Abstract
Central neurons in the brainstem and spinal cord are essential for the maintenance of sympathetic tone, the integration of responses to the activation of reflexes and central commands, and the generation of an appropriate respiratory motor output. Here, we will discuss work that aims to understand the role that metabotropic neurotransmitter systems play in central cardiorespiratory mechanisms. It is well known that blockade of glutamatergic, gamma-aminobutyric acidergic and glycinergic pathways causes major or even complete disruption of cardiorespiratory systems, whereas antagonism of other neurotransmitter systems barely affects circulation or ventilation. Despite the lack of an ‘all-or-none’ role for metabotropic neurotransmitters, they are nevertheless significant in modulating the effects of central command and peripheral adaptive reflexes. Finally, we propose that a likely explanation for the plethora of neurotransmitters and their receptors on cardiorespiratory neurons is to enable differential regulation of outputs in response to reflex inputs, while at the same time maintaining a tonic level of sympathetic activity that supports those organs that significantly autoregulate their blood supply, such as the heart, brain, retina and kidney. Such an explanation of the data now available enables the generation of many new testable hypotheses.
Keywords: cardiorespiratory integration, baroreflex, somatosympathetic, chemoreflex, peptide
1. Introduction
Sympathetic nerve activity is crucial for the regulation of many bodily functions including maintenance of arterial blood pressure, renal and reproductive function and vision. Despite decades of investigation, key questions about central cardiorespiratory regulation remain poorly understood and unexplored.
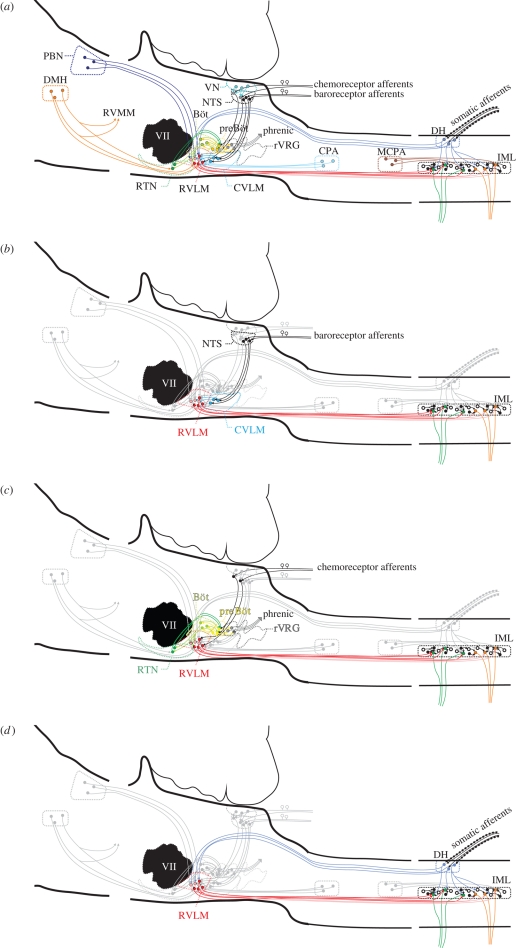
Figure 1a illustrates some pathways in the brainstem that can regulate the central control of the cardiorespiratory system. At the most simplistic level, central cardiovascular control is concerned with the maintenance of ‘tone’ in the cardiovascular system and the elaboration of reflex responses to sudden changes in blood pressure, oxygen, pH or other inputs such as regional requirements for increased oxygenation. Similarly, the same brainstem areas that regulate the cardiovascular system also contain neurons that generate a normal respiratory activity and are responsible for muscle tone in the upper airways, and for swallowing. The colocation of these functions could well be a consequence of their appearance in evolution rather than as a requirement for the control of the two systems. However, the colocation of all these vital systems, and their common blood supply, does mean that structural lesions in this region are commonly massively debilitating or fatal (Telerman-Toppet et al. 1982). Although our knowledge of the functional neuroanatomy of the ventrolateral medulla, which for the purposes of our discussion extends from the facial nucleus to the spinal cord is considerable, our knowledge of how the different groups of neurons form precise connections with other groups is still uncertain. Many of the ‘big’ questions that face cardiorespiratory neuroscientists today are the same as those that were prominent over the past decades. These intractable questions include:
How is blood pressure maintained at a mean of approximately 100 mm Hg so as to enable adequate perfusion of all organs?
What role do individual brainstem and spinal cord neurons play in the maintenance of blood pressure?
Why do so many of the important central neurons express so many different neurotransmitters and receptors?
Figure 1.
A diagram of pathways in the regulation of the cardiorespiratory system: (a) all pathways overlapped. The bulbospinal red pathways are in the RVLM (figure 2a) and integrate information from the centre and the periphery. The output from this nucleus is crucial for maintaining normal sympathetic tone. PBN, parabrachial nucleus; DMH, dorsomedial hypothalamus; CVLM, caudal ventrolateral medulla; VLM, ventrolateral medulla; rVRG, rostral ventral respiratory group; CPA, caudal pressor area; MCPA, medullo cervical pressor area; IML, intermediolateral cell column; RVMM, rostral ventromedial medulla; VII, facial nucleus; RTN, retrotrapezoid nucleus; preBöt, preBötzinger neurons; VN, vestibular nucleus. (b) The baroreflex pathway is shown on its own. Stretch receptor afferent neurons from the aortic arch and carotid sinus and the neurons synapse in the nucleus tractus solitarius (NTS). Neurons in the NTS then activate inhibitory neurons (blue) in the caudal ventrolateral medulla, which in turn inhibit the neurons in the RVLM; this intense gamma-aminobutyric acid (GABA)-mediated inhibition inhibits sympathetic outflow, causing blood pressure and sympathetic nerve activity to fall. Note also the yellow respiratory neurons that modulate the activity of the cardiovascular neurons (also in c). (c) The pathways for peripheral and central chemoreceptors are shown. Central chemoreceptors are highly responsive to changes in CO2 and are found in the retrotrapezoid nucleus. Many of these chemosensitive neurons (greater than 40%) are galaninergic and Phox2b positive, but all lack tyrosine hydroxylase (Stornetta et al. 2009; figure 2b). Peripheral chemoreception emanates from the carotid body. Neurons terminate in the medial NTS (like the baroreceptors). From here, the excitatory information passes to both respiratory and cardiovascular neurons. (d) The somatosympathetic pathway is shown in an abbreviated form. Afferent nociceptive pathways enter the spinal cord in the dorsal roots, activate circuits locally, and at several stations throughout the neuraxis including the RVLM. This pathway is excitatory and results in the appearance of a variable number of peaks in sympathetic nerve activity, depending on which nerve is recorded from. In the case of the greater splanchnic nerve, this is generally two peaks.
These questions are general, but their specific corollaries are no more tractable, and include:
Why do some sympathoexcitatory neurons in the rostroventrolateral medulla oblongata (RVLM) appear to have the capacity to synthesize adrenaline (Hökfelt et al. 1974; Phillips et al. 2001) and substance P (Pilowsky et al. 1986a,b; Milner et al. 1988a,b; Li et al. 2005), as well as many other neurotransmitters, in addition to glutamate?
Which brainstem neurons regulate which motor outputs?
Does the ‘chemical coding’ seen in different cardiorespiratory neurons correspond to a ‘functional fingerprint’?
The pot of gold at the end of this rainbow is a much better understanding of how the autonomic nervous system is regulated and consequently, the development of a deeper and more complete understanding of the pathophysiology of the underlying disorders of autonomic control, that range from hypertension to asthma, and cost our community considerable social and material capital.
First, it is necessary to take a step back to describe the system that we are investigating and then to select a manageable number of examples with which to develop the concepts discussed earlier. What is known about the central circuitry responsible for the maintenance of arterial blood pressure and the various reflex inputs that affect it? Normally, there is a continuous flow of excitation from the sympathetic nervous system to the periphery in order to maintain a basal level of tone to blood vessels, a certain amount of release of adreno-medullary hormones (adrenaline and noradrenaline) and a heart rate that can be varied up and down according to circumstance. Ventilation, on the other hand, is a discontinuous activity in the sense that the peripheral organs regulated by central respiratory generators stop their output entirely between each breath and, in certain circumstances, may not provide any output at all.
Figure 1a–d illustrates the neurons responsible for maintaining arterial blood pressure. The key thing to note is that a small nucleus in the rostral part of the RVLM, a tubular structure 0.6 mm in length and 0.2 mm in diameter in rats, defines its greater part. This nucleus is important because any intervention that decreases or eliminates its normal function causes sympathetic activity to fall to zero (acutely at least), all sympathetic reflex activity to be eliminated and blood pressure to fall to a level similar to that seen after high spinal cord transection (Schreihofer et al. 2005; Braga et al. 2007). This is the case in all vertebrates examined to date, from man (Telerman-Toppet et al. 1982) to rat (Suzuki et al. 1994; Pilowsky & Goodchild 2002; Miyawaki et al. 2003). Chronically, the contribution of the sympathetic nervous system in awake animals remains controversial, with some reports favouring an almost completely endocrine (angiotensin II, vasopressin) basis for the restoration of pressure, while others suggest a sympathetic component as well. Some studies favouring a role for sympathetic nerves in maintaining sympathetic nerve activity after spinal cord transection are based on pharmacological interventions with α and β adrenergic blockade. Modern studies suggest that sympathetic nerves are not active following acute or chronic cervical spinal cord transection (Trostel & Osborn 1994).
It has been clearly demonstrated that the RVLM is crucial for the maintenance of sympathetic tone and elaboration of reflex responses; both tone and reflex control are lost after acute destruction of the RVLM. On the other hand, there is little effect on arterial blood pressure or sympathetic nerve activity after the destruction of any other area unless the RVLM is also inactivated. Despite this, chemical inactivation of the RVLM, with the resultant immediate fall in sympathetic tone, blood pressure and abrogation of reflexes, does not eliminate the persistent potent hypertensive and sympathoexcitatory effects that can be elicited from other sites such as the medullo-cervical pressor area (Seyedabadi et al. 2006). Many other sites in the brain apart from the RVLM have direct or indirect inputs to sympathetic preganglionic neurons, but their activity, if any, appears insufficient to sustain any apparent sympathetic activity in the absence of the RVLM. A5 neurons, for example, have a direct spinal projection and are likely to innervate sympathetic preganglionic neurons (SPN); however, electrophysiological studies suggest that A5 neurons lack a cardiac-related rhythm in their firing pattern (Byrum et al. 1984). In fact, demonstrating a monosynaptic connection between an individual supraspinal neuron and an individual SPN has proved to be a very difficult problem. To date, only small numbers of barosensitive connections between the RVLM and the spinal cord have been revealed electrophysiologically (McAllen et al. 1994; Oshima et al. 2006, 2008). Similarly, the numbers of synapses between C1 neurons and SPN appear to be very small (Llewellyn-Smith et al. 1991). Figure 1b–d illustrates the sympathetic baro-, chemo- and somatosympathetic reflexes, respectively. The RVLM neurons and the inhibitory neurons in the caudal ventrolateral medulla also receive inputs that cause sympathetic activity to burst in phase with phrenic nerve discharge (inspiration; Haselton & Guyenet 1989; Miyawaki et al. 1995; Mandel & Schreihofer 2006).
