Abstract
The brainstem network for generating and modulating the respiratory motor pattern includes neurons of the medullary ventrolateral respiratory column (VRC), dorsolateral pons (PRG) and raphé nuclei. Midline raphé neurons are proposed to be elements of a distributed brainstem system of central chemoreceptors, as well as modulators of central chemoreceptors at other sites, including the retrotrapezoid nucleus. Stimulation of the raphé system or peripheral chemoreceptors can induce a long-term facilitation of phrenic nerve activity; central chemoreceptor stimulation does not. The network mechanisms through which each class of chemoreceptor differentially influences breathing are poorly understood. Microelectrode arrays were used to monitor sets of spike trains from 114 PRG, 198 VRC and 166 midline neurons in six decerebrate vagotomized cats; 356 were recorded during sequential stimulation of both receptor classes via brief CO2-saturated saline injections in vertebral (central) and carotid arteries (peripheral). Seventy neurons responded to both stimuli. More neurons were responsive only to peripheral challenges than those responsive only to central chemoreceptor stimulation (PRG, 20 : 4; VRC, 41 : 10; midline, 25 : 13). Of 16 474 pairs of neurons evaluated for short-time scale correlations, similar percentages of reference neurons in each brain region had correlation features indicative of a specific interaction with at least one target neuron: PRG (59.6%), VRC (51.0%) and raphé nuclei (45.8%). The results suggest a brainstem network architecture with connectivity that shapes the respiratory motor pattern via overlapping circuits that modulate central and peripheral chemoreceptor-mediated influences on breathing.
Keywords: chemoreceptor reflex, brainstem circuits, breathing
1. Introduction
The brainstem network for respiratory rhythm and motor pattern generation includes the medullary ventrolateral respiratory column (VRC) and the dorsolateral pontine respiratory group (PRG; Marckwald 1887; Lumsden 1923; Stella 1938; Cohen 1979; St John 1985, 1986, 1998; Smith et al. 1991, 2007; Dick et al. 1994, 2008; Bianchi et al. 1995; Feldman et al. 2003; Alheid et al. 2004). A recent study of functional connectivity within this pontomedullary network supported model-based hypotheses on circuit mechanisms for pontine influences on respiratory phase switching and drive (Rybak et al. 2008; Segers et al. 2008). Multi-array electrode technology and spike train analysis have also identified correlational linkages that support a model of respiratory network architecture with raphé circuits serving as a parallel system of ‘intermediate relays’ between the VRC and PRG (Nuding et al. 2009), as proposed by Bianchi et al. (1995). These results complement earlier evidence for functional relationships between the VRC and raphé nuclei, including that for efference copy of respiratory drive information to midline circuits and raphé modulation of phase timing and drive, and support the hypothesis that midline circuits maintain particular states or levels of neuronal activity that are subject to adjustment by afferent systems (Lindsey et al. 1992a,b,c, 1994, 1998; Morris et al. 1996a,b, 2001; Li et al. 1999b; Arata et al. 2000; Aungst et al. 2008).
Medullary raphé neurons respond to perturbations of both central and peripheral chemoreceptor systems. State-dependent changes in ventilation in response to local perturbations of raphé neurons support the hypothesis that midline neurons are elements of a distributed brainstem system of central chemoreceptors (Nattie & Li 2009), as well as modulators of central chemoreceptors at other sites, including the retrotrapezoid nucleus (Mulkey et al. 2004; Dias et al. 2008). Stimulation of peripheral chemoreceptors also evokes changes in raphé neuron activity (Morris et al. 1996a,b, 2001), and repeated intermittent stimulation of peripheral carotid body chemoreceptors or medullary raphé neurons can induce a long-term facilitation of phrenic nerve amplitude and cycling frequency (Millhorn et al. 1980; Morris et al. 1996a,b, 2001; Mitchell et al. 2001). Similar patterns of central chemoreceptor stimulation do not produce this respiratory memory (Millhorn 1986).
The network mechanisms through which each class of chemoreceptor differentially influences breathing and longer term changes in the respiratory motor pattern are not well understood. Given the results described above and the roles proposed for medullary raphé neurons, we addressed the hypothesis that midline brainstem circuits are connected to the PRG and VRC in a way appropriate for shaping the physiological responses evoked by the stimulation of both types of chemoreceptors. Here, we report simultaneous measurements of the firing rates of neurons in all three domains during sequential transient stimulation of central and peripheral chemoreceptors, together with results from spike train cross-correlation analysis indicative of paucisynaptic associations among them. A preliminary account of some of the results has been presented elsewhere (Nuding et al. 2007).
2. Methods
Data were obtained from six adult cats (2.8–5.6 kg) of either sex as part of a larger study on the brainstem respiratory network. A detailed description of the methods has been published elsewhere (Segers et al. 2008). Briefly, animals were initially anaesthetized with isoflurane (2–5%; n = 3) or with an intramuscular ketamine hydrochloride injection (5.5 mg kg−1; n = 3) followed by isoflurane and later decerebrated using a technique adapted from Kirsten & St John (1978). The level of anaesthesia was assessed periodically by noxious stimuli (toe pinch); if the withdrawal reflex occurred or there was an increase in blood pressure or respiration, the percentage of isoflurane in the inspired gas was increased until the response was absent. Animals were artificially ventilated through a tracheal cannula with a respirator. End-tidal CO2, rectal temperature and arterial blood pressure were monitored continuously; arterial PO2, PCO2 and pH were measured periodically. These parameters were maintained within normal limits. Prior to decerebration, an anaesthetic assessment was performed, animals were neuromuscularly blocked by pancuronium bromide (initial bolus of 0.1 mg kg−1 followed by 0.2 mg kg−1 h−1, IV) and the brainstem was immediately transected at the midcollicular level. Brain tissue rostral to the transection was aspirated. Isoflurane was removed from the inhaled gas circuit after the decerebration was complete. Animals were bilaterally vagotomized to eliminate vagal afferent feedback from pulmonary stretch receptors and aortic baroreceptors. At the end of the experiments, cats were killed by an injection of sodium pentobarbital (28 mg kg−1) followed by 5 ml of a saturated solution of KCl in water.
Efferent phrenic nerve activity was monitored together with signals from three arrays of extracellular electrodes with individual depth adjustment positioned in the dorsolateral pons (PRG), ventrolateral medullary respiratory column and medullary midline. Electrode placement was guided by appropriate stereotaxic coordinates (§3) derived from Berman (1968) and numerous previous studies as described in Segers et al. (2008) and herein. These signals, together with systemic arterial blood pressure, tracheal pressure and end-tidal CO2, were recorded; impulses from single neurons were converted to arrays of occurrence times with spike-sorting software.
Two statistical tests were used to evaluate each spike train for the presence of respiratory-modulated activity. The first test, a subjects-by-treatments analysis of variance, partitioned each respiratory cycle (‘subject’ variable) into twenty equal time segments (‘treatment’ variable; Netick & Orem 1981; Orem & Netick 1982). A complementary nonparametric sign test was also used to determine if the probability of occurrence of an increased firing rate in one half of the respiratory cycle, over the length of the recording, was greater than chance (Morris et al. 1996a). A neuron was classified as respiratory modulated if either test rejected the null hypothesis (p < 0.05); neurons with no preferred phase of maximum activity as assessed by both statistical tests were designated ‘non-respiratory modulated’ (NRM). Both standard and normalized respiratory cycle-triggered histograms (CTHs) were computed for each neuron and used to identify the phase (inspiration, I; expiration, E) or phase transition (IE and EI) in which the cell was more active. In keeping with previous conventions (e.g. Cohen 1968; Lindsey et al. 1992a; Segers et al. 2008), neurons with peak firing rates in the first half of the I or E phase were classified as ‘decrementing’ (Dec), whereas those cells with peak firing rates in the second half of the phase were grouped in the ‘augmenting’ (Aug) subcategory.
Both types of chemoreceptors were selectively stimulated by close injection of CO2-saturated 0.9 per cent saline solution (pH 5.0–5.2; 0.5–1.0 ml). Delivery catheters were placed so that blood flow forced the test solution through the carotid body (peripheral) or through the vertebral artery to the ventral surface of the medulla (central; figure 1a). Peripheral chemoreceptors were stimulated by injections delivered via a catheter inserted into the external carotid artery and advanced to a point immediately caudal to the carotid sinus (Arita et al. 1988; Li et al. 1999b); sectioning of the carotid sinus nerve has been shown to eliminate the phrenic response generated by this established technique for stimulating peripheral chemoreceptors (Morris et al. 1996a). To stimulate central chemoreceptors, a catheter was inserted into the left axillary artery and advanced until its tip was in the subclavian artery proximal to the origin of the left vertebral artery; other branches off the axillary artery were ligated (Kuwana & Natsui 1987). Injection of a CO2-saturated saline solution of similar pH into the vertebral artery has been shown to affect phrenic nerve activity (e.g. Hanson et al. 1982; Arita et al. 1988; Seller et al. 1990). Mean systemic arterial blood pressure was measured before the presentation of each stimulus and at the point of maximal change following the stimulus.
