Abstract
Control of the timing of the inspiratory/expiratory (IE) phase transition is a hallmark of respiratory pattern formation. In principle, sensory feedback from pulmonary stretch receptors (Breuer–Hering reflex, BHR) is seen as the major controller for the IE phase transition, while pontine-based control of IE phase transition by both the pontine Kölliker–Fuse nucleus (KF) and parabrachial complex is seen as a secondary or backup mechanism. However, previous studies have shown that the BHR can habituate in vivo. Thus, habituation reduces sensory feedback, so the role of the pons, and specifically the KF, for IE phase transition may increase dramatically.
Pontine-mediated control of the IE phase transition is not completely understood. In the present review, we discuss existing models for ponto-medullary interaction that may be involved in the control of inspiratory duration and IE transition. We also present intracellular recordings of pontine respiratory units derived from an in situ intra-arterially perfused brainstem preparation of rats. With the absence of lung inflation, this preparation generates a normal respiratory pattern and many of the recorded pontine units demonstrated phasic respiratory-related activity. The analysis of changes in membrane potentials of pontine respiratory neurons has allowed us to propose a number of pontine-medullary interactions not considered before. The involvement of these putative interactions in pontine-mediated control of IE phase transitions is discussed.
Keywords: respiratory rhythm, central pattern generator, Hebbian plasticity
1. The motor pattern of breathing
The pattern of centrally generated respiratory activity under conditions of normal or eupnoeic breathing comprises three phases of the respiratory cycle: inspiration, post-inspiration or passive expiration and late or active expiration (Richter 1982, 1996; Richter & Spyer 2001). The post-inspiratory (post-I) motor output controls the laryngeal adductor (constrictor) muscles (Harding 1984; Bartlett 1986; Dutschmann & Paton 2002), which counteract the recoil mechanisms of the expanded lung (Bartlett 1986; Dutschmann & Paton 2002). Laryngeal constriction dwindles with progression of the expiratory interval, and the air is evenly released from the lungs. Motor activity during late (active) expiration emerges during forced breathing and involves active contraction of abdominal and thoracic expiratory muscle groups. The active or late phase of expiration is always present centrally, but in terms of the motor act of exhalation, it may be only activated during high metabolic demands (Fortuna et al. 2008) or after experimental challenges involving intermittent hypoxia (Zoccal et al. 2008). However, active expiration under physiological conditions is always preceded by post-I and thus, the alternating phases of inspiration and expiration are largely governed by central oscillations between inspiratory and post-I neurons.
2. General pathways for inspiratory/expiratory phase transitions
According to our present knowledge, two main mechanisms contribute to the inspiratory/expiratory (IE) phase transition or the so-called inspiratory off-switch (IOS). A primary, well-investigated mechanism is afferent vagal feedback arising from slowly adapting pulmonary stretch receptors (PSRs) termed the Breuer–Hering reflex (BHR, Breuer 1868; Hering 1868; for recent review see, Kubin et al. 2006). The physiological significance of the BHR is underlined by the effect of vagotomy (PSR deafferation) or other physiological manoeuvres to suppress PSR feedback (cooling of the vagal nerve or no inflation tests) on the breathing pattern. Lack of BHR feedback triggers a prolonged inspiratory duration, an increase in burst amplitude and a decrease in respiratory frequency (Kubin et al. 2006). This effect on respiratory phase duration was confirmed in numerous studies and is taken as evidence that BHR feedback provides primary control of IE phase transition.