2. Historical aspects
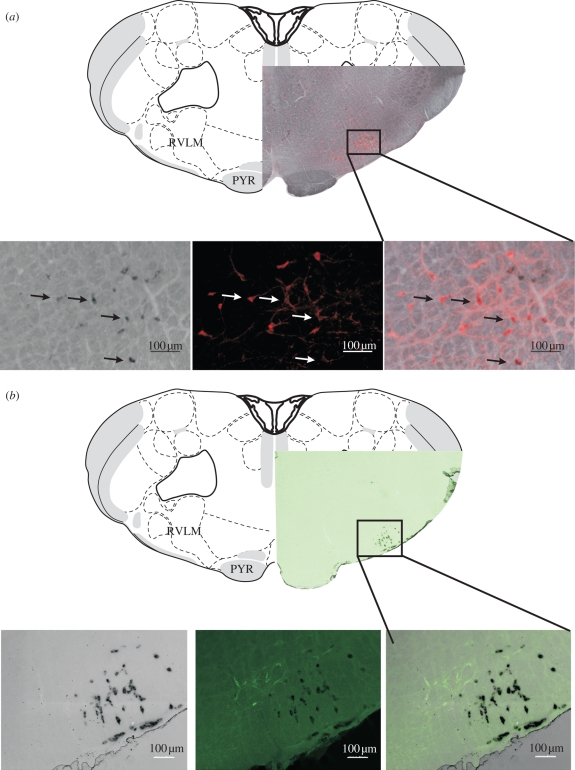
Early studies, in the late nineteenth century, identified the ventral brainstem as an area crucial for the tonic and reflex regulation of the cardiovascular system (Fye 1986; Seller 1996). Subsequently our understanding of the different compartments of the ventral brainstem has become more and more refined (Pilowsky & Goodchild 2002) as techniques such as drug microinjection (Goodchild et al. 1982; Lipski et al. 1988; Monnier et al. 2003) and electrophysiology combined with dye labelling, immunohistochemistry and tract tracing were applied to develop our understanding of these regions (Pilowsky et al. 1994b; Sun et al. 1994, 1995, 1997). The neurochemical and receptor content of barosensitive bulbospinal neurons in the RVLM has been the subject of intensive investigation over the past 30 years since the first report by Hökfelt and colleagues in 1974 (Hökfelt et al. 1974) that a population of neurons existed in the RVLM (figure 2) that contained the enzyme phenylethanolamine-N-methyltransferase (PNMT), which is the key (but not rate limiting) enzyme in the biosynthetic pathway for adrenaline. Subsequent studies combining immunohistochemistry, PCR and in situ hybridization revealed that many bulbospinal neurons in the RVLM contained all of the biosynthetic markers necessary for the production of adrenaline (Phillips et al. 2001). These PNMT-containing RVLM neurons are termed the C1 cell group (figure 2a). ‘A’ neurons (e.g. A1 neurons in the brainstem, A6 in the locus coeruleus or A10 that form the substantia nigra) lack PNMT and perform crucial functions throughout the neuraxis from the brainstem to the retina. ‘B’ neurons (B1, B2 and B3) synthesize serotonin and are located in the midline. Initially, it was believed that C1 neurons (Goodchild et al. 1984; Ross et al. 1984) were responsible for regulating sympathetic vasomotor pathways through the release of adrenaline in the spinal cord, but it eventually became clear that both C1 and non-C1 neurons also release glutamate. The actual role of adrenaline released at the level of sympathetic preganglionic neurons still remains unclear (Bolme et al. 1974). The possibility exists that it exerts complex effects depending on the post-synaptic receptor present, and if an inhibitory interneuron is interposed (Shi et al. 1988; Coote & Lewis 1995).
Figure 2.
Neurotransmitter phenotypes in the RVLM (PYR, pyramidal tract). (a) Neurons in the RVLM that express PNMT (in situ hybridization—black) and are also immunofluorescent for tyrosine hydroxylase (red). The third panel shows a merged image and demonstrates colocalization in many of the cells. The drawing is taken from map 59 of Swanson (1998). Note that the pyramidal tract is present but the olivary nucleus is absent. Note also that both the rostral pole of the nucleus ambiguus and the caudal pole of the facial nucleus are present. These ventral landmarks define the rostrocaudal location of the RVLM (as indicated by the boxed area). (b) Galanin (pre-progalanin-expressing—black) neurons in the retrotrapezoid nucleus are close to and partly intermingled with tyrosine hydroxylase (green) immunofluorescent neurons in the RVLM.
3. Physiological regulation of blood pressure and respiration
The objectives in the regulation of blood pressure and the circulation of blood to specific organs at specific times are related to the objectives of ventilation. The prime objective of the cardiovascular system is to ensure an adequate flow of blood and plasma through the various organs so as to achieve goals that include: removal of carbon dioxide and delivery of oxygen (pulmonary), delivery of local and systemic hormones (renal, adrenal pituitary and many others), delivery of metabolic waste to the kidney and, as a consequence, of these activities the maintenance of a normal electrolyte and fluid-balance status. It is not possible in this short review to elaborate in detail the synchrony necessary for all of the bodily functions in the maintenance of homeostasis. Ventilation on the other hand is crucial for moment-to-moment acid–base control and oxygenation, as well as other functions such as vocalization.
In order to achieve the objectives remarked upon earlier, three components are coupled in order to govern normal activity: afferent, integrative and motor.
(a). Afferent pathways to the autonomic nervous systems
The peripheral and central systems that provide a motor output to blood pressure and breathing pathways sample information from sensory neurons that can be in the periphery (e.g. baroreceptors, figure 1a,b) or central nervous system (e.g. chemoreceptors) and then relay this information to the autonomic centres in the brainstem that generate tone and bursting activity. Baroreceptor afferent pathways (figure 1a,b) arise as nerve endings on the adventitia of the aortic arch or carotid sinus (Ciriello 1983; McDonald 1983; Pilowsky & Goodchild 2002); when blood pressure increases, baroreceptor nerves increase their firing rate. The information is then transmitted to the medial subnucleus of the nucleus of the solitary tract: a nucleus in the dorsomedial medulla that integrates (Smith et al. 2002) information from many sources and relays it to many places in the central nervous system including the ventral medulla. The fidelity of transmission in this pathway is excellent. It was recently reported that neurons receiving inputs from aortic arch baroreceptors have properties that are consistent with inputs arising from a single branched axon, a finding supported by anterograde tracing (Andresen & Peters 2008). These and other findings demonstrating the presence of ionotropic glutamate receptors (Aicher et al. 2002) suggest that fast neurotransmitters coupled to ligand-gated ion channels underlie these phenomena. The electrophysiological and morphological data are also supported by pharmacological data demonstrating that the baroreceptor reflex is blocked following microinjection of antagonists to glutamate ionotropic receptors into the medial nucleus tractus solitarius (NTS) (Gordon & Leone 1991). Other evidence suggests that neuropeptides such as somatostatin may act as powerful longer-acting modulators of function at these sites (Chan et al. 1992). This means that while fast neurotransmission is essential for the full expression of the reflex, it is definitely not the case that the involvement of other regulators of cellular activity such as peptides is precluded. What remains to be determined is when and in what situations all of these neurotransmitters are released. These questions are clearly not restricted to the neural regulation of the cardiovascular system.
(b). Cardiorespiratory integration
A great deal has been written on this topic (Baekey et al. 2008). Respiratory modulation of sympathetic nerve activity was first convincingly demonstrated in recordings of sympathetic nerve activity in 1932 (Adrian et al. 1932). The morphological substrates that might enable such a phenomenon to occur are a connection between neurons with respiratory modulation and those responsible for cardiovascular regulation. Such connections have been reported in cats (Pilowsky et al. 1994b) and rats (Pilowsky et al. 1992; Sun et al. 1997). Barosensitive neurons in both the rostral (Haselton & Guyenet 1989; Miyawaki et al. 1995) and caudal ventrolateral medulla (Mandel & Schreihofer 2006) are known to have a respiratory modulation that is most probably derived centrally because it is not locked to the phase of the ventilator in vagotomized and paralysed animals.
The main sites of integration of central cardiorespiratory regulation are located in the ventral medulla oblongata close to the facial nucleus and then caudally to the junction of the brainstem and spinal cord. The effects of chemical activation of brainstem cardiovascular sites are diverse; in the RVLM, glutamate is pressor and sympathoexcitatory while more caudally, depressor and sympathoinhibitory responses are obtained (Ross et al. 1983; Goodchild et al. 1984; Pilowsky et al. 1985; Guyenet et al. 1989). Chemical inhibition of these sites causes opposite effects and blocks the aortic nerve baroreceptor reflex (Willette et al. 1984; Pilowsky et al. 1985). Approximately 1 mm caudally lie the GABAergic neurons of the CVLM that are an integral part of the sympathetic baroreflex (Schreihofer & Guyenet 2003). In the most caudal parts of the brainstem—close to the cervical spinal cord—potent pressor and sympathoexcitatory effects can be elicited by chemical stimulation (Seyedabadi et al. 2006). The RVLM is considered to be absolutely crucial for tonic control of autonomic function and for the regulation of almost all autonomic reflexes (figure 1a–d). Although destruction of the RVLM completely eliminates sympathetic tone and reflexes, it is not the only site from which independent increases in sympathetic activity can be obtained; even after complete destruction of the RVLM, stimulation of the medullo-cervical pressor area can still evoke pressor and sympathoexcitatory effects (Seyedabadi et al. 2006).
There is still no consensus on how basal sympathetic tone is maintained. There are at least three possibilities. First, neurons in the RVLM may have membrane properties that cause them to fire at a particular rate at all times. Second, the activity of neurons in the RVLM may simply represent the sum of all inputs at any one time, and third, activity may be derived from the neurons in the RVLM as a combination of both possibilities. In fact, appealing though the first possibility is, the evidence for it as the mainstay of activity generation remains uncertain. In brainstem slices from neonatal rats, 50 per cent of C1 neurons have pacemaker properties. The pacemaker properties are voltage dependent and not dependent on synaptic input. The pacemaker properties were attributed to the presence of a persistent sodium current (Kangrga & Loewy 1995). The second possibility also holds some appeal. In adult anaesthetized rats, sharp intracellular recording of bulbospinal neurons, inhibited by baroreceptor input, revealed that the neurons—many of which were C1 neurons—were continuously bombarded by inhibitory and excitatory post-synaptic potentials, but without any sign of pacemaker properties (Lipski et al. 1995, 1996). Calcium channels of all types also appear to be important in the normal functioning of C1 neurons and their ability to respond to a range of metabotropic neurotransmitters makes them attractive candidates in this regard (Li et al. 1998; Miyawaki et al. 2003). Perhaps the likeliest explanation of how these neurons operate is that they do have intrinsic biophysical properties that enable them to generate activity in certain circumstances, but that at most times they are regulated by external inputs to such an extent that these properties are less apparent. In certain preparations, such as slices from the neonatal rat, perhaps these other properties may become more evident. The recently described neurons in the retrotrapezoid nucleus (RTN; figures 1a,c and 2b) that are believed to be exquisitely chemosensitive have also been reported to have pacemaker-like activity in slices (Guyenet 2008). The pH sensitivity in both serotonin and RTN neurons is thought to be mediated by a K+ channel (TASK—in the case of serotonin neurons since the pH sensitivity is abolished in TASK knockout animals Mulkey et al. 2007b).
Other medullary sites, including neurons in the midline, may also be important in the control of blood pressure (Minson et al. 1987), with activation of sites towards the midline medulla that contain serotonin neurons causing an increase in blood pressure that is associated with a release of serotonin in the spinal cord (Pilowsky et al. 1986a,b).