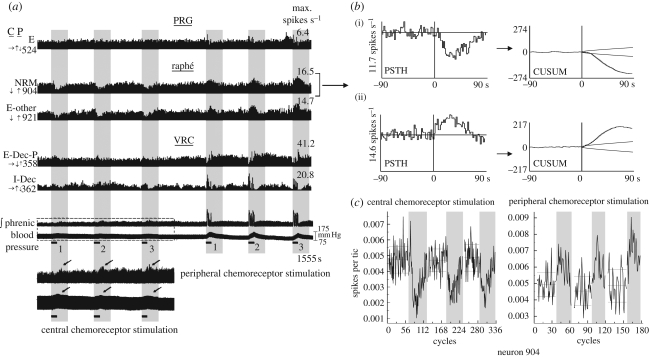
Figure 1.
Stimulus protocols and resulting changes in phrenic nerve activity and blood pressure. (a) Carotid chemoreceptors were stimulated selectively by 30 s injections of a CO2-saturated 0.9 per cent saline solution (range 0.5−1.0 ml) delivered just below the sinus via a catheter inserted into the external carotid artery. Central chemoreceptors were stimulated by 30 s injections (trials) of CO2-saturated saline (range 0.8−1.0 ml) into the left axillary artery at the level of the left vertebral artery. (b) Integrated phrenic nerve and blood pressure traces during two trials each of central (left) and peripheral (right) chemoreceptor stimulation in the same recording. Note spontaneous augmented phrenic bursts (arrows). (c,d) Detail of integrated phrenic activity traces during the central (c) and the peripheral (d) chemoreceptor stimulation marked by the boxes in (b). Phase graphs beneath these traces show the peak amplitude of integrated phrenic activity during cycles occurring before, during and after the same stimulus period. The dashed and solid horizontal lines indicate the average ±2 s.d. of the peak integrated phrenic amplitude during the interval before stimulation. Neuronal activity was examined for responses if the accompanying phrenic amplitude increased significantly by this analysis.
Most stimulus injections were 30 s in duration; one recording included 45 s stimulus durations. Neuronal responses, measured as changes in firing rates, were initially assessed with conventional peri-stimulus time (PSTH) and cumulative sum (CUSUM) histograms (e.g. figure 2b). Changes in activity exceeding CUSUM confidence bands at ±3 s.d. (Davey et al. 1986) were confirmed by a bootstrap statistical method (e.g. figure 2c). For each stimulus trial, the first, peak and maximum contiguous sum of deviations of rate per respiratory cycle in the response window from the control mean were used as measures of a response. p-Values were calculated using ordinary and autoregressive model-based bootstrap replications (Davison & Hinkley 1997). The order of the autoregressive model was determined using the finite sample information criterion (Broersen 2000). The p-value threshold (significance level) was set by controlling the false discovery rate to a level of 0.05 (Benjamini & Hochberg 1995).
Figure 2.
Simultaneously monitored neurons during repeated sequential stimulation of central and peripheral chemoreceptors. (a) Firing rate histograms of five simultaneously monitored neurons, integrated efferent phrenic nerve activity and arterial blood pressure during repeated sequential stimulation of central (left) and peripheral (right) chemoreceptors. Arrows to the left of each histogram indicate direction of significant change in firing rate in response to central (C) and peripheral (P) chemoreceptor stimulation: increase (↑), decrease (↓) or no change (→); see §2 for significance tests. Firing rates shown on the right refer to the tallest bin (i.e. the maximum rate) in the corresponding histogram. The horizontal black bars denote intervals when solutions were injected to stimulate central or peripheral chemoreceptors; vertical grey panels highlight the response periods. The boxed segment of the phrenic and blood pressure traces (shown with an expanded vertical scale below) indicates data collected during the vertebral injections; arrows denote phrenic-nerve firing rate changes (top trace) and blood pressure increases (bottom). (b) peri-stimulus time histograms (PSTH) and corresponding cumulative sum histograms (CUSUM) detailing the responses of neuron 904 to (i) central and (ii) peripheral chemoreceptor stimulation. The PSTH plots the average firing rate of the cell before and following stimulus onset over the three trials shown in (a); stimulus application began at 0. The CUSUM reflects the data trends seen in the PSTH. (c) Plotted counts of spikes per cycle for three paired control (white vertical panels) and stimulus periods (grey panels) used to calculate statistics; see §2 for details.
All pairs of simultaneously recorded spike trains were evaluated for short-time scale correlations (Perkel et al. 1967); shift-predictor ‘control’ correlograms were calculated for each pair using 20 cycles at a time with all possible shifts of these cycles and scaled so that the mean of the control equalled the mean of the correlogram over a respiratory cycle. Mean shift-control histograms (±3 s.d.) were superimposed over the original histogram during analysis. Significant cross-correlogram features (peaks and troughs) were identified using a ‘detectability index’: the ratio of the maximum amplitude of departure from the background to the standard deviation of the correlogram noise; features with index values ≥3 were considered significant (Aertsen & Gerstein 1985; Melssen & Epping 1987). Correlation linkage maps for groups of simultaneously monitored neurons were generated automatically by database queries using software employing the open source graph visualization tool Graphviz. Stereotaxic coordinates of recording sites were mapped into the three-dimensional space of a computer-based brainstem atlas derived from The Brain Stem of the Cat: A Cytoarchitectonic Atlas with Stereotaxic Coordinates, used with permission of the University of Wisconsin Press, as described in Segers et al. (2008).
3. Results
Central and peripheral chemoreceptors were selectively and sequentially stimulated by injection of CO2-saturated saline solution into the vertebral and carotid arteries, respectively (figure 1a). Chemoreceptor reflexes were identified by a change in the peak integrated phrenic nerve signal amplitude of at least 2 s.d. from the mean of pre-stimulus values. For central chemoreceptor stimulation trials, the mean time from the start of the injection to maximum peak phrenic amplitude was 49.8 ± 17.5 s. The corresponding value for peripheral chemoreceptor trials was 8.4 ± 3.3 s. Phrenic nerve and blood pressure traces during two trials each of central and peripheral chemoreceptor stimulation are illustrated in figure 1b. The boxed segments are shown with higher temporal resolution and different vertical scaling together with corresponding values of integrated peak activity following arterial injections (figure 1c,d). Changes in blood pressure were associated with some chemosensory stimulus presentations. The average increase in pressure associated with central chemoreceptor stimulation was 7.0 ± 2.6 mm Hg, while that for carotid stimulation was 22.1 ± 8.3 mm Hg; both changes were significant relative to pre-stimulus control values (two-sided Wilcoxon matched-pairs signed-ranks test, p = 0.02 and p = 0.008, respectively).
Seven of the eight recordings were made with both vertebral and carotid artery injections. Central chemoreceptors were stimulated first in five recordings. Peripheral chemoreceptors were stimulated first in three other recordings to control for possible influences of stimulus order; vertebral injections followed in two of these recordings. Neurons in each of the primary response categories were found in similar percentages irrespective of stimulus presentation order; therefore, responses to each type of stimulus were grouped for analysis.
(a). Identification of responses to chemoreceptor stimulation
Firing-rate histograms from five neurons recorded simultaneously during sequential stimulation of central and peripheral chemoreceptor are shown together with integrated efferent phrenic activity and arterial blood pressure in figure 2a. In this example, central chemoreceptor stimulation evoked transient declines in the firing rates of the two raphé neurons (904, 921); no other cell had a significant response when assessed using measurements from the three trials. In contrast, all five neurons responded to peripheral chemoreceptor stimulation; both raphé neurons exhibited increased activity. The PSTH plots the average firing rate of the cell before and following stimulus onset over the three trials shown in figure 2a; stimulus application began at 0. Both the PSTH and the CUSUM of the PSTH (figure 2b) show the average firing rate of raphé neuron 904 before and after the onset of central (top) and peripheral (bottom) chemoreceptor stimulation. The responses of raphé neuron 904 to central and peripheral chemoreceptor stimulation as determined by bootstrap statistics (figure 2c; plotted counts of spikes per cycle for three paired control (white vertical panels) and stimulus periods (grey panels); see §2 for details) were classified as a ‘decrease’ (↓) and an ‘increase’ (↑), respectively.
Vertebral and carotid artery injections each evoked biphasic changes in the firing rates of some neurons. These response patterns were characterized as increase–decrease (↑↓) or decrease–increase (↓↑) depending on the order of the direction of firing rate changes relative to pre-stimulus values. Three examples of biphasic responses (two ↑↓ and one ↓↑) to peripheral chemoreceptor stimulation are shown in figure 2a. A summary of the responses of neurons in each brain region to central and peripheral chemoreceptor stimulation is given in table 1. Owing to the relative paucity of biphasic responses, evoked changes in activity were tabulated based on the initial significant (primary) response direction. We note that a higher percentage of cells responding to carotid stimulation exhibited biphasic responses than did neurons that responded to vertebral stimulation (22/166, 13% versus 6/99, 6%). Overall, 70 neurons responded to both stimuli. Of these, 50 (71%) had firing rate changes in the same direction, with many more neurons responding with increases (57%) rather than decreases (14%) in activity. The remaining 20 cells (29%) responded with rate changes in opposite directions, with cells functionally inhibited by vertebral injections and excited by carotid injections outnumbering cells excited by vertebral injections and inhibited by carotid injections (14 versus 6).
Table 1.