The first description of central mechanisms controlling IE phase transition was provided by Marckwald (1887), one year after publication and demonstration of the BHR. Markwald showed that a lesion of the dorsolateral pons can transform the normal breathing pattern into apneusis, a breathing pattern characterized by a significantly prolonged inspiratory duration. The prolonged inspiratory phase duration indicates disruption of the central IOS mechanism involved in the proper timing of IE phase transition. Later, the potential physiological importance of the pons for IE phase transition was significantly hampered by the demonstration that apneusis after pontine lesions occurred only when vagal afferents, including those from the PSRs, were deactivated by focal cooling of the vagal nerve (Stella 1938). Finally, it was demonstrated that pontine respiratory neurons receive pronounced synaptic inhibition from PSR (Feldman & Gautier 1976; Feldman et al. 1976; Cohen & Feldman 1977). Thus, the dominant view is that under conditions of an intact sensory feedback from PSRs, the pontine activities are largely inhibited and therefore have no or only minor contributions to IE phase transition. The pontine circuits controlling IOS are thus considered a failsafe mechanism.
At the same time, it has been demonstrated that chemical or electrical stimulation of the medial parabrachial complex (mPB) or Kölliker–Fuse nucleus (KF) can evoke phase resetting and IOS in vagi intact animals (Oku & Dick 1992; Chamberlin & Saper 1994, 1998; Dutschmann & Herbert 1998, 2006; Okazaki et al. 2002). However, these pontine-mediated responses were attributed to various protective reflex behaviours (Dutschmann et al. 2004) and were not interpreted as a critical contribution of the pons to IOS and IE phase transition during normal breathing.
3. Neural interactions during inspiratory/expiratory phase transition
It was proposed that PSR feedback from the expanded lung during the later stage of lung inflation initiates the IE phase transition via excitation of late inspiratory (late-I) neurons (Richter 1982, 1996; Cohen et al. 1993; Haji et al. 2002; Rybak et al. 2004; Krolo et al. 2005) and post-I neurons (Richter 1982, 1996; Hayashi et al. 1996; Haji et al. 1999; Rybak et al. 2004). Thus, late-I neurons initiate the phase transition, and subsequent activation of the post-I neurons (also termed decrementing expiratory neurons, dec-E) and provides the irreversible completion of the transition from inspiration to expiration. The sequential activation of late-I and post-I neurons is a centrepiece of many models of respiratory rhythm and pattern generation (Richter 1982, 1996; Bianchi et al. 1995; Rybak et al. 1997, 2004).
Reports on cellular activity in the pons that could potentially contribute to IE phase transition are sparse. In the cat, it was shown that pontine IE phase-spanning neurons, located within the mPB/KF region, could be involved in IE phase transition (Cohen & Shaw 2004). The main hypothesis suggests that medullary late-I neurons which initiate IOS receive excitatory inputs from pontine IE or late-I neurons (Cohen & Shaw 2004). However, the pontine input to late-I neurons was suppressed by BHR feedback. Thus, it was suggested that ponto-medullary interaction may become important only under conditions with weak drive from PSR inputs (Cohen & Shaw 2004). A series of recent studies on pontine respiratory activity in cats using multi-electrode arrays and computational modelling supported this view (Dick et al. 2008; Rybak et al. 2008; Segers et al. 2008). These studies showed that the numbers of phasic respiratory activities in mPB and KF significantly increased after vagotomy or absence of lung inflation, while tonic activity with weak respiratory modulation dominated when vagi were intact or during lung inflation with intact vagi (Dick et al. 2008; Rybak et al. 2008). Nevertheless, reciprocal synaptic interactions between a variety of medullary and pontine respiratory cells could be established (Rybak et al. 2008; Segers et al. 2008). At the same time, a paucity of monosynaptic functional links between pontine and medullary neurons (Bianchi & St-John 1981, 1982; Segers et al. 1985, 2008) was also confirmed. The minor degree of pontomedullary interactions is somewhat surprising since anatomical tract tracing studies had suggested monosynaptic reciprocal coupling of pontine and medullary respiratory groups in cats and rats, but these were not functionally characterized as respiratory neurons (Smith et al. 1989; Ellenberger & Feldman 1990; Herbert et al. 1990; Nunez-Abades et al. 1993; Dobbins & Feldman 1994; Gaytan et al. 1997).