(c). Respiratory integration
In contrast to the uncertainty about how rhythmogenesis is generated in sympathetic outflow, there is greater consensus with respect to the respiratory system. As with the cardiovascular system, a column of nuclei present in the ventrolateral medulla is essential for the elaboration of normal (eupnoeic) respiratory activity. Put simply, phasic respiratory activity in different motor outputs is achieved through the sequential activation of populations of neurons that fire in the inspiratory or the expiratory phase. Both inspiratory-active and expiratory-active neurons may be either inhibitory or excitatory, depending on their neurotransmitter content and the precise phase of inspiration or expiration in which they are active. The key populations are the Bötzinger and pre-Bötzinger neurons (Smith et al. 1991; Sun et al. 1998; Koshiya & Smith 1999). Neurons in the preBötzinger region, particularly, have the electrical properties necessary to generate rhythm, and the morphological properties (extensive axon collateral arborizations) required to compose and distribute the generated activity into a form appropriate to the relevant output pathways (Pilowsky et al. 1990b). Thus, the motoneurons of the larynx will cause vocal cord dilation just prior to the start of diaphragmatic contraction (Berkowitz et al. 1999a,b). The ventrolateral medulla is not the only brainstem site that may be important in respiratory regulation; recently a site in the midline between the caudal poles of the facial nucleus was reported where chemical excitation potently inhibits respiration (Verner et al. 2004, 2008) with little effect on blood pressure. The physiological significance of this site remains to be determined. Conceivably, this site is the endogenous source of the substance P that can influence respiratory neurons (Holtman et al. 1984; Gatti et al. 1999; Guyenet & Wang 2001; Sun et al. 2003; Mulkey et al. 2007a). Medullary nuclei outside the RVLM may also play a role in respiratory control. In particular, serotonergic neurons in the midline (Severson et al. 2003; Richerson 2004) and noradrenergic neurons (Li & Nattie 2006) may also play a role in responding to changes in chemoreceptor activation and transmitting this information to cardiorespiratory regulatory regions. Many authors have examined the importance of supra-medullary regions on respiratory regulation (Dawid Milner et al. 2003; Voituron et al. 2005); these regions will not be discussed here.
(d). Motor pathways
The final step following generation of activity is to distribute the information generated to the relevant motor output pathways.
(i). Respiratory system
Motor output pathways in the respiratory system are relatively uncomplicated in that there is a direct connection between a respiratory-generating neuron and a motoneuron, or there is an interposed pre-motoneuron that in turn activates a motoneuron. The axons of respiratory motoneurons may be relatively uncomplicated with either few (Hilaire et al. 1983; Lipski et al. 1985; Pilowsky et al. 1990a) or extensive recurrent collateral arborizations (Hilaire et al. 1983).
(ii). Cardiovascular system
The terminology ‘pre-motoneuron’ or pre-sympathetic neuron is also used in cardiovascular regulation, but the nature of the physiological role played by all of the pre-sympathetic neurons is uncertain. The neurons in the RVLM that are spinally projecting and barosensitive are often depicted in diagrams as simple neurons that project to the spinal cord where they excite sympathetic preganglionic neurons. Commonly, when discussing the tonic and reflex regulation of the sympathetic nervous system, the main focus is the RVLM for reasons noted above; however, there are at least five other areas (above the spinal cord) that project caudally and are thought to be pre-sympathetic (Jansen et al. 1992; Krout et al. 2003; Seyedabadi et al. 2006). As noted above, this is part of the story, but by no means all of it. One very careful anterograde tracing study that used viral tracing combined with specific promoters so as to target only C1 neurons reported that while the expected dense projection to the intermediolateral cell column was indeed present, other targets also received an innervation, including a sparse but definite projection to the contralateral RVLM (Card et al. 2006). Evidence for an intramedullary projection of C1 neurons was also provided by Madden et al. (1999). who reported that following selective unilateral lesion of C1 neurons with antibodies to the adrenergic membrane protein dopamine-β-hydroxylase conjugated to the ribosomal neurotoxin saporin, there was a loss of some C1 neurons on the contralateral side. One plausible explanation for such data is that there is network activity or at least coordination between the two pre-motor cell groups in the same way as occurs in the respiratory system. However, there are other explanations—including the possibility that C1 neurons are indeed auto-active in some circumstances (Kangrga & Loewy 1995; Li et al. 1995), or that RVLM neurons simply act to integrate central and peripheral inputs without requiring intrinsic mechanisms to maintain activity. Clearly, much more work is needed to understand the extent to which these different possibilities are important parts of the whole (Lipski et al. 2002). At the moment, what is lacking is convincing evidence of a phenomenon of centrally generated sympathetic rhythms that require explanation in the same way that respiratory rhythms are needed. In fact, removal of baroreceptor inputs in conscious rats eliminates peaks in frequency spectra in arterial pressure apart from that caused by respiration (Kunitake & Kannan 2000), suggesting that there is no intrinsic oscillator that affects sympathetic output.
Evidence does exist for connections between functionally characterized sympathetic pre-motor neurons and sympathetic preganglionic neurons, although the precise role of individual connections between pre-motoneurons at supraspinal levels and sympathetic preganglionic neurons in the spinal cord is still uncertain. Monosynaptic connections using correlation techniques have been reported in cats (McAllen et al. 1994) and rats (Oshima et al. 2006). Recently, this result has been confirmed by spike-triggered averaging experiments combining extracellular recording from brainstem neurons with whole-cell patch clamp recording from sympathetic preganglionic neurons (Oshima et al. 2008). Of the many types of preganglionic neuron (Jänig & McLachlan 1992), cardiovascular sympathetic preganglionic neurons (as defined by the presence of bursts of excitatory post-synaptic potentials (EPSPs) in phase with phrenic nerve discharge and a slowly conducting axon) only form a small proportion of the total number of sympathetic preganglionic neurons in the spinal cord (approx. 7%). These cardiovascular sympathetic preganglionic neurons generally have small somata, but extremely extensive dendritic trees (Pilowsky et al. 1994a). The large amount of axonal arborizations from PNMT immunoreactive terminals in the intermediolateral cell column combined with the extensive dendritic arborizations of sympathetic preganglionic neurons suggests the possibility of considerable divergence in the bulbospinal input pathways. However, the electron-microscopic evidence in favour of an extensive input to sympathetic preganglionic neurons is not strong (Milner et al. 1988a,b; Llewellyn-Smith et al. 1991). There are strong teleological arguments in favour of this idea (ensuring that general vasoconstriction occurs where needed throughout vascular beds), but equally strong counterarguments (in that organ-specific vasoconstriction is also needed and that sympathetic activity can be controlled differentially). Clearly, additional experimentation—and possibly novel tools—is needed.
4. Neurotransmission
It is generally accepted that within the central nervous system three ionotropic neurotransmitters are primarily responsible for regulating activity in cardiorespiratory pathways viz. glutamate, GABA and glycine. Others, such as acetylcholine (Shao & Feldman 2001) and serotonin may also act on ligand-gated ion channels to exert rapid changes in membrane potential, but the effect of these latter neurotransmitters—as well as that of others—discussed below, is principally exerted on metabotropic receptors that are coupled to G proteins (Martin 1992; Pelat et al. 1999; Padley et al. 2005). Cannabinoids (Padley et al. 2003), gases such as nitric oxide (Zanzinger et al. 1995; Kishi et al. 2002; Gao et al. 2008) and other novel mediators are also part of the environment that influences the long- and short-term activity of cardiorespiratory neurons.
The ‘simplistic’ understanding of how G-protein-coupled receptors (GPCRs) work is clouded by the discovery that dimerization (both between the same receptor type and between different receptor types) can lead to activation of entirely different signal transduction pathways with effects that are the reverse of those normally seen (e.g. Duran-Prado et al. 2008). This is not the place for a detailed discussion of the complexities of G-protein signalling (Achour et al. 2008), but it is important to note that since they are the largest family of receptor-coupled proteins, abductive reasoning (Haig 2008) suggests that they play a very significant role in modulating the physiological interactions of neurons that are crucial for cardiorespiratory regulation.
In fact, almost all neurotransmitters exert their effects through multiple receptors that may be either ionotropic, metabotropic, inhibitory or excitatory. This ability of a neurotransmitter to exert more than one effect depending on the receptor expression profile of its target can be termed ‘pleiotropy’. The pleiotropic effects of neurotransmitters do not necessarily disprove Dale's principle as elaborated by Eccles (Burke 2006), that a neuron will release all of its neurotransmitters at all of its synapses, but it does seem to diminish the utility of the hypothesis if it means that the response to the release of such neurotransmitters may be completely different depending on the pre- or post-synaptic receptor profile. The hypothesis is further diminished by some more unusual circumstances where spatial and temporal release segregation of neurotransmitters from a neuron occurs (Sossin et al. 1990).
Because so much of the moment-to-moment control of neural networks appears to be under the control of ionotropic receptors that are operated by glutamate, GABA and glycine, we have attempted to define the role played by other neurotransmitters with a combination of microinjection into specific brain nuclei and analysis of specific reflexes. To achieve this, we generally use an adult ‘semi-reduced’ in vivo preparation in which only the sympathetic nervous system is active (achieved by vagotomy and atropine administration). We then record from at least one sympathetic nerve (generally the greater splanchnic) and the phrenic nerve, and activate baroreceptors (figure 1a,b), chemoreceptors (figure 1c) or somatic afferent neurons (figure 1d) before and after administration of agonist and antagonist agents. In this preparation, changes in heart rate may also represent a surrogate sympathetic output to the heart (integrating both direct neural input and influences from circulating catecholamines) as the vagi are cut.
Our findings reveal that there is a clear discrimination of different receptors on different types of reflexes, supporting our initial hypothesis. Here I will briefly survey some of these findings in relation to some of the neurotransmitters that we have examined.
5. Serotonin in cardiorespiratory regulation
Serotonin is a compound that has entered popular consciousness because of its positive effects on mood in patients suffering from depression. It is present in a restricted population of neurons in the brainstem, but the influence of these neurons is felt throughout the central nervous system. In the spinal cord, serotonin densely innervates phrenic motoneurons (Holtman 1988; Holtman et al. 1990; Pilowsky et al. 1990a) and sympathetic preganglionic motoneurons (Pilowsky et al. 1995a). It is released in the spinal cord following activation of cell bodies in the brainstem (Pilowsky et al. 1986a,b) and plays a role in plasticity in long-term potentiation of phrenic neural activity following intermittent hypoxia (Baker-Herman & Mitchell 2002). The precise mechanism of action of serotonin is not established in all systems because of its many receptors (Hoyer et al. 1994) and because of the many neurotransmitters that are coreleased with it (Jansen et al. 1995).
Does the anatomical finding of serotonin in the different spinal nuclei suggest specific functions? The answer here is unfortunately no. The neurons that provide the serotonergic input must come from the caudal Raphé as this is the only source of such cells (Pilowsky et al. 1995b; Lalley et al. 1997; Mason 1997; Richerson et al. 2001; Ootsuka et al. 2004). The many studies conducted on Raphé neurons suggest that individual cells may influence functions as diverse as control of pain, blood pressure and motor function. Furthermore, it seems that most serotonergic neurons contain other neurotransmitters including peptides and amino acids (Jansen et al. 1995) so that it is possible—even quite likely—that the majority of the effects mediated by Raphé neurons are not due to the release of serotonin. Thus, working out which Raphé neurons release which neurotransmitters, and under what circumstances, to mediate which effects, are all mysteries. The advantage of furthering our knowledge in this regard is that it may lead to the development of therapies that have greater specificity in targeting functions. One possibility is that serotonergic neurons play a system-wide role in raising tone in autonomic regions so that individual reflexes or functions become more or less sensitive depending on the activity of the inputs as suggested by workers using Fos studies and carbon dioxide exposure, who found a widespread activation of serotonin neurons (Haxhiu et al. 2001). Recently, it was reported that serotonin directly excited chemosensitive neurons, but that this occurred via a mechanism that was distinct from the ability of these chemosensors to detect change in pH (Mulkey et al. 2007a). The idea of a widespread role in modulating autonomic functions is further supported by reports that serotonergic, and noradrenergic, inputs are excitatory to hypoglossal motoneurons, and that the withdrawal of these inputs may be an underlying factor in muscle atonia in rapid-eye-movement sleep (REM sleep; Fenik et al. 2005). Moreover, in mice that lack the serotonin transporter, there is a disturbance in REM sleep compared with control mice (Wisor et al. 2003). A differential serotonergic input onto laryngeal motoneurons also exists, with constrictor motoneurons receiving a greater input than dilator motoneurons (Sun et al. 2002; Berkowitz et al. 2005).