Primary responses of single neurons to sequential central and peripheral chemoreceptor stimulation.
| response combinations |
location and numbers of neurons |
|||
|---|---|---|---|---|
| central | peripheral | PRG | raphé | VRC |
| ↑ | ↑ | 6 | 5 | 29 |
| → | ↑ | 14 | 18 | 26 |
| ↑ | → | 3 | 8 | 5 |
| ↓ | ↓ | 2 | 4 | 4 |
| → | ↓ | 6 | 7 | 15 |
| ↓ | → | 1 | 5 | 5 |
| ↑ | ↓ | 0 | 1 | 5 |
| ↓ | ↑ | 1 | 10 | 3 |
| → | → | 57 | 67 | 49 |
| Total | 90 | 125 | 141 | |
(b). Response profiles and respiratory-modulated discharge patterns of simultaneously recorded neurons in each brainstem region
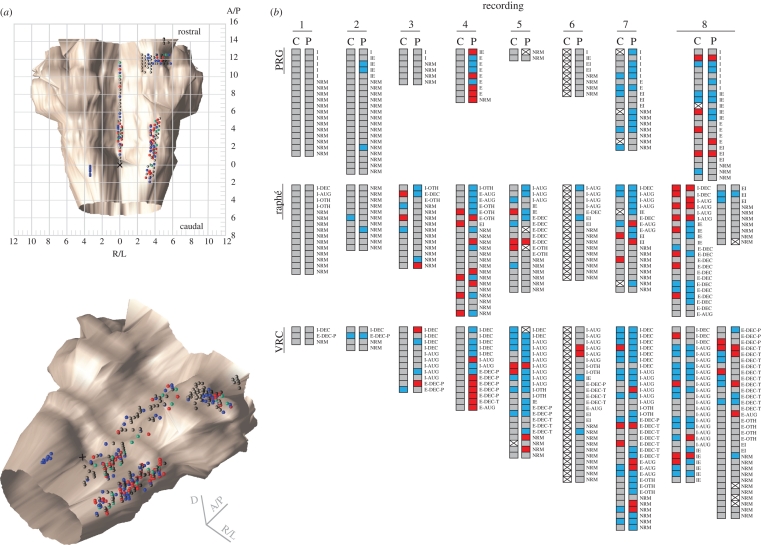
Figure 3a shows dorsal and isometric perspectives of the recording site coordinates of all neurons tested with sequential stimulation of central and peripheral chemoreceptors mapped in a standard brainstem atlas (Berman 1968). All raphé neurons were monitored within 0.2 mm of the midline. Cells recorded at the same coordinates, either simultaneously with the same electrode or during different experiments, are shown as vertically displaced spheres, colour coded to indicate the stimulus protocol(s) that evoked responses (central—blue, peripheral—green or both—red). Neurons without significant responses are represented by grey spheres.
Figure 3.
(Opposite.) Recording site locations and response profiles of neurons. (a) Dorsal (top) and isometric (bottom) views of 356 colour-coded spheres indicating the responses and stereotaxic coordinates of neurons tested with sequential stimulation of central and peripheral chemoreceptors. Neurons monitored at the same coordinates are represented by adjacent vertically ‘stacked’ spheres. The stereotaxic coordinates of the raphé recording sites (n = 125) were within 0.2 mm of the midline and extended from the obex to 11.6 mm rostral, and from 0.5 to 5.2 mm below the dorsal surface of the medulla. The recording sites in the PRG (n = 90) ranged from 1.8 mm anterior to 2.0 mm posterior to the caudal border of the inferior colliculus, 2.5–5.8 mm lateral to the midline and 1.3–3.6 mm below the dorsal surface of the pons. Cells recorded in the VRC (n = 141) were located 2.0 mm caudal to 5.2 mm rostral to the obex, 3.0–4.5 mm lateral to the midline and 2.5–5.9 mm below the dorsal surface of the medulla. Units are given in mm. Red circle, neurons responding to central and peripheral chemoreceptor stimulation (n = 70); blue circle, neurons responding to central chemoreceptor stimulation (n = 27); green circle, neurons responding to peripheral chemoreceptor stimulation (n = 86); grey circle, neurons not responsive to either stimulus (n = 173); cross, obex. (b) Neuron response profile diagrams from each recording (arranged in columns) reported by region (raphé, n = 166; PRG, n = 114; VRC, n = 198) and respiratory modulation. Each pair of shaded boxes represents a single neuron's responses to central (C) and peripheral (P) sequential chemoreceptor stimulation. Note that in one animal (recordings 1 and 2), only a few responsive neurons were identified, although robust motor responses were evoked in response to the stimulus protocols. Blue box, increase; red box, decrease; grey box, no change; box with cross, not tested.
Response profile diagrams generated for each recording and arranged by brain region (figure 3b) show the primary response for each neuron to each stimulus protocol and the cell's respiratory-modulated discharge pattern. The numbers of spike trains monitored simultaneously ranged from 36 to 116 in the eight recordings from six animals. The use of electrode arrays with individual depth adjustment allowed testing of many neurons simultaneously and under the same conditions.
Most of the neurons responsive to both central and peripheral stimulation were respiratory modulated (64/70), as were cells responsive only to peripheral (68/86) or central stimulation (19/27). A majority of the cells challenged with both stimulus protocols either did not respond to either stimulus (173/356; 49%) or responded to only one (carotid only: 86/356, 24%; vertebral only: 27/356, 8%). A subset of the raphé neurons responded to both peripheral and central chemoreceptor stimulation; most of these neurons were respiratory modulated (17/20; 85%). This percentage was greater than for respiratory-modulated raphé cells responsive only to peripheral (17/25; 68%) or central chemoreceptor stimulation (7/13; 54%). Among 19 inspiratory-modulated raphé neurons, 8 (42%) had increased activity in response to peripheral chemoreceptor stimulation and 3 (16%) to central chemoreceptor stimulation. Two were functionally excited by both protocols. Of 37 VRC I-Aug neurons tested, 14 (38%) responded with increased firing rates to both stimulus protocols. The activities of 20 (54%) increased during peripheral chemoreceptor stimulation; 16 (43%) were functionally excited by central chemoreceptor stimulation.
(c). Correlational linkages and neuronal responses
An aim of spike-train cross-correlation analysis is to define simple models of neuronal circuits that can reproduce experimentally observed features (Aertsen et al. 1989). Using simple model-based inferences, central peaks and troughs are considered indicative of shared influences of like and opposite sign, respectively; similarly, peaks and troughs offset relative to the correlogram origin may be interpreted as signs of functional excitation and inhibition (see e.g. Perkel et al. 1967; Moore et al. 1970; Balis et al. 1994; Duffin 2000; for further discussion).
Spike trains from a total of 16 474 pairs of neurons were evaluated for short-time scale correlations; 522 had primary offset features. For each brain region, the following numbers (percentages) of reference neurons had an offset-feature correlation with at least one other neuron: raphé nuclei (76, 45.8%), PRG (68, 59.6%) and VRC (101, 51.0%). The left panel in table 2 provides a summary of neuronal responses to central chemoreceptor stimulation and positive lag offset peaks and troughs in correlograms calculated using raphé, PRG or VRC neuron spike trains as reference events, arranged according to the brain region where the respective target neurons were recorded. The right half of table 2 is similarly organized, but summarizes correlations and primary responses to peripheral chemoreceptor stimulation. This arrangement is intended to facilitate the development of model circuits for chemoreceptor reflex modulation of the respiratory motor pattern. For example, the top row in table 2 (left panel) indicates that a total of five correlograms with offset peaks were identified for pairs composed of raphé reference (trigger) neurons and PRG or VRC target cells functionally excited by central chemoreceptor stimulation. Examples of correlogram features represented in the table are described and considered below.
Table 2.
Summary of offset correlation features for pairs of neurons tested with central and peripheral chemoreceptor stimulation.
