle with respect to the midline. Strikingly, periods of quiet breathing with small tidal volume consistently alternated with sequences of hyperpnoeic episodic breathing (figure 3), a cyclic waxing and waning of inspirations (Guimarães et al. 2007). This is a clinical trait widely reported in pre-term neonates, in patients with Joubert or Rett syndromes and in adults (Cheyne–Stokes respiration) with congestive heart failure and brainstem infarction. Human and animal studies have provided evidence supporting the importance of hypoxia, altitude acclimatization and hypocapnia in the induction of hyperpnoeic episodic breathing, so that ventilatory instability may result from the unbalanced control of the rhythm generators by peripheral and central afferent controls (Han et al. 2002). In mice treated with retinoic acid, exaggeration of this Cheyne–Stokes pattern upon silencing chemosensory receptors by hyperoxia (figure 3) suggests that the rostral pontine deficit interacts with peripheral chemosensory pathways.
Figure 3.
Deficit of the pontine control of inspiration may cause hyperpnoeic episodic breathing at post-natal stages (adapted from Guimarães et al. 2007). Plethysmographic records (one week after birth) of hyperpnoeic episodic breathing (triangles show single episodes) in normoxia (top trace) and hyperoxia (bottom trace). This pattern was consistently induced by treating embryos with retinoic acid at early pre-segmental stages (E 7.5, with doses as low as 0.5 mg kg−1). This treatment induces anatomical abnormalities restricted to the rostral pons resembling those in the human Joubert syndrome (Guimarães et al. 2007). Outside sequences of episodic breathing, the control of inspiration was altered (with small tidal volumes), but the respiratory frequency was normal. Apnoeas and survival were normal at neonatal stages. The hyperoxic test (bottom) aggravates the hyperpnoeic episodic pattern (apnoeas between episodes are not seen in normoxia), showing that both the pontine control (eliminated by the treatment with retinoic acid) and the peripheral chemosensory control (eliminated by hyperoxia) stabilize breathing.
Interestingly, anatomical anomalies of the superior cerebellar peduncle in mice also resemble the molar tooth sign reported in the Joubert syndrome, in which mal-oriented superior cerebellar peduncles give the appearance of a ‘molar tooth’ on axial brain MRI through the junction of the midbrain and hindbrain (Gleeson et al. 2004). The Joubert syndrome is clinically heterogeneous with breathing abnormalities including episodic hyperpnoeas (in 60–80% of the patients) in the neonatal period, oculomotor apraxia (abnormal eye movements in 75% of the patients), ataxia and mental retardation (Joubert et al. 1969). This is also a genetically heterogeneous syndrome, with different known loci (Ferland et al. 2004; Lagier-Tourenne et al. 2004). Although candidate gene approaches have failed so far to detect mutations in wnt1, en1, en2 and fgf8 genes of the patients (Blair et al. 2002), observations in mice suggest a possible link with the large Hox-related genetic network affected by retinoic acid during early embryonic development.
6. Control of inspirations by peripheral chemoreceptor afferent neurons
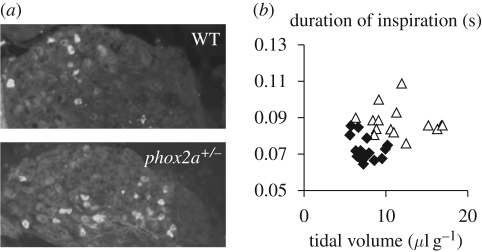
From the results mentioned earlier, we suspect an interaction between rostral pontine structures and sensory afferents in the nucleus tractus solitarii, in which the inspiratory shape is regulated by lung stretch receptors of the Breuer–Hering reflex, under control by peripheral chemoreceptor afferent neurons originating from the carotid bodies. Development of the petrosal ganglion, containing chemoreceptor afferent neurons, depends on the brain-derived neurotrophic factor (BDNF) as well as on the expression of the Phox2a and Phox2b transcription factors that are important for cell survival and specification of the dopaminergic fate (Brunet & Pattyn 2002). Our study of the petrosal ganglion was also prompted by previous findings of a small respiratory amplitude when the peripheral chemoreceptor afferent population was reduced by apoptosis following inactivation of the gene encoding the BDNF (Erickson et al. 1996, see also Thoby-Brisson et al. 2003). Mutations of Phox2b induce brainstem anomalies associated with defects of breathing behaviour at birth (Dubreuil et al. 2009). The role of Phox2a proteins in the establishment of brainstem cardiorespiratory neuronal pathways was unknown, largely because of the lethality of homozygous mutants. We therefore examined the effects of a haploinsufficiency of the Phox2a gene. Phox2a heterozygous mice survive and exhibit significantly larger and longer inspirations (figure 4b) compared with wild-type animals during both normoxic breathing and in response to hypoxia. This phenotype, accompanied by an unaltered frequency, is evident at birth and persists afterwards (it was followed until post-natal day 10). Morphological analysis of Phox2a+/− animals reveals no anomaly in the pons (parabrachial medial and Kölliker–Fuse nuclei, pediculo-pontine tegmentum, superior cerebellar peduncles) or in the locus coeruleus region, but highlighted an increase in the number of cells expressing the catecholamine-synthesizing enzyme—tyrosine hydroxylase, a marker of peripheral chemoreceptor afferent neurons (Katz & Black 1986; Erickson et al. 1996), in the petrosal sensory ganglion (figure 4a). These data indicate that Phox2a plays a critical role in the ontogeny of the reflex control of inspiration and that abnormality may independently affect rhythm generators and pontine/sensory inspiratory controllers as well as their peripheral reflex control.