In rats, if the serotonin 1a (5HT1a) receptor agonist 8-hydroxy-di-n-propylamino tetralin is microinjected bilaterally into the RVLM, there is a fall in arterial blood pressure and sympathetic blood pressure along with a decrease in the amplitude of phrenic nerve discharge (Miyawaki et al. 2001). The effect is by no means as large as the potent effects that can be achieved with GABA or glutamate, and on average, blood pressure only fell by 13 mm Hg. Despite this apparently modest effect on resting parameters, the effects on reflex function were profound. The two peak somatosympathetic reflexes seen in ensemble averages of splanchnic nerve activity were completely abolished, while baroreceptor function and hypoxia (10 s of 100% nitrogen instead of 100% oxygen) were unimpaired. All effects were prevented by pre-treatment with the 5HT1a antagonist NAN-190, which by itself had no effect on any measured parameters (Miyawaki et al. 2001).
6. Catecholamines in cardiorespiratory regulation
Catecholamines are also major players in the central regulation of cardiorespiratory neurons. Mainstays of the chemotherapy of hypertension such as alpha-methyldopamine, clonidine and moxonidine are thought to act through neurons in the RVLM. Clonidine, via activation of central alpha-2 receptors, causes a decrease in ventilation and is hypotensive and sympatholytic (Bolme et al. 1974; Koshiya & Guyenet 1995; Guyenet 1997; Grubb et al. 1998). Interestingly, with respect to cardiorespiratory integration, the post-inspiratory phase of sympathetic nerve activity is more sensitive to the sympatholytic effects of clonidine than in the inspiratory phase (Koshiya & Guyenet 1995), suggesting that sympathetic activity and arterial pressure are preserved preferentially during the inspiratory period; an effect that may serve to enhance oxygen delivery to tissue.
7. Peptides in cardiorespiratory regulation
Why look at peptides and other colocalized neurotransmitters if amino acids do all the work? The simple answer is that all of the ‘work’ is not done by short-acting transmitters. More importantly, there appears to be a segregation of function according to the different metabotropic transmitter receptors that are activated or inhibited. This means that one neuropeptide may selectively antagonize the somatosympathetic reflex (figure 1d) but not the baro- (figure 1b) or chemo-reflex (figure 1c). This is the general theme that we have been pursuing in our laboratory over the past 10 years.
It is not possible to survey all the peptides that have been implicated in cardiorespiratory regulation, so I will aim to mention only those for which there is at least some physiological or pharmacological evidence for a role in cardiorespiratory regulation.
(a). Opioids
Opioids are one of the first classes of peptides discovered and have a long history, scientifically (two Nobel prizes), socially (drug addiction) and in the literature (Dorothy falling asleep in a field of poppies in the Wizard of Oz). In combining all three, we need look no further than the occasionally opium- (as well as cocaine-) addicted, forensic scientist of literary fame: Sherlock Holmes. Opioids are famous as centrally acting cardiorespiratory depressants.
As a class of ligands, opioids bind to receptors (GPCRs) on the cell membrane that are coupled to G proteins (usually Gi/o in the case of opioid receptors; Wettschureck & Offermanns 2005) both inside and outside the nervous system (Wu & Wong 2005). The effect of the activation of opioid receptors is almost uniformly inhibitory and the intracellular mechanisms that mediate the cellular hyperpolarization that causes this inhibition depends on the opening of potassium channels, and inhibition of adenylate cyclases, among other things.
C1 neurons are themselves opioidergic and receive opioid inputs (Stasinopoulos et al. 2000; Stornetta et al. 2001). To address the relationships between opioid systems and cardiorespiratory neurons, we and others have used a range of approaches, including histological, pharmacological, electrophysiological and physiological. These studies reveal many facets of the way in which opioids can interact with cardiorespiratory neurons. Many inputs to C1 pre-sympathetic neurons are immunoreactive for the delta-opioid receptor, although the pre-sympathetic neurons themselves are not (Stasinopoulos et al. 2000). Microinjection of the delta agonist [D-Pen2;5]-enkephalin (DPDPE) has complex effects on central cardiovascular regulation (Miyawaki et al. 2002). Immediately following microinjection into the RVLM bilaterally, DPDPE causes a fall in arterial blood pressure and a reduction in lumbar sympathetic nerve activity (LSNA). The reduction in LSNA is principally associated with an almost complete loss of the post-inspiratory peak normally seen in the activity of this nerve. Testing of reflexes reveals that opioids that exert their effects via delta receptors have quite different effects from those seen following mu-agonist administration. While the somatosympathetic reflex is abolished, the sympathetic baroreflex and the chemoreflex (ventilation with 100% nitrogen for 10 s) are completely unaffected. A similar selective reduction in the somatosympathetic reflex can be achieved with hypercarbia (Makeham et al. 2004).
Mu-opioid receptors in the RVLM exert quite different effects when activated: arterial blood pressure and sympathetic nerve activity also fall, and the chemoreceptor reflex is also unaffected. However, in contrast to delta receptor agonism in the RVLM, mu-opioid agonism causes an attenuation of the sympathetic baroreceptor reflex with no effect on the somatosympathetic reflex (Miyawaki et al. 2002). Mu-opioid receptors are found both pre- and post-synaptically on neurons in the RVLM with a very marked post-synaptic preponderance (Aicher et al. 2001). Such a morphological arrangement would permit mu agonists to occlude baroreceptor inputs arising from inhibitory neurons in the caudal ventrolateral medulla (figure 1b); it remains to be determined in which of the afferent pathways mu receptors are found, but if they are absent from those mediating the somatosympathetic reflex and the chemoreceptor reflex, then the relative lack of effect on these reflexes would be more easily understood.
(b). Neuropeptide Y
Neuropeptide Y (NPY) is expressed in the RVLM (Agnati et al. 1988) and is colocalized with many C1 neurons that project to the hypothalamus (Li & Ritter 2004), but in only approximately 9 per cent of those that project to the spinal cord (Blessing et al. 1987; Stornetta et al. 1999). Most studies on a potential physiological role for NPY in the spinal cord have focused on its importance in mediating painful stimuli (Shi et al. 2006). Recently, it was found that intrathecal NPY will attenuate somatosympathetic (Kashihara et al. 2008) and noxious stimuli (Mahinda & Taylor 2004). Some authors report that NPY will induce pressor responses when delivered intrathecally, although this response is not always found (Mahinda & Taylor 2004). In any event, it is not known if any physiologically relevant pressor response to spinal NPY release is mediated by such a small population of bulbospinal sympathoexcitatory neurons, or by activation of ascending nociceptive pathways.
(c). Apelin
Apelin is a relatively new peptide. It acts through its own GPCR known as the APJ receptor and has effects peripherally and centrally. Despite some sequence similarities with the angiotensin II type 1 receptor, the two peptides do not bind to the others receptors. Generally, the effects of apelin in the periphery (Chandrasekaran et al. 2008) are opposite to those of angiotensin II, with apelin causing effects that are hypotensive. Few studies have examined possible roles for apelin in the central regulation of the cardiorespiratory system. Microinjection studies from our laboratory suggest that apelin has effects on blood pressure and phrenic nerve discharge in two key cardiorespiratory nuclei in the brainstem, viz., the NTS and the RVLM (Seyedabadi et al. 2002). A physiological role for apelin remains to be determined.
(d). Angiotensin II
Angiotensin peptides are among the most extensively investigated peptides in central cardiorespiratory regulation. Most studies focus on the effect of angiotensin II on angiotensin type-1 receptors. The roles of angiotensin II in homeostasis are broad. Here I will focus on only a few. Following intravenous injection, one of the effects of angiotensin II is to activate neurons in the circumventricular organs. This binding activates neuronal pathways that project from the hypothalamus either directly to the sympathetic motor pathways in the spinal cord or via a synapse in the RVLM (Li et al. 1992). The phenotype of the input to RVLM may be in part cholinergic (Kubo et al. 2002), but is not completely clarified. Qualitatively, the effect of vasoconstriction with angiotensin II is quite different from that seen with a peripherally acting alpha-1 agonist such as phenylephrine. Despite very high levels of arterial blood pressure that can be achieved with angiotensin II, sympathetic nerve activity is not abolished and baroreceptors are still effective in lowering arterial blood pressure and suppressing sympathetic nerve activity (McMullan et al. 2007). Equally surprising is the finding that some bulbospinal baroinhibited neurons in the RVLM are not inhibited following intravenous angiotensin II and some baroinhibited neurons are actually activated (McMullan et al. 2007).
Angiotensin II receptor activation is also known to exert clear effects in different parts of the ventrolateral medulla. Microinjection into the caudal ventrolateral medulla causes vasopressin release, although effects on respiration were not documented in that study (Allen et al. 1990). Microinjection of angiotensin II into the RVLM increases arterial blood pressure, an effect that is mediated by MAP kinase in normotensive rats and by both MAP kinase and PI3 kinase in hypertensive rats (Seyedabadi et al. 2001). When applied to individual C1 neurons in RVLM, angiotensin II causes a depolarization mediated by closure of potassium conductance (Li & Guyenet 1995, 1996). In a bath preparation, application of angiotensin II to the brainstem excites neurons, which in turn project to, and excite, sympathetic preganglionic neurons (Oshima et al. 2008).
(e). Other peptides
As noted earlier, many other peptides play an important role in the tonic and reflex regulation of the cardiorespiratory systems, including substance P (Gilbey et al. 1983; Makeham et al. 2001, 2005), pituitary adenylate cyclase-activating polypeptide (PACAP; Farnham et al. 2008), somatostatin (Burke et al. 2008), galanin (Stornetta et al. 2009; figure 2b), which is present in many chemosensitive neurons in the retrotrapezoid nucleus, and thyrotropin-releasing hormone (Murphy et al. 1995; Sun et al. 1995, 1996). The effects of these neuromodulators are site specific and in most cases their physiology is still very poorly understood.
8. Answers to questions
Can we provide any insights into the questions posed at the beginning? In answer to the question of why cardiorespiratory neurons appear to have multiple neurotransmitters, it seems that we are not well advanced. Can we tell if different brainstem neurons that contain different populations of neurotransmitters are targeted to different functional populations of motor outputs? The evidence in support of this proposition is weak, although we do know, for example, that C1 neurons are not respiratory neurons (Pilowsky et al. 1990b). Within the separate populations of cardiovascular and respiratory neurons, finer discrimination still eludes us. Can we associate different neurochemicals to specific populations of neurons? Here we are doing a little better, some C1 neurons (at least 26%) are definitely bulbospinal and inhibited by baroreceptors (Lipski et al. 1995). It also seems likely that most, if not all, bulbospinal C1 neurons are PACAP containing (Farnham et al. 2008) and that approximately 18 per cent of C1 neurons contain substance P (Li et al. 2005). Substance P (Solomon et al. 1999) and PACAP (Farnham et al. 2008) are both known to be sympathoexcitatory when injected intrathecally. The extent to which different populations of neurochemically identified neurons define specific functional pathways and the physiological roles that they may play remain mysterious and a challenge for future studies.