| location and response of target neuron |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| central |
peripheral |
||||||||||||||||||
| PRG |
raphé |
VRC |
PRG |
raphé |
VRC |
||||||||||||||
| response of reference neuron | peaks | troughs | peaks | troughs | peaks | troughs | peaks | troughs | peaks | troughs | peaks | troughs | |||||||
| central/peripheral | |||||||||||||||||||
| ↑ | 2 | ↑ | 0 | 0 | ↑ | 0 | 3 | ↑ | 0 | 3 | ↑ | 0 | 5 | ↑ | 0 | 6 | ↑ | 1 | |
| 0 | ↓ | 1 | 1 | ↓ | 0 | 1 | ↓ | 1 | 1 | ↓ | 1 | 2 | ↓ | 0 | 4 | ↓ | 2 | ||
| 0 | → | 0 | 0 | → | 1 | 5 | → | 2 | 7 | → | 0 | 3 | → | 1 | 6 | → | 1 | ||
| ↓ | 0 | ↑ | 1 | 3 | ↑ | 0 | 0 | ↑ | 0 | 0 | ↑ | 1 | 1 | ↑ | 1 | 2 | ↑ | 1 | |
| raphé | 0 | ↓ | 1 | 2 | ↓ | 0 | 0 | ↓ | 0 | 0 | ↓ | 2 | 0 | ↓ | 0 | 0 | ↓ | 0 | |
| 1 | → | 1 | 4 | → | 0 | 1 | → | 2 | 1 | → | 0 | 0 | → | 1 | 0 | → | 0 | ||
| → | 4 | ↑ | 1 | 4 | ↑ | 0 | 7 | ↑ | 2 | 4 | ↑ | 0 | 11 | ↑ | 0 | 10 | ↑ | 3 | |
| 2 | ↓ | 0 | 1 | ↓ | 0 | 5 | ↓ | 0 | 0 | ↓ | 0 | 1 | ↓ | 0 | 4 | ↓ | 0 | ||
| 13 | → | 3 | 17 | → | 3 | 14 | → | 1 | 14 | → | 5 | 12 | → | 1 | 11 | → | 0 | ||
| ↑ | 0 | ↑ | 1 | 1 | ↑ | 0 | 1 | ↑ | 3 | 0 | ↑ | 1 | 2 | ↑ | 1 | 0 | ↑ | 3 | |
| 0 | ↓ | 0 | 0 | ↓ | 0 | 1 | ↓ | 2 | 1 | ↓ | 0 | 0 | ↓ | 0 | 2 | ↓ | 0 | ||
| 0 | → | 0 | 0 | → | 1 | 2 | → | 2 | 11 | → | 1 | 1 | → | 1 | 6 | → | 3 | ||
| PRG | ↓ | 0 | ↑ | 0 | 1 | ↑ | 0 | 2 | ↑ | 0 | 0 | ↑ | 0 | 2 | ↑ | 0 | 0 | ↑ | 2 |
| 0 | ↓ | 0 | 1 | ↓ | 0 | 0 | ↓ | 0 | 0 | ↓ | 0 | 0 | ↓ | 0 | 0 | ↓ | 0 | ||
| 0 | → | 0 | 0 | → | 1 | 1 | → | 2 | 0 | → | 0 | 2 | → | 1 | 2 | → | 0 | ||
| → | 1 | ↑ | 0 | 2 | ↑ | 0 | 3 | ↑ | 1 | 4 | ↑ | 0 | 7 | ↑ | 1 | 2 | ↑ | 2 | |
| 0 | ↓ | 0 | 4 | ↓ | 2 | 1 | ↓ | 1 | 0 | ↓ | 0 | 0 | ↓ | 0 | 1 | ↓ | 0 | ||
| 37 | → | 4 | 7 | → | 5 | 11 | → | 3 | 28 | → | 3 | 5 | → | 6 | 9 | → | 4 | ||
| ↑ | 2 | ↑ | 0 | 6 | ↑ | 2 | 17 | ↑ | 8 | 5 | ↑ | 3 | 7 | ↑ | 2 | 20 | ↑ | 7 | |
| 2 | ↓ | 3 | 5 | ↓ | 2 | 3 | ↓ | 1 | 0 | ↓ | 0 | 1 | ↓ | 1 | 4 | ↓ | 4 | ||
| 2 | → | 0 | 6 | → | 0 | 10 | → | 3 | 3 | → | 1 | 7 | → | 5 | 10 | → | 3 | ||
| ↓ | 0 | ↑ | 1 | 2 | ↑ | 0 | 4 | ↑ | 1 | 3 | ↑ | 1 | 9 | ↑ | 2 | 5 | ↑ | 5 | |
| VRC | 0 | ↓ | 1 | 0 | ↓ | 2 | 1 | ↓ | 0 | 2 | ↓ | 0 | 2 | ↓ | 1 | 4 | ↓ | 1 | |
| 1 | → | 0 | 3 | → | 1 | 2 | → | 2 | 0 | → | 1 | 3 | → | 2 | 3 | → | 2 | ||
| → | 2 | ↑ | 1 | 3 | ↑ | 3 | 6 | ↑ | 5 | 3 | ↑ | 3 | 8 | ↑ | 3 | 6 | ↑ | 4 | |
| 1 | ↓ | 2 | 6 | ↓ | 3 | 1 | ↓ | 4 | 2 | ↓ | 0 | 1 | ↓ | 2 | 4 | ↓ | 4 | ||
| 17 | → | 4 | 11 | → | 7 | 23 | → | 9 | 14 | → | 6 | 8 | → | 4 | 21 | → | 9 | ||
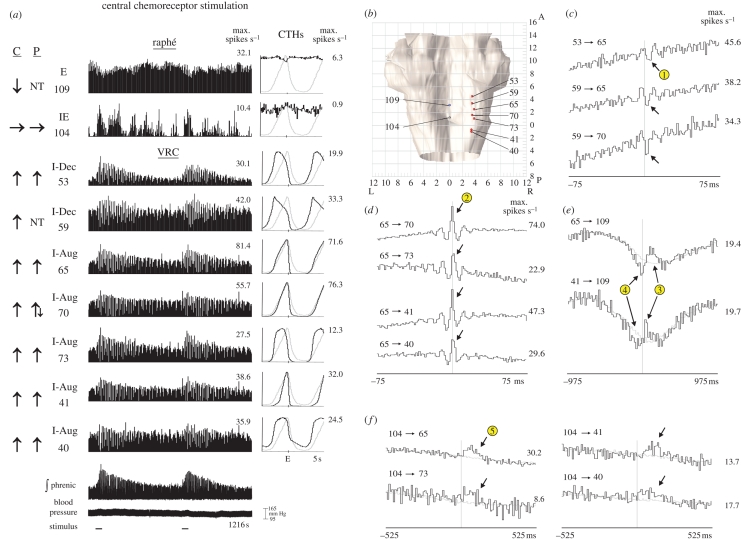
Correlation linkage maps generated from spike-train datasets acquired from neurons monitored at multiple sites revealed evidence of distributed functional relationships. One group composed of two raphé neurons with E and IE respiratory-modulated discharge patterns and seven VRC inspiratory neurons, including two with I-Dec and five with I-Aug discharge patterns, is represented in figure 4a. The raphé E neuron responded to central chemoreceptor stimulation with a decline in firing rate. The IE neuron had more variable activity and no specific response was detected. Each VRC neuron responded to both central and peripheral (not shown) chemoreceptor stimulation with increased activity; the I-Dec neuron designated 59 was not tested for responses to the latter stimulus because of a loss of signal. Stereotaxic coordinates of recording sites are shown in figure 4b.
Figure 4.
(Opposite.) Responses to central chemoreceptor stimulation and cross-correlograms selected from one large-scale multi-array recording. (a) Firing rate histograms for nine simultaneously monitored neurons, integrated phrenic nerve activity and blood pressure during two trials of central chemoreceptor stimulation; injection durations denoted by bars below analogue traces. Changes in firing rate in response to stimulation and maximum firing rates for each histogram are shown as in figure 2; some neurons were not tested (NT) with a particular stimulus. The respiratory modulation of a neuron is included above the neuron's ID code and depicted in the normalized CTH to the right of its corresponding firing rate histogram. The CTH of the neuron (black plot) is shown overlaid upon the CTH of the phrenic nerve (grey plot); the maximum firing rate for each neuronal CTH is shown to the right. For each CTH, 2047 respiratory cycles were averaged and the binwidth was 50 ms; the tic mark on the x-axis of each CTH indicates the occurrence of the E pulse. Numbers of spikes—neuron 109: 37 390 spikes; 104: 4653; 53: 54 748; 59: 91 313; 65: 134 036; 70: 131 610; 73: 26 550; 41: 37 389; 40: 79 657. (b) Dorsal view of brainstem showing stereotaxic coordinates of recording sites of neural activity shown in (a). Neuron balls are colour coded according to their primary response to central chemoreceptor stimulation: red, increase; blue, decrease; grey, no change. (c–f) Cross-correlation histograms (CCH) with central and offset features suggestive of functional connectivity among the neurons depicted in (a). Grey lines in some CCHs represent the mean activity in shifted cycles (§2). Maximum rates are shown to the right of each CCH. CCH plots were independently offset to facilitate display of multiple plots. Circled numbers match the particular correlogram features with inferred functional connections shown in figure 6.
Figure 4c–f shows cross-correlogram features (peaks and troughs) that departed from background firing probability, which is represented by the mean shift-predictor control trace in some plots. The correlograms in figure 4c were calculated using spikes in I-Dec neurons 53 and 59 as trigger events and impulses in two I-Aug neurons (65 and 70) as target events. The features in the correlograms for pairs 53→65, 59→65 and 59→70 include significant peaks and troughs, with primary troughs (arrows, circle 1) to the right of the correlogram origin in all three histograms. Circled numbers match the particular correlogram features with inferred functional connections represented in the ‘ball-and-stick’ models shown in figure 6 (§4).
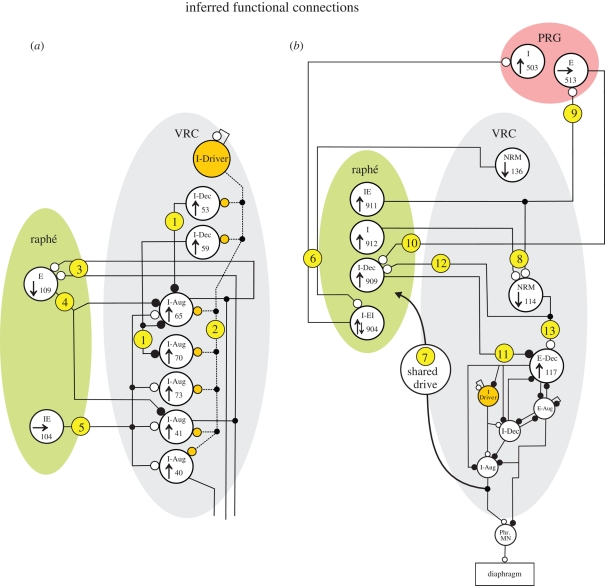
Figure 6.