In summary, accumulating evidence suggests that pontine respiratory-modulated activity is largely suppressed in the presence of BHR feedback mechanisms. On the other hand, some respiratory activity can be recorded in cats with intact BHR feedback (Sieck & Harper 1980; St-John 1987; Dick et al. 1994, 2008; Segers et al. 2008). A potential explanation for weak respiratory activity is provided by the finding that BHR feedback can also excite non-respiratory (Dick et al. 1994) and also respiratory modulated units (St-John 1987; Ezure 2004; Ezure & Tanaka 2006). In contrast to the cat, a recent study performed on rats showed substantial respiratory activity at the level of the dorsolateral pons with intact BHR circuitry (Ezure 2004; Ezure & Tanaka 2006). The discrepancy in the density of pontine respiratory activity between cats and rats could relate to some species differences. For instance, the cat shows respiratory-related behaviour such as purring and vomiting, while rats do not (particularly vomiting, see Andrews & Horn 2006). However, whether such subtle differences in respiratory behaviour can be linked to a different organization of the pontine respiratory group is currently matter of speculation.
4. Pontine-mediated inspiratory/expiratory phase transition: a physiological role revisited
Pharmacological manipulation of pontine regions that modulate IE phase transition in the absence of vagal feedback has received considerable attention. It was shown that post-synaptic blockade of NMDA-receptors (NMDA-Rs) substantially affects pontine-mediated phase transition (for review, see Bianchi et al. 1995; Bonham 1995; St-John 1998). Systemic or local blockade of NMDA-R can result in an apneustic breathing pattern (Foutz et al. 1989; Connelly et al. 1992; Pierrefiche et al. 1992, 1998; Fung et al. 1994; Ling et al. 1994; Borday et al. 1998) similar to that demonstrated by pontine lesions or transections. Systemic NMDA-R blockade was reported to trigger apneusis in the newborn kitten (Schweitzer et al. 1992), suggesting that NMDA-R-dependent mechanisms are already fully established at birth. However, systemic blockade of NMDA-R is as inconclusive as the lesion experiments described earlier, since both cannot distinguish which ascending or descending connections between pons and medulla were interrupted or blocked. Some studies supported the idea that systemic NMDA-R blockade suppressed the excitatory drive from the pons to the medulla (Foutz et al. 1989; Borday et al. 1998), while other publications suggest that NMDA-R blockade does not affect the descending inputs from the pons to the medulla (Pierrefiche et al. 1998). In addition, systemic blockade of NMDA-R could change neurotransmission in other brainstem areas such as the nucleus tractus solitarius (NTS) and/or interfere with inputs from peripheral chemoreceptors, or with first-order processing of PSR inputs (for review, see Bonham et al. 2006). However, in our opinion, NMDA-R blockade largely interrupts ascending glutamatergic transmission from medullary respiratory centres to the pons and, in particular, to the KF. This suggestion is supported by the findings that local blockade of post-synaptic NMDA-R in the KF causes apneusis (Fung et al. 1994; Ling et al. 1994; M. Dutschmann, M. Mörschel, I. A. Rybak & T. E. Dick 2009, unpublished data). Furthermore, NMDA-Rs are very densely expressed in the KF (Monaghan & Cotman 1985; Guthman & Herbert 1999). We therefore suggest that pontine-mediated IE phase transition depends largely on excitatory glutamatergic inputs to the KF. A conceptual model for the pontine-mediated IE phase transition that largely depends on excitatory inputs from the medullary respiratory neurons is shown in figure 3.
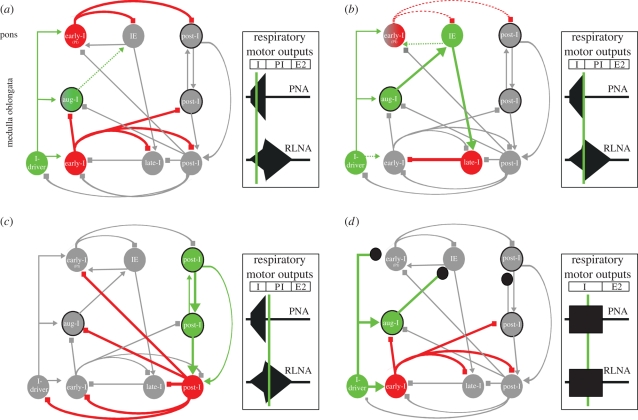
Figure 3.