Acknowledgements
Work in the authors laboratory is supported by grants from the National Health and Medical Research Council of Australia (457080, 457069) and the Garnett Passe and Rodney Williams Memorial Foundation. The authors are grateful to Mr Peter Burke for his comments on the manuscript.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Brainstem neural networks vital for life’.
References
- Achour L., Labbe-Jullie C., Scott M. G. H., Marullo S.2008An escort for GPCRs: implications for regulation of receptor density at the cell surface. Trends Pharmacol. Sci. 29, 528–535 (doi:10.1016/j.tips.2008.07.009) [DOI] [PubMed] [Google Scholar]
- Adrian E. D., Bronk D. W., Phillips G.1932Discharges in mammalian sympathetic nerves. J. Physiol. 74, 115–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnati L. F., Fuxe K., Zoli M., Zini I., Härfstrand A., Toffano G., Goldstein M.1988Morphometrical and microdensitometrical studies on phenylethanolamine-N-methyltransferase- and neuropeptide Y-immunoreactive neurons in the rostral medulla oblongata of the adult and old male rat. Neuroscience 26, 461–478 (doi:10.1016/0306-4522(88)90162-5) [DOI] [PubMed] [Google Scholar]
- Aicher S. A., Schreihofer A. M., Kraus J. A., Sharma S., Milner T. A., Guyenet P. G.2001Mu-opioid receptors are present in functionally identified sympathoexcitatory neurons in the rat rostral ventrolateral medulla. J. Comp. Neurol. 433, 34–47 (doi:10.1002/cne.1123) [DOI] [PubMed] [Google Scholar]
- Aicher S. A., Sharma S., Mitchell J. L.2002Co-localization of AMPA receptor subunits in the nucleus of the solitary tract in the rat. Brain Res. 958, 454–458 (doi:10.1016/S0006-8993(02)03693-4) [DOI] [PubMed] [Google Scholar]
- Allen A. M., Mendelsohn F. A. O., Gieroba Z. J., Blessing W. W.1990Vasopressin release following microinjection of angiotensin II into the caudal ventrolateral medulla oblongata in the anaesthetized rabbit. J. Neuroendocrinol. 2, 867–873 (doi:10.1111/j.1365-2826.1990.tb00653.x) [DOI] [PubMed] [Google Scholar]
- Andresen M. C., Peters J. H.2008Comparison of baroreceptive to other afferent synaptic transmission to the medial solitary tract nucleus. Am. J. Physiol. 295, H2032–H2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekey D. M., Dick T. E., Paton J. F. R.2008Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp. Physiol. 93, 803–816 (doi:10.1113/expphysiol.2007.041400) [DOI] [PubMed] [Google Scholar]
- Baker-Herman T. L., Mitchell G. S.2002Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J. Neurosci. 22, 6239–6246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz R. G., Sun Q. J., Chalmers J., Pilowsky P.1999aIdentification of posterior cricoarytenoid motoneurons in the rat. Ann. Otol. Rhinol. Laryngol. 108, 1033–1041 [DOI] [PubMed] [Google Scholar]
- Berkowitz R. G., Sun Q. J., Chalmers J., Pilowsky P.1999bIntracellular recording from posterior cricoarytenoid motoneurons in the rat. Ann. Otol. Rhinol. Laryngol. 108, 1120–1125 [DOI] [PubMed] [Google Scholar]
- Berkowitz R. G., Sun Q. J., Goodchild A. K., Pilowsky P. M.2005Serotonin inputs to laryngeal constrictor motoneurons in the rat. Laryngoscope 115, 105–109 (doi:10.1097/01.mlg.0000150695.15883.a4) [DOI] [PubMed] [Google Scholar]
- Blessing W. W., Oliver J. R., Hodgson A. H., Joh T. H., Willoughby J. O.1987Neuropeptide Y-like immunoreactive C1 neurons in the rostral ventrolateral medulla of the rabbit project to sympathetic preganglionic neurons in the spinal cord. J. Auton. Nerv. Syst. 18, 121–129 (doi:10.1016/0165-1838(87)90099-3) [DOI] [PubMed] [Google Scholar]
- Bolme P., Corrodi H., Fuxe K.1974Possible involvement of central adrenaline neurons in vasomotor and respiratory control. Studies with clonidine and its interactions with piperoxane and yohimbine. Eur. J. Pharmacol. 28, 89–94 (doi:10.1016/0014-2999(74)90116-2) [DOI] [PubMed] [Google Scholar]
- Braga V. A., Paton J. F. R., Machado B. H.2007Ischaemia-induced sympathoexcitation in spinalyzed rats. Neurosci. Lett. 415, 73–76 (doi:10.1016/j.neulet.2006.12.045) [DOI] [PubMed] [Google Scholar]
- Burke R. E.2006John Eccles' pioneering role in understanding central synaptic transmission. Prog. Neurobiol. 78, 173–188 (doi:10.1016/j.pneurobio.2006.02.002) [DOI] [PubMed] [Google Scholar]
- Burke P. G. R., Li Q., Mcmullan S., Costin M., Pilowsky P. M., Goodchild A. K.2008Somatostatin 2A receptor expressing presympathetic neurons in the rat rostral ventrolateral medulla maintain blood pressure. Hypertension 52, 1127–1133 (doi:10.1161/HYPERTENSIONAHA.108.118224) [DOI] [PubMed] [Google Scholar]
- Byrum C. E., Stornetta R., Guyenet P. G.1984Electrophysiological properties of spinally-projecting A5 noradrenergic neurons. Brain Res. 303, 15–29 (doi:10.1016/0006-8993(84)90206-3) [DOI] [PubMed] [Google Scholar]
- Card J. P., Sved J. C., Craig B., Raizada M., Vazquez J., Sved A. F.2006Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: implications for the central control of cardiovascular regulation. J. Comp. Neurol. 499, 840–859 (doi:10.1002/cne.21140) [DOI] [PubMed] [Google Scholar]
- Chan J. Y. H., Lin S., Chan S. H. H.1992Reversal by pertussis toxin and N-ethylmaleimide of the facilitation of baroreceptor reflex response by somatostatin in the rat. Neurosci. Lett. 134, 267–270 (doi:10.1016/0304-3940(92)90532-C) [DOI] [PubMed] [Google Scholar]
- Chandrasekaran B., Dar O., Mcdonagh T.2008The role of apelin in cardiovascular function and heart failure. Eur. J. Heart Fail. 10, 725–732 (doi:10.1016/j.ejheart.2008.06.002) [DOI] [PubMed] [Google Scholar]
- Ciriello J.1983Brainstem projections of aortic baroreceptor afferent fibers in the rat. Neurosci. Lett. 36, 37–42 (doi:10.1016/0304-3940(83)90482-2) [DOI] [PubMed] [Google Scholar]
- Coote J. H., Lewis D. I.1995Bulbospinal catecholamine neurons and sympathetic pattern generation. J. Physiol. Pharmacol. 46, 259–271 [PubMed] [Google Scholar]
- Dawid Milner M. S., Lara J. P., López de Miguel M. P., López-González M. V., Spyer K. M., González-Barón S.2003A5 region modulation of the cardiorespiratory responses evoked from parabrachial cell bodies in the anaesthetised rat. Brain Res. 982, 108–118 (doi:10.1016/S0006-8993(03)03005-1) [DOI] [PubMed] [Google Scholar]
- Duran-Prado M., Malagon M. M., Gracia-Navarro F., Castano J. P.2008Dimerization of G protein-coupled receptors: new avenues for somatostatin receptor signalling, control and functioning. Mol. Cell Endocrinol. 286, 63–68 (doi:org/10.1016/j.mce.2007.12.006) [DOI] [PubMed] [Google Scholar]
- Farnham M. M., Li Q., Goodchild A. K., Pilowsky P. M.2008PACAP is expressed in sympathoexcitatory bulbospinal C1 neurons of the brain stem and increases sympathetic nerve activity in vivo. Am. J. Physiol. 294, R1304–R1311 [DOI] [PubMed] [Google Scholar]
- Fenik V. B., Davies R. O., Kubin L.2005REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am. J. Respir. Crit. Care Med. 172, 1322–1330 (doi:10.1164/rccm.200412-1750OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fye W. B.1986Carl Ludwig and the Leipzig Physiological Institute: ‘a factory of new knowledge’. Circulation 74, 920–928 [DOI] [PubMed] [Google Scholar]
- Gao L., Wang W., Zucker I. H.2008Simvastatin inhibits central sympathetic outflow in heart failure by a nitric-oxide synthase mechanism. J. Pharmacol. Exp. Ther. 326, 278–285 (doi:10.1124/jpet.107.136028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti P. J., Llewellyn-Smith I. J., Sun Q. J., Chalmers J., Pilowsky P.1999Substance P-immunoreactive boutons closely appose inspiratory protruder hypoglossal motoneurons in the cat. Brain Res. 834, 155–159 (doi:10.1016/S0006-8993(99)01515-2) [DOI] [PubMed] [Google Scholar]
- Gilbey M. P., Mckenna K. E., Schramm L. P.1983Effects of substance P on sympathetic preganglionic neurons. Neurosci. Lett. 41, 157–159 (doi:10.1016/0304-3940(83)90239-2) [DOI] [PubMed] [Google Scholar]
- Goodchild A. K., Dampney R. A. L., Bandler R.1982A method for evoking physiological responses by stimulation of cell bodies, but not axons of passage, within localized regions of the central nervous system. J. Neurosci. Methods 6, 351–363 (doi:10.1016/0165-0270(82)90036-X) [DOI] [PubMed] [Google Scholar]
- Goodchild A. K., Moon E. A., Dampney R. A., Howe P. R.1984Evidence that adrenaline neurons in the rostral ventrolateral medulla have a vasopressor function. Neurosci. Lett. 45, 267–272 (doi:10.1016/0304-3940(84)90237-4) [DOI] [PubMed] [Google Scholar]
- Gordon F. J., Leone C.1991Non-NMDA receptors in the nucleus of the tractus solitarius play the predominant role in mediating aortic baroreceptor reflexes. Brain Res. 568, 319–322 (doi:10.1016/0006-8993(91)91418-Z) [DOI] [PubMed] [Google Scholar]
- Grubb M. C., Stornetta R. L., Pence R., Baertschi A. J., Guyenet P. G.1998Antagonist precipitated clonidine withdrawal in rat: effects on locus coeruleus neurons, sympathetic nerves and cardiovascular parameters. J. Auton. Nerv. Syst. 71, 85–95 (doi:10.1016/S0165-1838(98)00065-4) [DOI] [PubMed] [Google Scholar]
- Guyenet P. G.1997Is the hypotensive effect of clonidine and related drugs due to imidazoline binding sites? Am. J. Physiol. 42, R1580–R1584 [DOI] [PubMed] [Google Scholar]
- Guyenet P. G.2008The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J. Appl. Physiol. 105, 404–416 (doi:10.1152/japplphysiol.90452.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet P. G., Wang H.2001Pre-Bötzinger neurons with preinspiratory discharges ‘in vivo’ express NK1 receptors in the rat. J. Neurophysiol. 86, 438–446 [DOI] [PubMed] [Google Scholar]
- Guyenet P. G., Haselton J. R., Sun M. K.1989Sympathoexcitatory neurons of the rostroventrolateral medulla and the origin of the sympathetic vasomotor tone. Prog. Brain Res. 81, 105–116 (doi:10.1016/S0079-6123(08)62002-6) [DOI] [PubMed] [Google Scholar]