Figure 4.
The Phox2a heterozygous mutation increases the number of peripheral chemoreceptor afferent neurons in the petrosal ganglia (a) and impairs inspiratory control (b; adapted from Wrobel et al. 2007). (a) Peripheral chemoreceptor afferent cell bodies identified by immunohistochemistry of tyrosine hydroxylase in the petrosal ganglion at post-natal day 10. The effect of the mutation was rather selective because the size of the ganglion and the total number of unstained neurons were not affected. (b) Respiratory deficits in mutant include long inspirations (in ordinates) and large amplitudes (tidal volume in abscissa). Large amplitudes (illustrated here at post-natal day 1) were seen until adulthood. Respiratory frequency, neonatal apnoeas and survival were normal. Interestingly, the reverse phenotype (a small tidal volume) has been reported after the decrease (instead of increase) in the peripheral chemoreceptor afferent population by the invalidation of the BDNF (Erickson et al. 1996). Diamonds, WT; triangles, Phox2a+/−.
7. Conclusion
Although transient during early development, the rhombomeric patterning strongly and irreversibly influences the establishment of the neuronal circuitry of the mature brainstem. Reconfiguration of neurons and synapses during foetal and post-natal development is unable to restore viable neural functions when the basic scaffold is altered during early development. Thus, from studies of hindbrain development, we are gaining an understanding of how genes govern the early embryonic specification of neuronal networks, how they might specify patterns of motor activities operating normally throughout life and how early developmental dysfunction might cause hereditary syndromes including post-natal breathing abnormalities relevant to those seen in humans.
Acknowledgements
Work in J.C.'s laboratory is supported by grants from the ‘Center National pour la Recherche Scientifique’ (CNRS), the European Community (grant QLG2-CT- 2001-01467 ‘Brainstem Genetics’) and the Agence Nationale pour la Recherche (grant no. ANR-07-Neuro-007-01 to G.F.).
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Brainstem neural networks vital for life’.