Hypothetical network model for pontine-mediated IE phase transition. For the theoretical model presented, the following predictions were made: the pontine early-I population receives excitatory synaptic input (efference copy 1) from I-driver neurons located in the pre-Bötzinger complex. The pontine early-I populations are inhibitory interneurons of the pons. The pontine early-I neurons inhibit the pontine IE phase-spanning neuron and pontine post-I pre-motoneurons (post-I) to prevent the initiation of phase transition during early and mid-inspiration (a). With ongoing inspiration, the pontine I/E neurons receive increasing excitatory drive (efference copy 2) from medullary aug-I pre-motor neurons that override the inhibition of early-I, causing firing onset around late inspiration. The pontine IE neurons are, in turn, excitatory interneurons that activate the medullary late-I neurons to initiate the IE phase transition (b). Finally, the inhibitory late-I neurons of the medulla terminate the activity of the medullary early-I neurons and release the medullary post-I population (inhibitory interneurons) from synaptic inhibition. These inhibitory post-I neurons inhibit all the inspiratory population (medullary and pontine). Consequently, the pontine and medullary post-I pre-motor population start firing. According to our previous publication (Dutschmann et al. 2008), we suggest that the pontine population could dominate the medullary populations (c). The effect of post-synaptic blockade of glutamatergic neurotransmission (e.g. NMDA-R antagonism) within the dorsolateral pons is illustrated in (d). Local blockade of excitatory synaptic interaction (black circles) in the pons suppresses the efference copies 1–2, causing blockade of the descending excitatory synaptic input from the pontine IE neurons to the medullary late-I population. This abolishes the pontine-mediated timing of the IE phase transition causing arrest in the inspiratory phase (apneusis). Note that other expiratory neurons that could be involved in the termination of the inspiratory burst are not considered in the theoretical sketch. Red circles, inhibitory interneurons; red lines, the associated connectivity; green circles without frame, excitatory interneurons; green circles with black frame, pre-motor neurons; green lines, excitatory connectivity. The right-hand frames of (a–d) illustrate the motor outputs of the phrenic nerve (PNA) and recurrent laryngeal nerve (RCNA). The green line indicates the point in time of the three-phase respiratory cycle (I, inspiration; PI, post-inspiration; E2, late expiration) that corresponds to the illustrated ponto-medullary synaptic interactions.
Recent publications from our laboratory showed significant postnatal changes in the subunit composition of the heteromeric NMDA-R that correlate with the cellular mechanism for synaptic plasticity (Kron et al. 2008). In addition, blockade of NMDA-R within KF can disrupt the plasticity associated with the processing of BHR feedback (M. Dutschmann, M. Mörschel, I. A. Rybak & T. E. Dick 2009, unpublished data). This is in accordance with our general working hypothesis of postnatal emergence of synaptic plasticity affecting two convergent and parallel pathways mediating IE phase transition: the vagal feedback associated with BHR and the intrinsic IOS mechanisms generated within the dorsolateral pons.
Poon and his co-workers (Siniaia et al. 2000; Song & Poon 2004) have recently demonstrated habituation of vagal feedback that probably occurs within the NTS at the level of the first synapse of PSR afferents. This habituation may also affect the dorsolateral pons. The waxing and waning of habituation and sensitization within the circuitry described earlier can cause a dynamic and state-dependent shift from a dominance of vagal input-dependent to pontine-dependent IOS mechanisms (for review, see Dutschmann et al. 2008).