- Haig B. D.2008Precis of ‘an abductive theory of scientific method’. J. Clin. Psychol. 64, 1019–1022 (doi:10.1002/jclp.20506) [DOI] [PubMed] [Google Scholar]
- Haselton J. R., Guyenet P. G.1989Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am. J. Physiol. 256, R739–R750 [DOI] [PubMed] [Google Scholar]
- Haxhiu M. A., Tolentino-Silva F., Pete G., Kc P., Mack S. O.2001Monoaminergic neurons, chemosensation and arousal. Respir. Physiol. 129, 191–209 (doi:10.1016/S0034-5687(01)00290-0) [DOI] [PubMed] [Google Scholar]
- Hilaire G., Khatib M., Monteau R.1983Spontaneous respiratory activity of phrenic and intercostal Renshaw cells. Neurosci. Lett. 43, 97–101 (doi:10.1016/0304-3940(83)90135-0) [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Fuxe K., Goldstein M., Johansson O.1974Immunohistochemical evidence for the existence of adrenaline neurons in the rat brain. Brain Res. 66, 235–251 (doi:10.1016/0006-8993(74)90143-7) [Google Scholar]
- Holtman J. R., Jr1988Immunohistochemical localization of serotonin- and substance P-containing fibers around respiratory muscle motoneurons in the nucleus ambiguus of the cat. Neuroscience 26, 169–178 (doi:10.1016/0306-4522(88)90135-2) [DOI] [PubMed] [Google Scholar]
- Holtman J. R., Jr, Norman W. P., Skirboll L., Dretchen K. L., Cuello C., Visser T. J., Hokfelt T., Gillis R. A.1984Evidence for 5-hydroxytryptamine, substance P, and thyrotropin-releasing hormone in neurons innervating the phrenic motor nucleus. J. Neurosci. 4, 1064–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman J. R., Vascik D. S., Maley B. E.1990Ultrastructural evidence for serotonin-immunoreactive terminals contracting phrenic motoneurons in the cat. Exp. Neurol. 109, 269–272 (doi:10.1016/S0014-4886(05)80016-0) [DOI] [PubMed] [Google Scholar]
- Hoyer D., Clarke D. E., Fozard J. R., Hartig P. R., Martin G. R., Mylecharane E. J., Saxena P. R., Humphrey P. P.1994International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 46, 157–203 [PubMed] [Google Scholar]
- Jänig W., McLachlan E. M.1992Characteristics of function-specific pathways in the sympathetic nervous system. Trends Neurosci. 15, 475–481 (doi:10.1016/0166-2236(92)90092-M) [DOI] [PubMed] [Google Scholar]
- Jansen A. S. P., Ter Horst G. J., Mettenleiter T. C., Loewy A. D.1992CNS cell groups projecting to the submandibular parasympathetic preganglionic neurons in the rat: a retrograde transneuronal viral cell body labeling study. Brain Res. 572, 253–260 (doi:10.1016/0006-8993(92)90479-S) [DOI] [PubMed] [Google Scholar]
- Jansen A. S. P., Wessendorf M. W., Loewy A. D.1995Transneuronal labeling of CNS neuropeptide and monoamine neurons after pseudorabies virus injections into the stellate ganglion. Brain Res. 683, 1–24 (doi:10.1016/0006-8993(95)00276-V) [DOI] [PubMed] [Google Scholar]
- Kangrga I. M., Loewy A. D.1995Whole-cell recordings from visualized C1 adrenergic bulbospinal neurons: ionic mechanisms underlying vasomotor tone. Brain Res. 670, 215–232 (doi:10.1016/0006-8993(94)01282-M) [DOI] [PubMed] [Google Scholar]
- Kashihara K., McMullan S., Lonergan T., Goodchild A. K., Pilowsky P. M.2008Neuropeptide Y in the rostral ventrolateral medulla blocks somatosympathetic reflexes in anesthetized rats. Auton. Neurosci 142, 64–70 (doi:10.1016/j.autneu.2008.05.002) [DOI] [PubMed] [Google Scholar]
- Kishi T., Hirooka Y., Ito K., Sakai K., Shimokawa H., Takeshita A.2002Cardiovascular effects of overexpression of endothelial nitric oxide synthase in the rostral ventrolateral medulla in stroke-prone spontaneously hypertensive rats. Hypertension 39, 264–268 (doi:10.1161/hy0202.102701) [DOI] [PubMed] [Google Scholar]
- Koshiya N., Guyenet P. G.1995Sympatholytic effect of clonidine depends on the respiratory phase in rat splanchnic nerve. J. Auton. Nerv. Syst. 53, 82–86 (doi:10.1016/0165-1838(94)00181-I) [DOI] [PubMed] [Google Scholar]
- Koshiya N., Smith J. C.1999Neuronal pacemaker for breathing visualized in vitro. Nature 400, 360–363 (doi:10.1038/22540) [DOI] [PubMed] [Google Scholar]
- Krout K. E., Mettenleiter T. C., Loewy A. D.2003Single CNS neurons link both central motor and cardiosympathetic systems: a double-virus tracing study. Neuroscience 118, 853–866 (doi:10.1016/S0306-4522(02)00997-1) [DOI] [PubMed] [Google Scholar]
- Kubo T., Hagiwara Y., Endo S., Fukumori R.2002Activation of hypothalamic angiotensin receptors produces pressor responses via cholinergic inputs to the rostral ventrolateral medulla in normotensive and hypertensive rats. Brain Res. 953, 232–245 (doi:10.1016/S0006-8993(02)03297-3) [DOI] [PubMed] [Google Scholar]
- Kunitake T., Kannan H.2000Discharge pattern of renal sympathetic nerve activity in the conscious rat: spectral analysis of integrated activity. J. Neurophysiol. 84, 2859–2867 [DOI] [PubMed] [Google Scholar]
- Lalley P. M., Benacka R., Bischoff A. M., Richter D. W.1997Nucleus raphe obscurus evokes 5-HT-1A receptor-mediated modulation of respiratory neurons. Brain Res. 747, 156–159 (doi:10.1016/S0006-8993(96)01233-4) [DOI] [PubMed] [Google Scholar]
- Li Y. W., Guyenet P. G.1995Neuronal excitation by angiotensin II in the rostral ventrolateral medulla of the rat in vitro. Am. J. Physiol. 268, R272–R277 [DOI] [PubMed] [Google Scholar]
- Li Y. W., Guyenet P. G.1996Angiotensin II decreases a resting K+ conductance in rat bulbospinal neurons of the C1 area. Circ. Res. 78, 274–282 [DOI] [PubMed] [Google Scholar]
- Li A., Nattie E.2006Catecholamine neurons in rats modulate sleep, breathing, central chemoreception and breathing variability. J. Physiol. 570, 385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A. J., Ritter S.2004Glucoprivation increases expression of neuropeptide Y mRNA in hindbrain neurons that innervate the hypothalamus. Eur. J. Neurosci. 19, 2147–2154 (doi:10.1111/j.1460-9568.2004.03287.x) [DOI] [PubMed] [Google Scholar]
- Li Y. W., Polson J. W., Dampney R. A. L.1992Angiotensin-II excites vasomotor neurons but not respiratory neurons in the rostral and caudal ventrolateral medulla. Brain Res. 577, 161–164 (doi:10.1016/0006-8993(92)90551-J) [DOI] [PubMed] [Google Scholar]
- Li Y. W., Bayliss D. A., Guyenet P. G.1995C1 neurons of neonatal rats: intrinsic beating properties and alpha2-adrenergic receptors. Am. J. Physiol. 269, R1356–R1369 [DOI] [PubMed] [Google Scholar]
- Li Y. W., Guyenet P. G., Bayliss D. A.1998Voltage-dependent calcium currents in bulbospinal neurons of neonatal rat rostral ventrolateral medulla: modulation by alpha(2)-adrenergic receptors. J. Neurophysiol. 79, 583–594 [DOI] [PubMed] [Google Scholar]
- Li Q., Goodchild A. K., Seyedabadi M., Pilowsky P. M.2005Pre-protachykinin A mRNA is colocalized with tyrosine hydroxylase-immunoreactivity in bulbospinal neurons. Neuroscience 136, 205–216 (doi:10.1016/j.neuroscience.2005.07.057) [DOI] [PubMed] [Google Scholar]
- Lipski J., Fyffe R. E. W., Jodkowski J.1985Recurrent inhibition of cat phrenic motoneurons. J. Neurosci. 5, 1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J., Bellingham M. C., West M. J., Pilowsky P.1988Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J. Neurosci. Methods 26, 169–179 (doi:10.1016/0165-0270(88)90166-5) [DOI] [PubMed] [Google Scholar]
- Lipski J., Kanjhan R., Kruszewska B., Smith M.1995Barosensitive neurons in the rostral ventrolateral medulla of the rat in vivo: morphological properties and relationship to C1 adrenergic neurons. Neuroscience 69, 601–618 (doi:10.1016/0306-4522(95)92652-Z) [DOI] [PubMed] [Google Scholar]
- Lipski J., Kanjhan R., Kruszewska B., Rong W.-F.1996Properties of presympathetic neurons in the rostral ventrolateral medulla in the rat: an intracellular study ‘in vivo’. J. Physiol. 490, 729–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J., Lin J., Teo M. Y., Van Wyk M.2002The network vs. pacemaker theory of the activity of RVL presympathetic neurons—a comparison with another putative pacemaker system. Auton. Neurosci. 98, 85–89 (doi:10.1016/S1566-0702(02)00038-3) [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith I. J., Minson J. B., Pilowsky P. M., Chalmers J. P.1991There are few catecholamine- or neuropeptide Y-containing synapses in the intermediolateral cell column of rat thoracic spinal cord. Clin. Exp. Pharmacol. Physiol. 18, 111–115 (doi:10.1111/j.1440-1681.1991.tb01418.x) [DOI] [PubMed] [Google Scholar]
- Madden C. J., Ito S., Rinaman L., Wiley R. G., Sved A. F.1999Lesions of the C1 catecholaminergic neurons of the ventrolateral medulla in rats using anti-DbetaH-saporin. Am. J. Physiol. 277, R1063–R1075 [DOI] [PubMed] [Google Scholar]
- Mahinda T. B., Taylor B. K.2004Intrathecal neuropeptide Y inhibits behavioral and cardiovascular responses to noxious inflammatory stimuli in awake rats. Physiol. Behav. 80, 703–711 (doi:10.1016/j.physbeh.2003.12.007) [DOI] [PubMed] [Google Scholar]
- Makeham J. M., Goodchild A. K., Pilowsky P. M.2001NK1 receptor and the ventral medulla of the rat: bulbospinal and catecholaminergic neurons. Neuroreport 12, 3663–3667 (doi:10.1097/00001756-200112040-00012) [DOI] [PubMed] [Google Scholar]
- Makeham J. M., Goodchild A. K., Costin N. S., Pilowsky P. M.2004Hypercapnia selectively attenuates the somato-sympathetic reflex. Resp. Physiol. Neurobiol. 140, 133–143 (doi:10.1016/j.resp.2003.11.003) [DOI] [PubMed] [Google Scholar]
- Makeham J. M., Goodchild A. K., Pilowsky P. M.2005NK1 receptor activation in rat rostral ventrolateral medulla selectively attenuates somato-sympathetic reflex while antagonism attenuates sympathetic chemoreflex. Am. J. Physiol. 288, R1707–R1715 [DOI] [PubMed] [Google Scholar]