Ball-and-stick models of functional connectivity suggested or supported by the results. Simple interpretations of offset correlogram features and responses to (a) central or (b) peripheral chemoreceptor stimulation for two large-scale multi-array recordings. Individual neurons are shown as large circles labelled with the cell ID code, respiratory type and response to chemoreceptor stimulation. The small circles at the ends of the connecting lines indicate the particular synaptic relationship: excitatory and inhibitory synapses are shown as open and solid circles, respectively. Circled numbers refer to functional connections inferred from correlograms in figures 4 and 5. The circuit shown in the lower half of the VRC in (b) (circles with no cell ID codes) is a previously published summary of inferred actions among VRC neurons (Lindsey et al. 1998). The raphé I-EI neuron began its increase in discharge rate shortly before the E-to-I phase transition. Phr. MN, phrenic motor neurons. See text for further discussion. Open circle, offset peak; filled large circle, offset trough; filled small circle, network junction.
Central peaks were the primary feature in correlograms for several pairs of VRC I-Aug neurons; secondary bilateral troughs were also apparent (figure 4d). When spikes from two of these I-Aug neurons served as trigger events in correlograms with the expiratory-modulated raphé neuron 109, two significant peaks with positive lags were identified (figure 4e, circle 3). Troughs to the left of the peaks were also noted (circle 4); both had detectability index values greater than 3. The offset peaks in each correlogram in figure 4f (arrows) suggest functional connections between one unresponsive raphé IE neuron and four of the five VRC I-Aug neurons. Simple interpretations of the correlation features together with current models of the respiratory network suggest the circuit represented in figure 6a, which is considered further in §4.
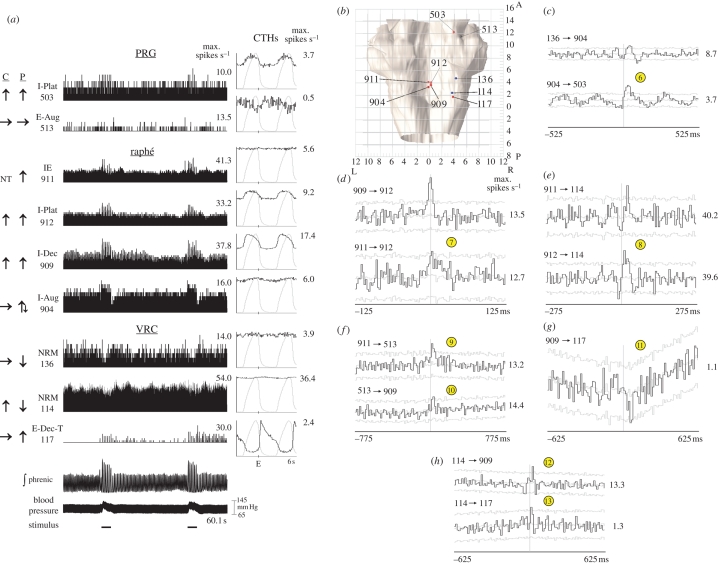
Responses and CTHs from another group of correlated neurons that exhibited various changes in firing rate during the stimulus periods are shown in figure 5a. Changes in firing rate during two peripheral chemoreceptor stimulation trials are illustrated. Stereotaxic coordinates of recording sites of these nine neurons are shown in figure 5b. Extended correlational linkages were identified among various neurons in this group; some are documented by the features shown in figure 5c–h. For example, the offset peak with the positive lag in the 136→904 correlogram (figure 5c) is followed by a trough. The 904→503 correlogram has secondary peaks and troughs in addition to a primary peak with a positive lag (figure 5c). The remaining features illustrated in the correlograms include central peaks (figure 5d), primary offset peaks (figure 5e,f,h) and an offset trough (figure 5g). Simple circuits consistent with this feature set are shown in figure 6b and considered in §4.
Figure 5.
(Opposite.) Responses to peripheral chemoreceptor stimulation and cross-correlograms selected from one large-scale multi-array recording. (a) Firing rate histograms for nine simultaneously monitored neurons, integrated phrenic nerve activity and blood pressure during two trials of peripheral chemoreceptor stimulation; labelling conventions are as in figure 4. For each CTH, 2758 respiratory cycles were averaged and the binwidth was 60 ms. Numbers of spikes—neuron 503: 25 902 spikes; 513: 3526; 911: 49 505; 912: 72 647; 909: 125 495; 904: 51 885; 136: 33 409; 114: 329 666; 117: 9588. (b) Dorsal view of brainstem showing stereotaxic coordinates of recording sites of neural activity shown in (a). Neuron balls are colour coded according to their primary response to peripheral chemoreceptor stimulation: red, increase; blue, decrease; grey, no change. (c–h) Cross-correlograms with central and offset features suggestive of functional connectivity among the neurons depicted in (a). Grey lines in CCHs represent the mean activity ±3 s.d. in shuffled cycles. Maximum rates are shown to the right of each CCH. CCH plots were independently offset to facilitate display of multiple plots. Circled numbers are the same as in figure legend 4.
4. Discussion
Overall, the results of this study suggest a brainstem network architecture with connectivity for the modulation of the respiratory motor pattern by overlapping circuits that promote and limit central and peripheral chemoreceptor-mediated influences on breathing. The response profiles of neurons distributed in the raphé-pontomedullary respiratory network show that overlapping populations are differentially influenced by selective, sequential, transient stimulation of central and peripheral chemoreceptors. Stimulation of both classes of receptors resulted in increased VRC, raphé and PRG inspiratory neuron activity, although particular inspiratory neurons did respond differently to the two stimuli. Correlations between neuronal spike trains indicate paucisynaptic interactions among neurons responsive to stimulation of either or both types of chemoreceptors; a functional projection to at least one other neuron could be inferred for approximately 50 per cent of the cells in each brain region. Detected correlational linkages suggest multiple feedback loops between different categories of neurons in the VRC and cells in the PRG and brainstem midline, supporting recent models of respiratory network architecture (Smith et al. 2007; Rybak et al. 2008; Nuding et al. 2009).
Sequential observations of single neurons cannot distinguish between responsive cells with a putative ‘relay’ or excitatory function and neurons with similar responses that may act to suppress or limit the activity of their targets. Our use of electrode arrays contributed to experimental efficiency (i.e. more neurons recorded per experiment) and enabled the detection of multiple correlations among the simultaneously monitored spike trains. Moreover, for each monitored group of neurons, changes in activity and the respiratory motor pattern in response to a particular stimulus were measured under the same history-dependent conditions, and, therefore, were not confounded by possible changes in the state of the animal. We also note that our approach screened neurons for responses to chemoreceptor perturbations using measurements of firing rate. Circuit architectures that promote changes in impulse synchrony with or without changes in firing rate could also be involved in brainstem sensory processing of chemoreceptor information (Morris et al. 2001) and would not necessarily be detected with this approach, as has also been noted elsewhere in studies on baroreceptors (Arata et al. 2000) and pulmonary stretch receptor reflexes (Dick et al. 2008) in the control of breathing. This possibility remains an area for future investigation.
(a). Functional implications
Medullary raphé neurons constitute a major modulatory system in the control of breathing (Holtman et al. 1986a,b; Lalley 1986b; Millhorn 1986). Both anatomical and physiological evidence support a role for the raphé nuclei in respiratory regulation. Numerous anatomical studies in both cat (Lalley 1986a) and rat (Connelly et al. 1989; Holtman et al. 1990) have demonstrated projections between raphé nuclei and other regions of the nervous system involved in respiratory control. Their putative functions include: (i) permissive or enabling roles in respiratory rhythmogenesis (Lalley et al. 1997; Lovick 1997; Peña & Ramirez 2002); (ii) a respiratory memory associated with increased ventilation in response to repeated intermittent peripheral chemoreceptor stimulation or hypoxia (Millhorn et al. 1980; Millhorn 1986; Morris et al. 1996b, 2001, 2003; Mitchell & Johnson 2003); (iii) baroreceptor modulation of the respiratory motor pattern (Lindsey et al. 1998); (iv) network reconfiguration during cough (Shannon et al. 1998, 2000; Baekey et al. 2003); and (v) a central chemoreceptor function (Bernard et al. 1996; Wang et al. 1998, 2002; Nattie 1999; Bradley et al. 2002). More recently, it has been shown that focal acidification of the rostral medullary raphé nuclei via microdialysis of CO2 stimulates breathing (Nattie & Li 2001); ventilation was unchanged when the same technique was applied solely to the more caudal raphé obscurus nucleus (Dias et al. 2008). However, high-CO2 dialysis applied simultaneously to the chemoreceptive retrotrapezoid nucleus and caudal raphé obscurus nucleus produced a greater increase in ventilation than when the retrotrapezoid nucleus alone was stimulated (Dias et al. 2008), suggesting a modulatory role for the caudal medullary raphé with respect to central chemoreception at the retrotrapezoid nucleus.