References
- Bianchi A. L., Denavit-Saubié M., Champagnat J.1995Neurobiology of the central control of breathing in mammals: neuronal circuitry, membrane properties and neurotransmitters involved. Physiol. Rev. 75, 1–45 [DOI] [PubMed] [Google Scholar]
- Blair I. P., Gibson R. R., Bennett C. L., Chance P. F.2002Search for genes involved in Joubert syndrome: evidence that one or more major loci are yet to be identified and exclusion of candidate genes EN1, EN2, FGF8 and BARHL1. Am. J. Med. Genet. 107, 190–196 (doi:10.1002/ajmg.10145) [PubMed] [Google Scholar]
- Borday C., Wrobel L., Fortin G., Champagnat J., Thaëron-Antono C., Thoby-Brisson M.2004Developmental gene control of brainstem function: views from the embryo. Prog. Biophys. Mol. Biol. 84, 89–106 (doi:10.1016/j.pbiomolbio.2003.11.002) [DOI] [PubMed] [Google Scholar]
- Borday C., Chatonnet F., Thoby-Brisson M., Champagnat J., Fortin G.2005Neural tube patterning by Krox20 and emergence of a respiratory control. Respir. Physiol. and Neurobiol. 149, 63–72 (doi:10.1016/j.resp.2005.02.014) [DOI] [PubMed] [Google Scholar]
- Borday C., Coutinho A., Germon I., Champagnat J., Fortin G.2006Pre-/post-otic rhombomeric interactions control the emergence of a fetal-like respiratory rhythm in the mouse embryo. J. Neurobiol. 66, 1285–1301 (doi:10.1002/neu.20271) [DOI] [PubMed] [Google Scholar]
- Boudinot E., Yamada M., Wess J., Champagnat J., Foutz A. S.2004aVentilatory pattern and chemosensitivity in M1 and M3 muscarinic receptor knockout mice. Respir. Physiol. Neurobiol. 139, 237–245 (doi:10.1016/j.resp.2003.10.006) [DOI] [PubMed] [Google Scholar]
- Boudinot E., Emery M. J., Mouisel E., Chatonnet A., Champagnat J., Escourrou P., Foutz A. S.2004bIncreased ventilation and CO2 chemosensitivity in acetylcholinesterase knockout mice. Respir. Physiol. Neurobiol. 140, 231–241 (doi:10.1016/j.resp.2004.03.002) [DOI] [PubMed] [Google Scholar]
- Boudinot E., Taysse L., Daulon S., Chatonnet A., Champagnat J., Foutz A. S.2005Effects of acetylcholinesterase and butyrylcholinesterase inhibition on breathing in mice adapted or not to reduced acetylcholinesterase. Pharmacol. Biochem. Behav. 80, 53–61 (doi:10.1016/j.pbb.2004.10.014) [DOI] [PubMed] [Google Scholar]
- Boudinot E., Champagnat J., Foutz A. S.2008M1/M3 and M2/M4 muscarinic receptor double-knockout mice present distinct respiratory phenotypes. Respir. Physiol. Neurobiol. 161, 54–61 (doi:10.1016/j.resp.2007.12.001) [DOI] [PubMed] [Google Scholar]
- Brimblecombe R. W.1981Drugs acting on cholinergic mechanisms and affecting respiration. In Respiratory pharmacology (ed. Widdicombe J.), pp. 175–184 Oxford, UK: Pergamon Press [Google Scholar]
- Brunet J. F., Pattyn A.2002Phox2 genes—from patterning to connectivity. Curr. Opin. Genet. Dev. 12, 435–440 (doi:10.1016/S0959-437X(02)00322-2) [DOI] [PubMed] [Google Scholar]
- Chatonnet F., Dominguez del Toro E., Voilescu O., Charnay P., Champagnat J.2002Different respiratory control systems are affected in homozygous and heterozygous kreisler mutant mice. Eur. J. Neurosci. 15, 684–692 (doi:10.1046/j.1460-9568.2002.01909.x) [DOI] [PubMed] [Google Scholar]
- Chatonnet F., Boudinot E., Chatonnet A., Taysse L., Daulon S., Champagnat J., Foutz A. S.2003aRespiratory survival mechanisms in acetylcholinesterase knockout mouse. Eur. J. Neurosci. 18, 1419–1427 (doi:10.1046/j.1460-9568.2003.02867.x) [DOI] [PubMed] [Google Scholar]
- Chatonnet F., Domínguez del Toro E., Thoby-Brisson M., Champagnat J., Fortin G., Rijli F. M., Thaëron-Antôno C.2003bFrom hindbrain segmentation to breathing after birth, developmental patterning in rhombomeres 3 and 4. Mol. Neurobiol. 28, 277–293 (doi:10.1385/MN:28:3:277) [DOI] [PubMed] [Google Scholar]
- Chatonnet F., Borday C., Wrobel L., Thoby-Brisson M., Fortin G., McLean H., Champagnat J.2006Ontogeny of central rhythm generation in chicks and rodents. Respir. Physiol. Neurobiol. 154, 37–46 (doi:10.1016/j.resp.2006.02.004) [DOI] [PubMed] [Google Scholar]