- Mandel D. A., Schreihofer A. M.2006Central respiratory modulation of barosensitive neurons in rat caudal ventrolateral medulla. J. Physiol. 572, 881–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. R.1992Pressor response to posterior hypothalamic administration of carbachol is mediated by muscarinic M3 receptor. Eur. J. Pharmacol. 215, 83–91 (doi:10.1016/0014-2999(92)90612-8) [DOI] [PubMed] [Google Scholar]
- Mason P.1997Physiological identification of pontomedullary serotonergic neurons in the rat. J. Neurophysiol. 77, 1087–1098 [DOI] [PubMed] [Google Scholar]
- McAllen R. M., Habler H. J., Michaelis M., Peters O., Janig W.1994Monosynaptic excitation of preganglionic vasomotor neurons by subretrofacial neurons of the rostral ventrolateral medulla. Brain Res. 634, 227–234 (doi:10.1016/0006-8993(94)91925-9) [DOI] [PubMed] [Google Scholar]
- McDonald D. M.1983Morphology of the rat carotid sinus nerve. I. Course, connections, dimensions and ultrastructure. J. Neurocytol. 12, 345–372 (doi:10.1007/BF01159380) [DOI] [PubMed] [Google Scholar]
- McMullan S., Goodchild A. K., Pilowsky P. M.2007Circulating angiotensin II attenuates the sympathetic baroreflex by reducing the barosensitivity of medullary cardiovascular neurons. J. Physiol. 582, 711–722 (doi:10.1113/jphysiol.2007.128983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner T. A., Morrison S. F., Abate C., Reis D. J.1988aPhenylethanolamine N-methyltransferase-containing terminals synapse directly on sympathetic preganglionic neurons in the rat. Brain Res. 448, 205–222 (doi:10.1016/0006-8993(88)91258-9) [DOI] [PubMed] [Google Scholar]
- Milner T. A., Pickel V. M., Abate C., Joh T. H., Reis D. J.1988bUltrastructural characterization of substance P-like immunoreactive neurons in the rostral ventrolateral medulla in relation to neurons containing catecholamine-synthesizing enzymes. J. Comp. Neurol. 270, 427–445 (doi:10.1002/cne.902700311) [DOI] [PubMed] [Google Scholar]
- Minson J. B., Chalmers J. P., Caon A. C., Renaud B.1987Separate areas of rat medulla oblongata with populations of serotonin- and adrenaline-containing neurons alter blood pressure after L-glutamate stimulation. J. Auton. Nerv. Syst. 19, 39–50 (doi:10.1016/0165-1838(87)90143-3) [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Pilowsky P., Sun Q. J., Minson J., Suzuki S., Arnolda L., Llewellyn-Smith I., Chalmers J.1995Central inspiration increases barosensitivity of neurons in rat rostral ventrolateral medulla. Am. J. Physiol., 268, R909–R918 [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Goodchild A. K., Pilowsky P. M.2001Rostral ventral medulla 5-HT1A receptors selectively inhibit the somatosympathetic reflex. Am. J. Physiol. 280, R1261–R1268 [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Goodchild A. K., Pilowsky P. M.2002Activation of mu-opioid receptors in rat ventrolateral medulla selectively blocks baroreceptor reflexes while activation of delta opioid receptors blocks somato-sympathetic reflexes. Neuroscience 109, 133–144 (doi:10.1016/S0306-4522(01)00439-0) [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Goodchild A. K., Pilowsky P. M.2003Maintenance of sympathetic tone by a nickel chloride-sensitive mechanism in the rostral ventrolateral medulla of the adult rat. Neuroscience 116, 455–464 (doi:10.1016/S0306-4522(02)00705-4) [DOI] [PubMed] [Google Scholar]
- Monnier A., Alheid G. F., McCrimmon D. R.2003Defining ventral medullary respiratory compartments with a glutamate receptor agonist in the rat. J. Physiol. 548, 859–874 (doi:10.1113/jphysiol.2002.038141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey D. K., Rosin D. L., West G., Takakura A. C., Moreira T. S., Bayliss D. A., Guyenet P. G.2007aSerotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J. Neurosci. 27, 14 128–14 138 (doi:10.1523/JNEUROSCI.4167-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey D. K., et al. 2007bTASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J. Neurosci. 27, 14 049–14 058 (doi:10.1523/JNEUROSCI.4254-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. M., Pilowsky P. M., Sun Q. J., Llewellyn-Smith I. J.1995Thyrotropin-releasing hormone-immunoreactive varicosities synapse on rat phrenic motoneurons. J. Comp. Neurol. 359, 310–322 (doi:10.1002/cne.903590209) [DOI] [PubMed] [Google Scholar]
- Ootsuka Y., Nalivaiko E., Blessing W. W.2004Spinal 5-HT2A receptors regulate cutaneous sympathetic vasomotor outflow in rabbits and rats; relevance for cutaneous vasoconstriction elicited by MDMA (3,4-methylenedioxymethamphetamine, ‘Ecstasy’) and its reversal by clozapine. Brain Res. 1014, 34–44 (doi:10.1016/j.brainres.2004.03.058) [DOI] [PubMed] [Google Scholar]
- Oshima N., McMullan S., Goodchild A. K., Pilowsky P. M.2006A monosynaptic connection between baroinhibited neurons in the RVLM and IML in Sprague–Dawley rats. Brain Res. 1089, 153–161 (doi:10.1016/j.brainres.2006.03.024) [DOI] [PubMed] [Google Scholar]
- Oshima N., et al. 2008Monosynaptic excitatory connection from the rostral ventrolateral medulla to sympathetic preganglionic neurons revealed by simultaneous recordings. Hypertens. Res. 31, 1445–1454 (doi:10.1291/hypres.31.1445) [DOI] [PubMed] [Google Scholar]
- Padley J. R., Li Q., Pilowsky P. M., Goodchild A. K.2003Cannabinoid receptor activation in the rostral ventrolateral medulla oblongata evokes cardiorespiratory effects in anaesthetised rats. Br. J. Pharmacol. 140, 384–394 (doi:10.1038/sj.bjp.0705422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padley J. R., Overstreet D. H., Pilowsky P. M., Goodchild A. K.2005Impaired cardiac and sympathetic autonomic control in rats differing in acetylcholine receptor sensitivity. Am. J. Physiol. 289, H1985–H1992 [DOI] [PubMed] [Google Scholar]
- Pelat M., Lazartigues E., Tran M. A., Gharib C., Montastruc J. L., Montastruc P., Rascol O.1999Characterization of the central muscarinic cholinoceptors involved in the cholinergic pressor response in anesthetized dogs. Eur. J. Pharmacol. 379, 117–124 (doi:10.1016/S0014-2999(99)00508-7) [DOI] [PubMed] [Google Scholar]
- Phillips J. K., Goodchild A. K., Dubey R., Sesiashvili E., Takeda M., Chalmers J., Pilowsky P. M., Lipski J.2001Differential expression of catecholamine biosynthetic enzymes in the rat ventrolateral medulla. J. Comp. Neurol. 432, 20–34 (doi:10.1002/cne.1086) [DOI] [PubMed] [Google Scholar]
- Pilowsky P. M., Goodchild A. K.2002Baroreceptor reflex pathways and neurotransmitters: 10 years on. J. Hypertens. 20, 1675–1688 (doi:10.1097/00004872-200209000-00002) [DOI] [PubMed] [Google Scholar]
- Pilowsky P. M., West M. J., Chalmers J. P.1985Renal sympathetic nerve responses to stimulation, inhibition and destruction of the ventrolateral medulla in the rabbit. Neurosci. Lett. 60, 51–55 (doi:10.1016/0304-3940(85)90380-5) [DOI] [PubMed] [Google Scholar]
- Pilowsky P. M., Kapoor V., Minson J. B., West M. J., Chalmers J. P.1986aSpinal cord serotonin release and raised blood pressure after brainstem kainic acid injection. Brain Res. 366, 354–357 (doi:10.1016/0006-8993(86)91318-1) [DOI] [PubMed] [Google Scholar]
- Pilowsky P. M., Minson J. B., Hodgson A. J., Howe P. R. C., Chalmers J. P.1986bDoes substance P coexist with adrenaline in neurons of the rostral ventrolateral medulla in the rat? Neurosci. Lett. 71, 293–298 (doi:10.1016/0304-3940(86)90636-1) [DOI] [PubMed] [Google Scholar]
- Pilowsky P. M., Decastro D., Llewellyn-Smith I., Lipski J., Voss M. D.1990aSerotonin immunoreactive boutons make synapses with feline phrenic motoneurons. J. Neurosci. 10, 1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky P. M., Jiang C., Lipski J.1990bAn intracellular study of respiratory neurons in the rostral ventrolateral medulla of the rat and their relationship to catecholamine-containing neurons. J. Comp. Neurol. 301, 604–617 (doi:10.1002/cne.903010409) [DOI] [PubMed] [Google Scholar]
- Pilowsky P. M., Wakefield B., Minson J., Llewellyn-Smith I., Chalmers J.1992Axonal projections from respiratory centres towards the rostral ventrolateral medulla in the rat. Clin. Exp. Pharmacol. Physiol. 19, 335–338 (doi:10.1111/j.1440-1681.1992.tb00466.x) [DOI] [PubMed] [Google Scholar]
- Pilowsky P. M., Llewellyn-Smith I. J., Arnolda L., Minson J., Chalmers J.1994aIntracellular recording from sympathetic preganglionic neurons in cat lumbar spinal cord. Brain Res. 656, 319–328 (doi:10.1016/0006-8993(94)91476-1) [DOI] [PubMed] [Google Scholar]
- Pilowsky P. M., Llewellyn-Smith I. J., Lipski J., Minson J., Arnolda L., Chalmers J.1994bProjections from inspiratory neurons of the ventral respiratory group to the subretrofacial nucleus of the cat. Brain Res. 633, 63–71 (doi:10.1016/0006-8993(94)91522-9) [DOI] [PubMed] [Google Scholar]
- Pilowsky P. M., Llewellyn-Smith I. J., Minson J. B., Arnolda L. F., Chalmers J. P.1995aSubstance P and serotonergic inputs to sympathetic preganglionic neurons. Clin. Exp. Hypertens. 17, 335–344 (doi:10.3109/10641969509087075) [DOI] [PubMed] [Google Scholar]
- Pilowsky P. M., Miyawaki T., Minson J. B., Sun Q. J., Arnolda L. F., Llewellyn-Smith I. J., Chalmers J. P.1995bBulbospinal sympatho-excitatory neurons in the rat caudal raphe. J. Hypertens. 13, 1618–1623 (doi:org/10.1097/00004872-199512010-00020) [PubMed] [Google Scholar]
- Richerson G. B.2004Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat. Rev. Neurosci. 5, 449–461 (doi:10.1038/nrn1409) [DOI] [PubMed] [Google Scholar]
- Richerson G. B., Wang W., Tiwari J., Bradley S. R.2001Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir. Physiol. 129, 175–189 (doi:10.1016/S0034-5687(01)00289-4) [DOI] [PubMed] [Google Scholar]
- Ross C. A., Ruggiero D. A., Joh T. H., Park D. H., Reis D. J.1983Adrenaline synthesizing neurons in the rostral ventrolateral medulla: a possible role in tonic vasomotor control. Brain Res. 273, 356–361 (doi:10.1016/0006-8993(83)90862-4) [DOI] [PubMed] [Google Scholar]