Previous results from this laboratory have demonstrated the influence of peripheral chemoreceptors (Morris et al. 1996a,b, 2001) and baroreceptors (Lindsey et al. 1998; Arata et al. 2000) and their functional convergence on raphé neurons (Li et al. 1999a). Other work has shown that raphé neurons change their discharge patterns when the respiratory network is reconfigured during cough (Baekey et al. 2001, 2003) and during the alteration or absence of pulmonary stretch receptor feedback (Morris et al. 2007).
The present results lend further support to the hypothesis that raphé neurons are organized into functionally connected groups that regulate breathing. The ball-and-stick models in figure 6a,b represent simple circuit configurations suggested by the correlograms and responses to chemoreceptor stimulation shown in figures 4 and 5, respectively. Connections in the models are labelled with circled numbers that correspond to the supporting correlograms.
The results shown in figure 4c,d and represented in figure 6a reinforce our previous work within the VRC (Segers et al. 1987; Li et al. 1999a). The offset troughs in the correlograms in figure 4c provide evidence for functional inhibition of I-Aug neurons 65 and 70 by I-Dec cells (figure 6a, circle 1). The central peaks in figure 4d are consistent with input(s) shared by VRC I-Aug neurons 65, 70, 41 and 40; shown in figure 6a (circle 2), these synchronizing inputs (dashed lines) are postulated to come from an I-Driver population, described previously as VRC neurons whose activity increases shortly before phrenic nerve discharge, peaks in the early part of inspiration and then slowly decrements before abruptly ceasing at the I-to-E phase transition (Segers et al. 1987; Morris et al. 1996a). All four VRC I-Aug cells had an increase in firing rate in response to central chemoreceptor stimulation.
This study also identified evidence for specific feedback loops that could influence responses evoked by chemoreceptor stimulation. The peaks and troughs to the right and left, respectively, of the midline in the two correlograms in figure 4e suggest a complex relationship between a raphé E neuron and two VRC I-Aug cells. These correlation features are consistent with facilitatory inputs (circle 3 in figure 6a) from VRC cells 65 and 41 to raphé neuron 109, which, in turn, provided inhibitory feedback (circle 4) to the same VRC cells. This circuit and the decreased discharge rate of neuron 109 in response to vertebral CO2 injection would serve to promote the response of the VRC neurons to central chemoreceptor stimulation via disinhibition of the I-Aug cells. Evidence for excitatory modulation of the same two VRC inspiratory neurons by a raphé IE neuron that did not respond to either chemoreceptor challenge (figure 4f; circle 5) suggests that non-responding inputs can bias the reaction of target neurons to chemoreceptor stimulation.
Bianchi et al. (1995) proposed that midline raphé circuits serve as ‘intermediate relays’ in PRG–VRC interactions. Previous results from our laboratory support this hypothesis (Nuding et al. 2009). An example of an ‘indirect’ pathway between the VRC and PRG involving intermediary raphé cells is included in the circuit model in figure 6b (circle 6) and reflects the correlogram features in figure 5c. Both correlograms contain an offset peak, suggesting that each reference neuron had an excitatory effect upon its target cell, resulting in a VRC→raphé→PRG functional connectivity chain. VRC neuron 136 decreased and raphé cell 904 first increased and then decreased its firing rate in response to peripheral chemoreceptor stimulation. The delayed portion of the biphasic response of neuron 904 could be due to the reduction of excitatory input from cell 136 (disfacilitation).
Our data are consistent with the concept of efference copy of VRC inspiratory drive serving as a conduit for the transference of chemoreceptor stimulation effects to raphé neurons (Lindsey et al. 1994). Results from one group of neurons documented enhanced inspiratory drive to diverse raphé neurons that had functional connections with the PRG and VRC. The central peaks in figure 5d suggest that raphé neurons 909, 911 and 912 shared an input source, perhaps from efference copy reflecting changes in VRC output inspiratory drive (circle 7 in figure 6b); all three cells responded to peripheral chemoreceptor stimulation with an increased firing rate.
The correlograms of several neuron pairs in figure 5 provide evidence of convergent and divergent functional connections of raphé neurons. The offset peaks in figure 5e can be interpreted as a convergent excitatory effect of raphé neurons 911 and 912 upon VRC cell 114 (circle 8 in figure 6b). Both of the raphé cells increased in activity while the discharge rate of the VRC neuron decreased in response to peripheral chemoreceptor stimulation, suggesting that the activity decline in the VRC neuron following the stimulation was limited by the increased rates of the raphé cells. The offset peak in the upper correlogram in figure 5f suggests that, in addition to its effect upon VRC cell 114, midline neuron 911 had a divergent excitatory influence upon PRG cell 513 (circle 9), which, in turn, had an excitatory effect on raphé cell 909 (figure 5f, circle 10). The intermediate position of cell 513 in this raphé →PRG→ raphé loop could serve to gate the transference of increased activity from neuron 911 to cell 909 in response to peripheral chemoreceptor stimulation.
The results also support the hypothesis that midline brainstem neurons are connected to the PRG and VRC in ways appropriate for shaping the physiological responses evoked by both types of chemoreceptors. VRC E-Dec neurons are proposed to play a key role in the regulation of expiratory duration in current respiratory network models (Rybak et al. 2008); changes in the firing rate and/or discharge pattern of such a cell would have a bearing on phase switching and breathing rate. Our data suggest that raphé neurons have a modulatory effect upon VRC E-Dec cells. Interpretations of the three correlograms in figure 5g,h are shown in figure 6b as circles 11, 12 and 13 and suggest that VRC cell 114 had a direct excitatory as well as an indirect inhibitory, self-limiting effect through raphé neuron 909 upon VRC E-Dec cell 117. Changes in the gain of intermediary raphé neuron 909 (by the loop discussed above, for example) would modulate the level of functional inhibition imposed upon the VRC E-Dec cell by neuron 114.
(b). Other relationships to previous studies
Long-term respiratory facilitation can be induced by repeated brief stimulation of peripheral chemoreceptors or medullary raphé neurons (Millhorn et al. 1980; Morris et al. 1996a,b, 2001; Mitchell et al. 2001) and is associated with enhanced efficacy of shared drive, leading to increased synchrony of inspiratory neurons and amplified activity in respiratory motor neurons. A previous study identified raphé neurons with transient increases in firing rate at the onset of carotid chemoreceptor stimulation that subsequently declined as a delayed increase in other raphé neurons developed (Morris et al. 1996a,b, 2001). These distinct response properties and related short-time scale correlations led to a ‘ratchet’ model (Morris et al. 2001, fig. 7c): Transiently responding neurons contribute to the generation of the facilitated state during periods of high firing rate, while inhibitory actions of delayed responders limit the amount of potentiation induced with each stimulus.
The dissimilar responses of some raphé neurons to central and peripheral chemoreceptor stimulation and the connectivity inferred by this study suggest interactions that differentially promote and limit chemoreceptor interactions. We found raphé neurons stimulated by carotid injection but functionally inhibited by central chemoreceptor activation (e.g. figure 2a). These distinct responses are consistent with the proposed role of raphé neurons in the ratchet hypothesis of long-term facilitation induction and the inability of central chemoreceptor stimulation to evoke this respiratory memory (Millhorn 1986). A related ‘push–pull’ proposal based on raphé cell culture data has been suggested for the regulation of respiration and other pH-sensitive central nervous system functions by Richerson et al. (2001). Our findings are also relevant to the observation of Eldridge et al. (1981) that the magnitude of the response to a chemoreceptor stimulus is inversely related to the level of pre-existing activity. That earlier work postulated that this hypoadditive interaction occurs at the level of the central respiratory controller after convergence of CO2 inputs but prior to medullary output. Our new results emphasize the need for further studies to test the broader hypothesis that midline neuronal assemblies stabilize and regulate the gain of motor output in cardiorespiratory control (Lindsey et al. 1992c, 1998; Morris et al. 1996a, 2001).
Acknowledgements
Experiments were performed under protocols approved by the University of South Florida's Institutional Animal Care and Use Committee and with strict adherence to all American Association for Accreditation of Laboratory Animal Care International (AAALAC), National Institutes of Health and National Research Council guidelines.
We thank Peter Barnhill, Kimberly Ruff, Kathryn Ross, Andrew Ross, Mackenzie Ott and Carl Strohmenger for excellent technical assistance. This work was supported by NIH grants R37NS19814 and RO1NS046062.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Brainstem neural networks vital for life’.