- Chatonnet F., Wrobel L., Mézières V., Pasqualetti M., Ducret S., Taillebourg E., Charnay P., Rijli F., Champagnat J.2007Distinct roles of Hoxa2 and Krox20 in the development of rhythmic neural networks controlling inspiratory depth, respiratory frequency and jaw opening. Neural Dev. 2, 19 (doi:10.1186/1749-8104-2-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez del Toro E., Borday V., Davenne M., Neun R., Rijli F., Champagnat J.2001Generation of a novel functional neuronal circuit in Hoxa1 mutant mice. J. Neurosci. 21, 5637–5642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V., Barhanin J., Goridis C., Brunet J.-F.2009Breathing with Phox2b. Phil. Trans. R. Soc. B 364, 2477–2483 (doi:10.1098/rstb.2009.0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. T., Conover J. C., Borday V., Champagnat J., Katz D. M.1996Mice lacking BDNF exhibit visceral sensory neurone losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J. Neurosci. 16, 5361–5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. L., Del Negro C. A.2006Looking for inspiration: new perspectives on respiratory rhythm. Nat. Rev. Neurosci. 7, 232–242 (doi:10.1038/nrn1871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland R. J., et al. 2004Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat. Genet. 36, 1008–1013 (doi:10.1038/ng1419) [DOI] [PubMed] [Google Scholar]
- Gleeson J. G., Keeler L. C., Parisi M. A., Marsh S. E., Chance P. F., Glass I. A., Graham J. M., Jr, Maria B. L., Barkovich A. J., Dobyns W. B.2004Molar tooth sign of the midbrain–hindbrain junction: occurrence in multiple distinct syndromes. Am. J. Med. Genet. 125A, 125–134 (doi:10.1002/ajmg.a.20437) [DOI] [PubMed] [Google Scholar]
- Gray P. A., Janczewski W. A., Mellen N., McCrimmon D. R., Feldman J. L.2001Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat. Neurosci. 4, 927–930 (doi:10.1038/nn0901-927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães L., Dominguez del Toro E., Chatonnet F., Wrobel L., Pujades C., Monteiro L. S., Champagnat J.2007Exposure to retinoic acid at the onset of hindbrain segmentation induces episodic breathing in mice. Eur. J. Neurosci. 25, 3526–3536 (doi:10.1111/j.1460-9568.2007.05609.x) [DOI] [PubMed] [Google Scholar]
- Han F., Subramanian S., Price E. R., Nadeau J., Strohl K. P.2002Periodic breathing in the mouse. J. Appl. Physiol. 92, 1133–1140 [DOI] [PubMed] [Google Scholar]
- Jacquin T. D., Borday V., Schneider-Maunoury S., Topilko P., Kato F., Ghilini G., Charnay P., Champagnat J.1996Reorganization of pontine rhythmogenic neuronal network in Krox-20 knockout mice. Neuron 17, 747–758 (doi:10.1016/S0896-6273(00)80206-8) [DOI] [PubMed] [Google Scholar]
- Joubert M., Eisenring J. J., Robb J. P., Andermann F.1969Familial agenesis of the cerebellar vermis. A syndrome of episodic hyperpnoea, abnormal eye movements, ataxia, and retardation. Neurology 19, 813–825 [DOI] [PubMed] [Google Scholar]
- Katz D. M., Black I. B.1986Expression and regulation of catecholaminergic traits in primary sensory neurons: relationship to target innervation in vivo. J. Neurosci. 6, 983–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier-Tourenne C., Boltshauser E., Breivik N., Gribaa M., Betard C., Barbot C., Koenig M.2004Homozygosity mapping of a third Joubert syndrome locus to 6q23. J. Med. Genet. 41, 273–277 (doi:10.1136/jmg.2003.014787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Y. H., Joyner A. L.2001Otx2 and Gbx2 are required for refinement and not induction of midhindbrain gene expression. Development 128, 4979–4991 [DOI] [PubMed] [Google Scholar]
- Lumsden A., Krumlauf R.1996Patterning the vertebrate neuraxis. Science 274, 1109–1115 (doi:10.1126/science.274.5290.1109) [DOI] [PubMed] [Google Scholar]
- Manzanares M., Cordes S., Kwan C. T., Sham M. H., Barsh G. S., Krumlauf R.1997Segmental regulation of Hoxb3 by kreisler. Nature 387, 191–195 (doi:10.1038/387191a0) [DOI] [PubMed] [Google Scholar]
- McCrimmon D. R., Milsom W. K., Alheid G. F.2004The rhombencephalon and breathing: a view from the pons. Respir. Physiol. Neurobiol. 143, 103–337 (doi:10.1016/j.resp.2004.06.007) [DOI] [PubMed] [Google Scholar]