In summary, several recent studies support the view that pontine circuits may not serve as a simple failsafe mechanism for the BHR feedback, but instead play a significant role in controlling the generation of the respiratory motor pattern in mammals (Cohen & Shaw 2004; Dutschmann et al. 2004, 2008; Poon & Young 2006).
5. Studying pontine-mediated inspiratory/expiratory phase transitions using the in situ perfused brainstem preparation of rats
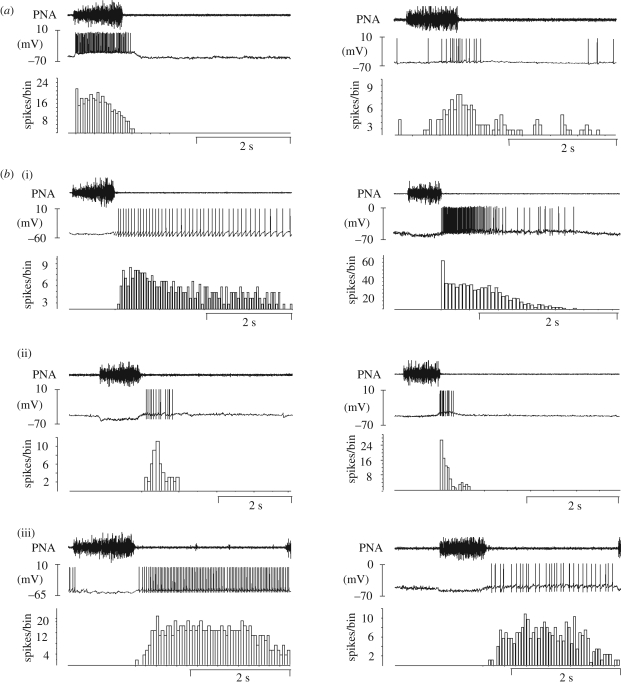
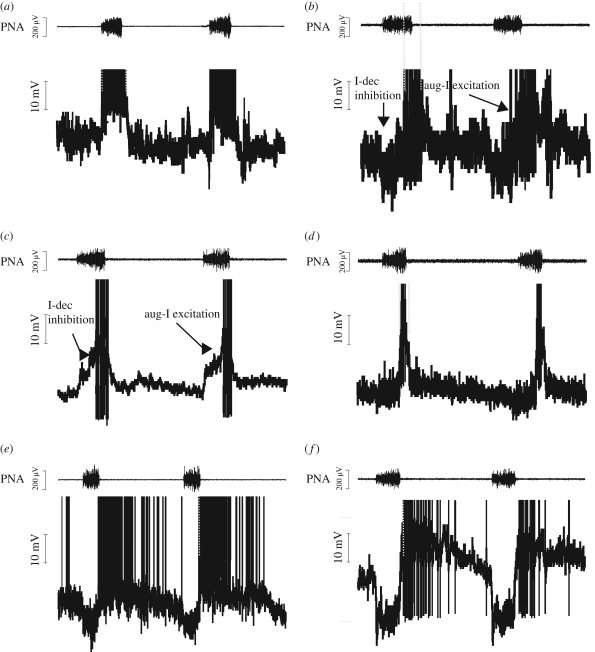
The physiological role of the pontine-mediated mechanisms involved in IE phase transition has not received much attention, and reports of synaptic interactions required for mediating IE phase transitions are rare. Although several computational models were developed to explain possible pontine mechanisms (Rybak et al. 2004, 2008), little is known about intra-pontine synaptic interactions, biophysical properties of pontine respiratory neurons and their interaction with the primary rhythm and pattern-generating circuits in the medulla. For example, intracellular recordings of pontine respiratory neurons, which provide insights into their potential involvement in IE transition, have not been performed except in a single study in the cat (Dick et al. 1994). The perfused brainstem preparation (Paton 1996) circumnavigates many technical difficulties and provides new experimental approaches for the investigation of the pontine mechanisms for IE phase transition. Despite the lack of inputs from higher brain centres and phasic Breuer–Hering afferent feedback, this preparation generates a normal three-phase pattern of respiratory activity as described originally (Richter 1982). Because of the lack of BHR feedback, IE phase transition in this preparation may strongly depend on synaptic interaction between medullary and pontine respiratory neurons. This assumption is substantiated by the demonstration that a transient pharmacological lesion of the KF triggers apneusis and loss of any post-I activity in the associated motor output (Dutschmann & Herbert 2006). Transection of the dorsolateral pons in this preparation also causes apneustic breathing in juvenile (Smith et al. 2007) or neonatal (Dutschmann et al. 2000) rat preparations. Further, systemic or local blockade of NMDA-R in the KF in the in situ preparation can also transform the normal three-phase breathing pattern to apneusis (M. Dutschmann, M. Mörschel, I. A. Rybak & T. E. Dick 2009, unpublished data). This confirms that the arterially perfused in situ brainstem preparation reflects the in vivo situation as observed in other studies (Foutz et al. 1989; Connelly et al. 1992; Pierrefiche et al. 1992, 1998; Fung et al. 1994; Ling et al. 1994; Borday et al. 1998). Cellular recordings in the in situ preparation from the KF revealed neurons