References
- Aertsen A. M., Gerstein G. L.1985Evaluation of neuronal connectivity: sensitivity of cross-correlation. Brain Res. 340, 341–354 (doi:10.1016/0006-8993(85)90931-X) [DOI] [PubMed] [Google Scholar]
- Aertsen A. M., Gerstein G. L., Habib M. K., Palm G.1989Dynamics of neuronal firing correlation: modulation of ‘effective connectivity’. J. Neurophysiol. 61, 900–917 [DOI] [PubMed] [Google Scholar]
- Alheid G. F., Milsom W. K., McCrimmon D. R.2004Pontine influences on breathing: an overview. Respir. Physiol. Neurobiol. 143, 105–114 (doi:10.1016/j.resp.2004.06.016) [DOI] [PubMed] [Google Scholar]
- Arata A., Hernandez Y. M., Lindsey B. G., Morris K. F., Shannon R.2000Transient configurations of baroresponsive respiratory-related brainstem neuronal assemblies in the cat. J. Physiol. 525, 509–530 (doi:10.1111/j.1469-7793.2000.t01-1-00509.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita H., Kogo N., Ichikawa K.1988Locations of medullary neurons with non-phasic discharges excited by stimulation of central and/or peripheral chemoreceptors and by activation of nociceptors in cat. Brain Res. 442, 1–10 (doi:10.1016/0006-8993(88)91426-6) [DOI] [PubMed] [Google Scholar]
- Aungst J., Ptak K., Yamanishi T., Milescu L. S., Zhang R., Richerson G. B., Smith J. C.2008Raphé neurons stimulate respiratory circuit activity by endogenously released serotonin and substance P which are critical for respiratory rhythm generation. Program No. 476.6. In 2008 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience. Online; [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekey D. M., Morris K. F., Gestreau C., Li Z., Lindsey B. G., Shannon R.2001Medullary respiratory neurons and control of laryngeal motoneurons during fictive eupnoea and cough in the cat. J. Physiol. 534, 565–581 (doi:10.1111/j.1469-7793.2001.t01-1-00565.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekey D. M., Morris K. F., Nuding S. C., Segers L. S., Lindsey B. G., Shannon R.2003Medullary raphé neuron activity is altered during fictive cough in the decerebrate cat. J. Appl. Physiol. 94, 93–100 (doi:10.1152/japplphysiol.00341.2002) [DOI] [PubMed] [Google Scholar]
- Balis U. J., Morris K. F., Koleski J., Lindsey B. G.1994Simulations of a ventrolateral medullary neural network for respiratory rhythmogenesis inferred from spike train cross-correlation. Biol. Cybern. 70, 311–327 (doi:10.1007/BF00200329) [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y.1995Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. B 57, 289–300 [Google Scholar]
- Berman A. L.1968The brain stem of the cat: a cytoarchitectonic atlas with stereotaxic coordinates Madison, WI: University of Wisconsin Press [Google Scholar]
- Bernard D. G., Li A., Nattie E. E.1996Evidence for central chemoreception in the midline raphé. J. Appl. Physiol. 80, 108–115 [DOI] [PubMed] [Google Scholar]
- Bianchi A. L., Denavit-Saubie M., Champagnat J.1995Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol. Rev. 75, 1–45 [DOI] [PubMed] [Google Scholar]
- Bradley S. R., Pieribone V. A., Wang W., Severson C. A., Jacobs R. A., Richerson G. B.2002Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat. Neurosci. 5, 401–402 (doi:10.1038/nn848) [DOI] [PubMed] [Google Scholar]
- Broersen P. M. T.2000Finite sample criteria for autoregressive order selection. Signal Process. IEEE Trans. 48, 3550–3558 (doi:10.1109/78.887047) [Google Scholar]
- Cohen M. I.1968Discharge patterns of brain-stem respiratory neurons in relation to carbon dioxide tension. J. Neurophysiol. 31, 142–165 [DOI] [PubMed] [Google Scholar]
- Cohen M. I.1979Neurogenesis of respiratory rhythm in the mammal. Physiol. Rev. 59, 1105–1173 [DOI] [PubMed] [Google Scholar]
- Connelly C. A., Ellenberger H. H., Feldman J. L.1989Are there serotonergic projections from raphé and retrotrapezoid nuclei to the ventral respiratory group in the rat? Neurosci. Lett. 105, 34–40 (doi:10.1016/0304-3940(89)90007-4) [DOI] [PubMed] [Google Scholar]
- Davey N. J., Ellaway P. H., Stein R. B.1986Statistical limits for detecting change in the cumulative sum derivative of the peristimulus time histogram. J. Neurosci. Methods 17, 153–166 (doi:10.1016/0165-0270(86)90068-3) [DOI] [PubMed] [Google Scholar]
- Davison A. C., Hinkley D. V. Bootstrap methods and their application. Cambridge, UK:: Cambridge University Press; 1997. [Google Scholar]
- Dias M. B., Li A., Nattie E.2008Focal CO2 dialysis in raphé obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus. J. Appl. Physiol. 105, 83–90 (doi:10.1152/japplphysiol.00120.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick T. E., Bellingham M. C., Richter D. W.1994Pontine respiratory neurons in anesthetized cats. Brain Res. 636, 259–269 (doi:10.1016/0006-8993(94)91025-1) [DOI] [PubMed] [Google Scholar]
- Dick T. E., Shannon R., Lindsey B. G., Nuding S. C., Segers L. S., Baekey D. M., Morris K. F.2008Pontine respiratory-modulated activity before and after vagotomy in decerebrate cats. J. Physiol. 586, 4265–4282 (doi:10.1113/jphysiol.2008.152108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J.2000Simulation of cross-correlograms resulting from synaptic connections between neurons. J. Neurosci. Methods 99, 65–70 (doi:10.1016/S0165-0270(00)00213-2) [DOI] [PubMed] [Google Scholar]
- Eldridge F. L., Gill-Kumar P., Millhorn D. E.1981Input–output relationships of central neural circuits involved in respiration in cats. J. Physiol. 311, 81–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. L., Mitchell G. S., Nattie E. E.2003Breathing: rhythmicity, plasticity, chemosensitivity. Annu. Rev. Neurosci. 26, 239–266 (doi:10.1146/annurev.neuro.26.041002.131103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. A., Nye P. C., Torrance R. W.1982The effects on respiration in the cat of the sudden excitation of cerebral vascular nociceptors by carbon dioxide. Clin. Sci. 63, 505–511 [DOI] [PubMed] [Google Scholar]
- Holtman J. R., Jr, Anastasi N. C., Norman W. P., Dretchen K. L.1986aEffect of electrical and chemical stimulation of the raphe obscurus on phrenic nerve activity in the cat. Brain Res. 362, 214–220 (doi:org/10.1016/0006-8993(86)90446-4) [DOI] [PubMed] [Google Scholar]
- Holtman J. R., Jr, Dick T. E., Berger A. J.1986bInvolvement of serotonin in the excitation of phrenic motoneurons evoked by stimulation of the raphe obscurus. J. Neurosci. 6, 1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman J. R., Jr, Marion L. J., Speck D. F.1990Origin of serotonin-containing projections to the ventral respiratory group in the rat. Neuroscience 37, 541–552 (doi:org/10.1016/0306-4522(90)90422-Z) [DOI] [PubMed] [Google Scholar]
- Kirsten E. B., St John W. M.1978A feline decerebration technique with low mortality and long-term homeostasis. J. Pharmacol. Methods 1, 263–268 (doi:10.1016/0160-5402(78)90057-8) [Google Scholar]
- Kuwana S., Natsui T.1987Effect of hypercapnic blood injection into the vertebral artery on the phrenic nerve activity in cats. Jpn J. Physiol. 37, 155–159 (doi:10.2170/jjphysiol.37.155) [DOI] [PubMed] [Google Scholar]
- Lalley P. M.1986aResponses of phrenic motoneurons of the cat to stimulation of medullary raphe nuclei. J. Physiol. 380, 349–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley P. M.1986bSerotoninergic and non-serotoninergic responses of phrenic motoneurons to raphe stimulation in the cat. J. Physiol. 380, 373–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley P. M., Benacka R., Bischoff A. M., Richter D. W.1997Nucleus raphe obscurus evokes 5-HT1A receptor-mediated modulation of respiratory neurons. Brain Res. 747, 156–159 (doi:10.1016/S0006-8993(96)01233-4) [DOI] [PubMed] [Google Scholar]
- Li Z., Morris K. F., Baekey D. M., Shannon R., Lindsey B. G.1999aMultimodal medullary neurons and correlational linkages of the respiratory network. J. Neurophysiol. 82, 188–201 [DOI] [PubMed] [Google Scholar]
- Li Z., Morris K. F., Baekey D. M., Shannon R., Lindsey B. G.1999bResponses of simultaneously recorded respiratory-related medullary neurons to stimulation of multiple sensory modalities. J. Neurophysiol. 82, 176–187 [DOI] [PubMed] [Google Scholar]
- Lindsey B. G., Hernandez Y. M., Morris K. F., Shannon R.1992aFunctional connectivity between brain stem midline neurons with respiratory-modulated firing rates. J. Neurophysiol. 67, 890–904 [DOI] [PubMed] [Google Scholar]
- Lindsey B. G., Hernandez Y. M., Morris K. F., Shannon R., Gerstein G. L.1992bDynamic reconfiguration of brain stem neural assemblies: respiratory phase-dependent synchrony versus modulation of firing rates. J. Neurophysiol. 67, 923–930 [DOI] [PubMed] [Google Scholar]
- Lindsey B. G., Hernandez Y. M., Morris K. F., Shannon R., Gerstein G. L.1992cRespiratory-related neural assemblies in the brain stem midline. J. Neurophysiol. 67, 905–922 [DOI] [PubMed] [Google Scholar]
- Lindsey B. G., Segers L. S., Morris K. F., Hernandez Y. M., Saporta S., Shannon R.1994Distributed actions and dynamic associations in respiratory-related neuronal assemblies of the ventrolateral medulla and brain stem midline: evidence from spike train analysis. J. Neurophysiol. 72, 1830–1851 [DOI] [PubMed] [Google Scholar]