- McKay L. C., Janczewski W. A., Feldman J. L.2005Sleep-disordered breathing after targeted ablation of preBötzinger complex neurons. Nat. Neurosci. 8, 1142–1144 (doi:10.1038/nn1517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen N. M., Janczewski W. A., Bocchiaro C. M., Feldman J. L.2003Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 37, 821–826 (doi:10.1016/S0896-6273(03)00092-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H., Homma I.2003A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J. Neurosci. 23, 1478–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oury F., Murakami Y., Renaud J. S., Pasqualetti M., Charnay P., Ren S. Y., Rijli F. M.2006Hoxa2- and rhombomere-dependent development of the mouse facial somatosensory map. Science 313, 1408–1413 (doi:10.1126/science.1130042) [DOI] [PubMed] [Google Scholar]
- Potts J. T., Rybak I. A., Paton J. F.2005Respiratory rhythm entrainment by somatic afferent stimulation. J. Neurosci. 25, 1965–1978 (doi:10.1523/jneurosci.3881-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadl V. S., Sing A., Mar L., Jin F., Cordes S. P.2003Analysis of hindbrain patterning defects caused by the kreislerenu mutation reveals multiple roles of Kreisler in hindbrain segmentation. Dev. Dyn. 227, 134–142 (doi:10.1002/dvdy.10279) [DOI] [PubMed] [Google Scholar]
- Schneider-Maunoury S., Gilardi-Hebenstreit P., Charnay P.1998How to build a vertebrate hindbrain. Lessons from genetics. C. R. Acad. Sci. III 321, 819–834 (doi:10.1016/S0764-4469(99)80022-5) [DOI] [PubMed] [Google Scholar]
- Smith J. C., Ellenberger H. H., Ballanyi K., Richter D. W., Feldman J. L.1991Pre-Bötzinger complex: a region that may generate respiratory rhythm in mammals. Science 254, 726–729 (doi:10.1126/science.1683005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillebourg E., Buart S., Charnay P.2002Conditional, floxed allele of the Krox20 gene. Genesis 32, 112–113 (doi:10.1002/gene.10062) [DOI] [PubMed] [Google Scholar]
- Tan W., Janczewski W. A., Yang P., Shao X. M., Callaway E. M., Feldman J. L.2008Silencing preBötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat. Neurosci. 11, 538–540 (doi:10.1038/nn.2104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M., Greer J. J.2008Anatomical and functional development of the pre-Bötzinger complex in prenatal rodents. J. Appl. Physiol. 104, 1213–1219 (doi:10.1152/japplphysiol.01061.2007) [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M., Cauli B., Champagnat J., Fortin G., Katz D. M.2003Expression of functional TrkB receptors by rhythmically active respiratory neurons in the pre-Bötzinger complex of neonatal mice. J. Neurosci. 23, 7685–7689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M., Trinh J. B., Champagnat J., Fortin G.2005Emergence of the pre-Bötzinger respiratory rhythm generator in the mouse embryo. J. Neurosci. 25, 4307–4313 (doi:10.1523/JNEUROSCI.0551-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M., Karlén M., Wu N., Charnay P., Champagnat J., Fortin G.In press Genetic identification of an embryonic parafacial oscillator coupling to the preBötzinger complex. Nature Neurosci [DOI] [PubMed] [Google Scholar]
- Voiculescu O., Charnay P., Schneider-Maunoury S.2000Expression pattern of a Krox-20/Cre knock-in allele in the developing hindbrain, bones, and peripheral nervous system. Genesis 26, 123–126 (doi:10.1002/(SICI)1526-968X(200002)26:2<123::AID-GENE7>3.0.CO;2-O) [DOI] [PubMed] [Google Scholar]
- Wallen-Mackenzie A., Gezelius H., Thoby-Brisson M., Nygard A., Enjin A., Fujiyama F., Fortin G., Kullander K.2006Vesicular glutamate transporter 2 is required for central respiratory rhythm generation. J. Neurosci. 26, 12 294–12 307 (doi:10.1523/JNEUROSCI.3855-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel L., Ogier M., Chatonnet F., Autran S., Mézières V., Thoby-Brisson M., McLean H., Thaeron C., Champagnat J.2007Abnormal inspiratory depth in Phox2a haploin sufficient mice. Neuroscience 145, 384–392 (doi:10.1016/j.neuroscience.2006.11.055) [DOI] [PubMed] [Google Scholar]