exhibiting a variety of phasic respiratory activities (Dutschmann & Herbert 2006). The majority of KF respiratory neurons (out of a total of 96 units, 82% recorded intracellularly) exhibit either early-inspiratory (early-I, also termed decrementing inspiratory) (17%) IE phase-spanning (22%) or post-I (39%) activities, while neurons with other discharge pattern (expiratory units with constant firing pattern, augmenting-inspiratory (aug-I), inspiratory modulated) were seen in only 22 per cent of the recordings. Figure 1 depicts intracellular recordings from the three most prominent cell types. The density of IE phase-spanning neurons in the pons is in general accordance with reports from pontine recordings in cats and rats in vivo (Ezure & Tanaka 2006; Dick et al. 2008). Nevertheless, the phase-spanning neuron types found in the KF in situ could be specific to this preparation (i.e. decerebrated, and no BHR). Membrane potential voltage changes of key neurons during the IE phase transition are illustrated in figure 2. Early-I neurons showed a decrementing depolarization and hyperpolarization during post-I (figure 2a). Some of the pontine IE phase-spanning neurons were characterized by a small hyperpolarization during early-I followed by depolarization with an augmenting slope (5/11, figure 2b), while others were not clearly inhibited during early-I and showed augmenting depolarization during inspiration (figure 2c). Comparison with the discharge pattern of medullary late-I neurons suggests that they receive synaptic inhibition during the inspiratory phase (or at least no excitatory drive) and thus showed a strong depolarization only during late-I (figure 2d). The shape of the excitatory post-synaptic potentials (EPSPs) largely corresponded to the discharge pattern of the pontine IE phase-spanning neurons. This allows the suggestion that the medullary late-I neurons involved in IE phase transition (Richter 1982, 1996; Cohen et al. 1993; Haji et al. 2002; Krolo et al. 2005) receive synaptic input from the pontine IE phase-spanning neurons (Haji et al. 2002; Cohen & Shaw 2004; Rybak et al. 2004, 2008). The pontine and medullary post-neurons had comparable discharge pattern and voltage changes (figure 2e, f ).
Figure 1.
Discharge patterns of pontine and medullary respiratory neurons. (a) Peri-stimulus triggered histogram (PSTH) derived from at least 10 consecutive respiratory cycles shows the discharge pattern of a pontine decrementing inspiratory with post-I after discharge (left-hand side) and late-inspiratory/post-I (right-hand side) phase-spanning neurons. (b) PSTHs of pontine (left-hand side) and medullary (right-hand side), (i)–(ii) post-I neurons (both with (i) long decrementing discharge and (ii) with short discharge) and (iii) constant expiratory neurons (con-E). PNA, phrenic nerve activity.
Figure 2.