- Lindsey B. G., Arata A., Morris K. F., Hernandez Y. M., Shannon R.1998Medullary raphé neurons and baroreceptor modulation of the respiratory motor pattern in the cat. J. Physiol. 512, 863–882 (doi:10.1111/j.1469-7793.1998.863bd.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovick T. A.1997The medullary raphe nuclei: a system for integration and gain control in autonomic and somatomotor responsiveness? Exp. Physiol. 82, 31–41 [DOI] [PubMed] [Google Scholar]
- Lumsden T.1923Observations on the respiratory centres in the cat. J. Physiol. 57, 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marckwald M.1887Die Athembewegunge und deren Innervation beim Kaninchen. Ztschr. Biol. 23, 149–283 [Google Scholar]
- Melssen W. J., Epping W. J.1987Detection and estimation of neural connectivity based on crosscorrelation analysis. Biol. Cybern. 57, 403–414 (doi:10.1007/BF00354985) [DOI] [PubMed] [Google Scholar]
- Millhorn D. E.1986Stimulation of raphe (obscurus) nucleus causes long-term potentiation of phrenic nerve activity in cat. J. Physiol. 381, 169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhorn D. E., Eldridge F. L., Waldrop T. G.1980Prolonged stimulation of respiration by endogenous central serotonin. Respir. Physiol. 42, 171–188 (doi:10.1016/0034-5687(80)90113-9) [DOI] [PubMed] [Google Scholar]
- Mitchell G. S., Johnson S. M.2003Plasticity in respiratory motor control. Invited review: neuroplasticity in respiratory motor control. J. Appl. Physiol. 94, 358–374 [DOI] [PubMed] [Google Scholar]
- Mitchell G. S., Baker T. L., Nanda S. A., Fuller D. D., Zabka A. G., Hodgeman B. A., Bavis R. W., Mack K. J., Olson E. B., Jr2001Intermittent hypoxia and respiratory plasticity. J. Appl. Physiol. 90, 2466–2475 [DOI] [PubMed] [Google Scholar]
- Moore G. P., Segundo J. P., Perkel D. H., Levitan H.1970Statistical signs of synaptic interaction in neurons. Biophys. J. 10, 876–899 (doi:10.1016/S0006-3495(70)86341-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris K. F., Arata A., Shannon R., Lindsey B. G.1996aInspiratory drive and phase duration during carotid chemoreceptor stimulation in the cat: medullary neuron correlations. J. Physiol. 491, 241–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris K. F., Arata A., Shannon R., Lindsey B. G.1996bLong-term facilitation of phrenic nerve activity in cats: responses and short time scale correlations of medullary neurons. J. Physiol. 490, 463–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris K. F., Baekey D. M., Nuding S. C., Segers L. S., Dick T. E., Shannon R., Lindsey B. G.2001Integration of cardiorespiratory responses to carotid chemoreceptor stimulation by medullary and pontine neural networks in cats. Soc. Neurosci. Abstr. 27, 172.8 [Google Scholar]
- Morris K. F., Baekey D. M., Nuding S. C., Dick T. E., Shannon R., Lindsey B. G.2003Plasticity in respiratory motor control. Invited review: neural network plasticity in respiration control. J. Appl. Physiol. 94, 1242–1252 [DOI] [PubMed] [Google Scholar]
- Morris K. F., Lindsey B. G., Baekey D. M., Nuding S. C., Segers L. S., Shannon R., O'Connor R. E., Dick T. E.2007Cardiorespiratory rhythms in spike trains of caudal raphe and pontine neurons in cats: insights from computational models of acute vagotomy. Program No. 230.11. In 2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience [Google Scholar]
- Mulkey D., Stornetta R., Weston M., Simmons J., Parker A., Bayliss D., Guyenet P.2004Respiratory control by ventral surface chemoreceptor neurons in rats. Nat. Neurosci. 7, 360–1369 (doi:org/10.1038/nn1357) [DOI] [PubMed] [Google Scholar]
- Nattie E. E.1999CO2, brainstem chemoreceptors and breathing. Prog. Neurobiol. 59, 299–331 (doi:10.1016/S0301-0082(99)00008-8) [DOI] [PubMed] [Google Scholar]
- Nattie E. E., Li A.2001CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J. Appl. Physiol. 90, 1247–1257 [DOI] [PubMed] [Google Scholar]
- Nattie E. E., Li A.2009Central chemoreception is a complex system function that involves multiple brainstem sites. J. Appl. Physiol. 106, 1464–1466 (doi:10.1152/japplphysiol.00112.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netick A., Orem J.1981Erroneous classification of neuronal activity by the respiratory modulation index. Neurosci. Lett. 21, 301–306 (doi:10.1016/0304-3940(81)90221-4) [DOI] [PubMed] [Google Scholar]
- Nuding S. C., Segers L. S., Shannon R., Lindsey B. G., Morris K. F.2007Raphé neuronal circuits and responses to central and peripheral chemoreceptor stimulation. Exp. Biol. Meet. Abstr. 761.11 [Google Scholar]
- Nuding S. C., Segers L. S., Baekey D. M., Dick T. E., Solomon I. C., Shannon R., Morris K. F., Lindsey B. G.2009Pontine—ventral respiratory column interactions through raphé circuits detected using multi-array spike train recordings. J. Neurophysiol. 101, 2943–2960 (doi:10.1152/jn.91305.2008,2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orem J., Netick A.1982Characteristics of midbrain respiratory neurons in sleep and wakefulness in the cat. Brain Res. 244, 231–241 (doi:10.1016/0006-8993(82)90082-8) [DOI] [PubMed] [Google Scholar]
- Peña F., Ramirez J.2002Endogenous activation of serotonin2A receptors is required for respiratory rhythm generation in vitro. J. Neurosci. 22, 11 055–11 064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P.1967Neuronal spike trains and stochastic point processes II: simultaneous spike trains. Biophys. J. 7, 419–440 (doi:10.1016/S0006-3495(67)86597-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson G. B., Wang W., Tiwari J., Bradley S. R.2001Chemosensitivity of serotonergic neurons in the rostral ventral medulla. Respir. Physiol. 129, 175–189 (doi:10.1016/S0034-5687(01)00289-4) [DOI] [PubMed] [Google Scholar]
- Rybak I. A., et al. 2008Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J. Neurophysiol. 100, 1770–1799 (doi:10.1152/jn.90416.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers L. S., Shannon R., Saporta S., Lindsey B. G.1987Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. I. Evidence for excitatory and inhibitory actions of inspiratory neurons. J. Neurophysiol. 57, 1078–1100 [DOI] [PubMed] [Google Scholar]
- Segers L. S., Nuding S. C., Dick T. E., Shannon R., Baekey D. M., Solomon I. C., Morris K. F., Lindsey B. G.2008Functional connectivity in the pontomedullary respiratory network. J. Neurophysiol. 100, 1749–1769 (doi:10.1152/jn.90414.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seller H., König S., Czachurski J.1990Chemosensitivity of sympathoexcitatory neurons in the rostroventrolateral medulla of the cat. Pflugers Arch. Eur. J. Physiol. 416, 735–741 (doi:org/10.1007/BF00370623) [DOI] [PubMed] [Google Scholar]
- Shannon R., Baekey D. M., Morris K. F., Lindsey B. G.1998Ventrolateral medullary respiratory network and a model of cough motor pattern generation. J. Appl. Physiol. 84, 2020–2035 [DOI] [PubMed] [Google Scholar]
- Shannon R., Baekey D. M., Morris K. F., Li Z., Lindsey B. G.2000Functional connectivity among ventrolateral medullary respiratory neurons and responses during fictive cough in the cat. J. Physiol. 525, 207–224 (doi:10.1111/j.1469-7793.2000.00207.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Ellenberger H. H., Ballanyi K., Richter D. W., Feldman J. L.1991Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254, 726–729 (doi:10.1126/science.1683005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Abdala A. P. L., Koizumi H., Rybak I. A., Paton J. F. R.2007Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J. Neurophysiol. 98, 3370–3387 (doi:10.1152/jn.00985.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John W. M.1985Maintenance of respiratory modulation by pneumotaxic mechanisms in deep anesthesia. Exp. Neurol. 87, 382–386 (doi:10.1016/0014-4886(85)90229-8) [DOI] [PubMed] [Google Scholar]
- St John W. M.1986Diffuse pathways convey efferent activity from rostral pontile pneumotaxic center to medullary respiratory regions. Exp. Neurol. 94, 155–165 (doi:10.1016/0014-4886(86)90279-7) [DOI] [PubMed] [Google Scholar]
- St John W. M.1998Neurogenesis of patterns of automatic ventilatory activity. Prog. Neurobiol. 56, 97–117 (doi:10.1016/S0301-0082(98)00031-8) [DOI] [PubMed] [Google Scholar]
- Stella G.1938On the mechanism of production, and the physiological significance of ‘apneusis’. J. Physiol. 93, 10–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Pizzonia J. H., Richerson G. B.1998Chemosensitivity of rat medullary raphé neurons in primary tissue culture. J. Physiol. 511, 433–450 (doi:10.1111/j.1469-7793.1998.433bh.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Bradley S. R., Richerson G. B.2002Quantification of the response of rat medullary raphe neurons to independent changes in pHo and PCO2. J. Physiol. 540, 951–970 (doi:10.1113/jphysiol.2001.013443) [DOI] [PMC free article] [PubMed] [Google Scholar]