- Ross C. A., Ruggiero D. A., Park D. H., Joh T. H., Sved A. F., Fernandez-Pardal J., Saavedra J. M., Reis D. J.1984Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J. Neurosci. 4, 474–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreihofer A. M., Guyenet P. G.2003Baro-activated neurons with pulse-modulated activity in the rat caudal ventrolateral medulla express GAD67 mRNA. J. Neurophysiol. 89, 1265–1277 (doi:10.1152/jn.00737.2002) [DOI] [PubMed] [Google Scholar]
- Schreihofer A. M., Ito S., Sved A. F.2005Brain stem control of arterial pressure in chronic arterial baroreceptor-denervated rats. Am. J. Physiol. 289, 58–56 [DOI] [PubMed] [Google Scholar]
- Seller H.1996Carl Ludwig and the localization of the medullary vasomotor center: old and new concepts of the generation of sympathetic tone. Pflugers Arch. 432, R94–R98 [PubMed] [Google Scholar]
- Severson C. A., Wang W., Pieribone V. A., Dohle C. I., Richerson G. B.2003Midbrain serotonergic neurons are central pH chemoreceptors. Nat. Neurosci. 6, 1139–1140 (doi:10.1038/nn1130) [DOI] [PubMed] [Google Scholar]
- Seyedabadi M., Goodchild A. K., Pilowsky P. M.2001Differential role of kinases in brain stem of hypertensive and normotensive rats. Hypertension 38, 1087–1092 (doi:10.1161/hy1101.096054) [DOI] [PubMed] [Google Scholar]
- Seyedabadi M., Goodchild A. K., Pilowsky P. M.2002Site-specific effects of apelin-13 in the rat medulla oblongata on arterial pressure and respiration. Auton. Neurosci. 101, 32–38 (doi:10.1016/S1566-0702(02)00178-9) [DOI] [PubMed] [Google Scholar]
- Seyedabadi M., Li Q., Padley J. R., Pilowsky P. M., Goodchild A. K.2006A novel pressor area at the medullo-cervical junction that is not dependent on the RVLM: efferent pathways and chemical mediators. J. Neurosci. 26, 5420–5427 (doi:10.1523/JNEUROSCI.1190-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X. M., Feldman J. L.2001Mechanisms underlying regulation of respiratory pattern by nicotine in preBötzinger complex. J. Neurophysiol. 85, 2461–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Lewis D. I., Coote J. H.1988Effects of activating spinal alpha-adrenoreceptors on sympathetic nerve activity in the rat. J. Auton. Nerv. Syst. 23, 69–78 (doi:10.1016/0165-1838(88)90168-3) [DOI] [PubMed] [Google Scholar]
- Shi T. J. S., Li J., Dahlström A., Theodorsson E., Ceccatelli S., Decosterd I., Pedrazzini T., Hökfelt T.2006Deletion of the neuropeptide Y Y1 receptor affects pain sensitivity, neuropeptide transport and expression, and dorsal root ganglion neuron numbers. Neuroscience 140, 293–304 (doi:10.1016/j.neuroscience.2006.02.009) [DOI] [PubMed] [Google Scholar]
- Smith J. C., Ellenberger H. H., Ballanyi K., Richter D. W., Feldman J. L.1991Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254, 726–729 (doi:10.1126/science.1683005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. N., Davis S. F., van den Pol A. N., Xu W.2002Selective enhancement of excitatory synaptic activity in the rat nucleus tractus solitarius by hypocretin 2. Neuroscience 115, 707–714 (doi:10.1016/S0306-4522(02)00488-8) [DOI] [PubMed] [Google Scholar]
- Solomon S., Llewellyn-Smith I., Minson J., Arnolda L., Chalmers J., Pilowsky P.1999Neurokinin-1 receptors and spinal cord control of blood pressure in spontaneously hypertensive rats. Brain Res. 815, 116–120 (doi:10.1016/S0006-8993(98)01107-X) [DOI] [PubMed] [Google Scholar]
- Sossin W. S., Sweet-Cordero A., Scheller R. H.1990Dale's hypothesis revisited: different neuropeptides derived from a common prohormone are targeted to different processes. Proc. Natl Acad. Sci. USA 87, 4845–4848 (doi:10.1073/pnas.87.12.4845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasinopoulos T., Goodchild A. K., Christie M. J., Chalmers J., Pilowsky P. M.2000Delta opioid receptor immunoreactive boutons appose bulbospinal C1 neurons in the rat. Neuroreport 11, 887–891 (doi:10.1097/00001756-200003200-00045) [DOI] [PubMed] [Google Scholar]
- Stornetta R. L., Akey P. J., Guyenet P. G.1999Location and electrophysiological characterization of rostral medullary adrenergic neurons that contain neuropeptide Y mRNA in rat medulla. J. Comp. Neurol. 415, 482–500 (doi:10.1002/(SICI)1096-9861(19991227)415:4<482::AID-CNE5>3.0.CO;2-4) [DOI] [PubMed] [Google Scholar]
- Stornetta R. L., Schreihofer A. M., Pelaez N. M., Sevigny C. P., Guyenet P. G.2001Preproenkephalin mRNA is expressed by C1 and non-C1 barosensitive bulbospinal neurons in the rostral ventrolateral medulla of the rat. J. Comp. Neurol. 435, 111–126 (doi:10.1002/cne.1196) [DOI] [PubMed] [Google Scholar]
- Stornetta R. L., Spirovski D., Moreira T. S., Takakura A. C., West G. H., Gwilt J. M., Pilowsky P. M., Guyenet P. G.2009Galanin is a selective marker of the retrotrapezoid nucleus in rats. J. Comp. Neurol. 512, 373–383 (doi:10.1002/cne.21897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q. J., Pilowsky P., Minson J., Arnolda L., Chalmers J., Llewellyn-Smith I. J.1994Close appositions between tyrosine hydroxylase immunoreactive boutons and respiratory neurons in the rat ventrolateral medulla. J. Comp. Neurol. 340, 1–10 (doi:10.1002/cne.903400102) [DOI] [PubMed] [Google Scholar]
- Sun Q. J., Pilowsky P., Llewellyn-Smith I. J.1995Thyrotropin-releasing hormone inputs are preferentially directed towards respiratory motoneurons in rat nucleus ambiguus. J. Comp. Neurol. 362, 320–330 (doi:10.1002/cne.903620303) [DOI] [PubMed] [Google Scholar]
- Sun Q. J., Llewellyn-Smith I. J., Minson J. B., Arnolda L. F., Chalmers J. P., Pilowsky P. M.1996Thyrotropin-releasing hormone immunoreactive boutons form close appositions with medullary expiratory neurons in the rat. Brain Res. 715, 136–144 (doi:10.1016/0006-8993(95)01569-8) [DOI] [PubMed] [Google Scholar]
- Sun Q. J., Minson J., Llewellyn-Smith I. J., Arnolda L., Chalmers J., Pilowsky P.1997Bötzinger neurons project towards bulbospinal neurons in the rostral ventrolateral medulla of the rat. J. Comp. Neurol. 388, 23–31 (doi:10.1002/(SICI)1096-9861(19971110)388:1<23::AID-CNE2>3.0.CO;2-Q) [DOI] [PubMed] [Google Scholar]
- Sun Q. J., Goodchild A. K., Chalmers J. P., Pilowsky P. M.1998The pre-Bötzinger complex and phase-spanning neurons in the adult rat. Brain Res. 809, 204–213 (doi:10.1016/S0006-8993(98)00872-5) [DOI] [PubMed] [Google Scholar]
- Sun Q. J., Berkowitz R. G., Goodchild A. K., Pilowsky P. M.2002Serotonin inputs to inspiratory laryngeal motoneurons in the rat. J. Comp. Neurol. 451, 91–98 (doi:10.1002/cne.10329) [DOI] [PubMed] [Google Scholar]
- Sun Q. J., Berkowitz R. G., Goodchild A. K., Pilowsky P. M.2003Substance P inputs to laryngeal motoneurons in the rat. Resp. Physiol. Neurobiol. 137, 11–18 (doi:10.1016/S1569-9048(03)00136-8) [DOI] [PubMed] [Google Scholar]
- Suzuki S., Pilowsky P., Minson J., Arnolda L., Llewellyn-Smith I., Chalmers J.1994C-Fos antisense in rostral ventral medulla reduces arterial blood pressure. Am. J. Physiol. 266, R1418–R1422 [DOI] [PubMed] [Google Scholar]
- Swanson L. W.1998Brain maps: structure of the rat brain: a laboratory guide with printed and electronic templates for data, models, and schematics Amsterdam, The Netherlands: Elsevier Science Ltd [Google Scholar]
- Telerman-Toppet N., Vanderhaeghen J. J., Warszawski M.1982Orthostatic hypotension with lower brain stem glioma. J. Neurol. Neurosurg. Psychiat. 45, 1147–1150 (doi:10.1136/jnnp.45.12.1147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trostel K. A., Osborn J. W.1994Does the spinal cord generate functionally significant sympathetic activity in the awake rat? Am. J. Physiol. 266, R1102–R1110 [DOI] [PubMed] [Google Scholar]
- Verner T. A., Goodchild A. K., Pilowsky P. M.2004A mapping study of cardiorespiratory responses to chemical stimulation of the midline medulla oblongata in ventilated and freely breathing rats. Am. J. Physiol. 287, R411–R421 [DOI] [PubMed] [Google Scholar]
- Verner T. A., Pilowsky P. M., Goodchild A. K.2008Retrograde projections to a discrete apneic site in the midline medulla oblongata of the rat. Brain Res. 1208, 128–136 (doi:10.1016/j.brainres.2008.02.028) [DOI] [PubMed] [Google Scholar]
- Voituron N., Frugiere A., Gros F., Macron J. M., Bodineau L.2005Diencephalic and mesencephalic influences on ponto-medullary respiratory control in normoxic and hypoxic conditions: an in vitro study on central nervous system preparations from newborn rat. Neuroscience 132, 843–854 (doi:10.1016/j.neuroscience.2004.12.011) [DOI] [PubMed] [Google Scholar]
- Wettschureck N., Offermanns S.2005Mammalian G proteins and their cell type specific functions. Physiol. Rev. 85, 1159–1204 (doi:10.1152/physrev.00003.2005) [DOI] [PubMed] [Google Scholar]
- Willette R. N., Punnen S., Krieger A. J., Sapru H. N.1984Interdependence of rostral and caudal ventrolateral medullary areas in the control of blood pressure. Brain Res. 321, 169–174 (doi:10.1016/0006-8993(84)90696-6) [DOI] [PubMed] [Google Scholar]
- Wisor J. P., Wurts S. W., Hall F. S., Lesch K. P., Murphy D. L., Uhl G. R., Edgar D. M.2003Altered rapid eye movement sleep timing in serotonin transporter knockout mice. Neuroreport 14, 233–238 (doi:10.1097/00001756-200302100-00015) [DOI] [PubMed] [Google Scholar]
- Wu E. H. T., Wong Y. H.2005Activation of delta-, kappa-, and mu-opioid receptors induces phosphorylation of tuberin in transfected HEK 293 cells and native cells. Biochem. Biophys. Res. Commun. 334, 838–844 (doi:10.1016/j.bbrc.2005.06.184) [DOI] [PubMed] [Google Scholar]
- Zanzinger J., Czachurski J., Seller H.1995Inhibition of basal and reflex-mediated sympathetic activity in the RVLM by nitric oxide. Am. J. Physiol. 268, R958–R962 [DOI] [PubMed] [Google Scholar]