Voltage characteristics of key pontine and medullary neurons for IE phase transitions. (a) Pontine early-I neuron, (b) pontine IE phase-spanning neuron, (c) a second IE phase-spanning neuron that clearly showed augmenting excitatory post-synaptic potentials (EPSPs) with the onset of inspiration, (d) medullary late-I neuron, (e) medullary decrementing post-I neuron, and (f) pontine decrementing post-I neuron. Note that pontine IE neurons showed augmenting EPSPs during inspiration, while late-I medullary neurons were inhibited during 70 per cent of inspiratory phase and received excitatory synaptic input only during the late-I phase.
6. A hypothetical model for pontine-mediated inspiratory/expiratory phase transition
Based on our recordings (figures 1 and 2), data provided by other groups (Smith et al. 2007; Dick et al. 2008; Segers et al. 2008) and existing computational models (Rybak et al. 2004, 2008), we suggest a theoretical scheme for mechanisms responsible for IE phase transition in the perfused brainstem preparation. The scheme is based on the following assumptions. First, the pontine early-I neurons are inhibitory, and both medullary and pontine early-I neurons receive synaptic drive from inspiratory-driver (I-driver) neurons. This is based on the fact that early-I neurons are inhibitory interneurons as was the case in all previously published models (Richter 1982; Smith et al. 2007; Rybak et al. 2008). Therefore, the pontine early-I neurons should receive input from the I-driver population like those in the medulla. These I-driver neurons could be those neurons with conditional pacemaker properties located within the pre-Bötzinger complex (Feldman & Del Negro 2006; Rybak et al. 2008) and termed pre-I neurons of the pre-Bötzinger complex (Rybak et al. 2004; Smith et al. 2007). These neurons are governed by synaptic interaction under eupnoeic conditions, but can generate gasping during severe hypoxia (Paton et al. 2006). Second, we suggest that I-driver neurons also interact with the pontine early-I respiratory populations. This could be important for rapid re-establishment of ponto-medullary interactions after recovery from severe hypoxia. The next prediction is that the pontine early-I cells inhibit IE phase-spanning neurons and post-I neurons of the pons via local connections. This connectivity prevents initiation of phase transition during the early-I phase (figure 3a). In addition, IE neurons receive increasing excitatory drive from aug-I pre-motor population of the rostral ventral respiratory group. This excitatory drive counterbalances the early-I inhibition and causes firing of the neurons during the late-I phase. In turn, IE neurons are excitatory and trigger activation of late-I neurons that initiate the IE phase transition at the medullary level. This is followed by the ‘classic’ cascade of synaptic interactions that mediate the IOS in most of the previously suggested models (Richter 1982; Rybak et al. 2004, figure 3b). Late-I neurons switch off the early-I neurons via synaptic inhibition. This releases inhibitory (interneurons) and excitatory (pre-motoneurons) post-I neurons from synaptic inhibition provided by the early-I population, reflecting the irreversible transition from inspiration to expiration as described by Richter (1996). The pontine and medullary pre-motor post-I populations are reciprocally coupled via excitatory connectivity (see the following).
In summary, the present model is based on the assumption that pontine neurons receive excitatory synaptic inputs from two major populations, augmenting-I and post-I neurons. In this model, the drive from the medullary inspiratory pre-motor population is adjusted by only one intra-pontine inhibitory synaptic interaction, which shapes the activity pattern of IE neurons that transmit the crucial signal for inspiratory termination to the medulla as proposed previously (Cohen & Shaw 2004; Rybak et al. 2004, 2008). The experimental verification of the intra-pontine connectivity remains to be established and is a challenge for future experiments. At the moment, we suggest that the configuration presented in our scheme could be the core for ponto-medullary interactions in vivo. The phasic activities of the pons are most probably heavily influenced by the BHR pathway and by various other sources providing tonic inputs and may thus be very difficult to detect under the experimental conditions in vivo (Dick et al. 2008; Segers et al. 2008).
Finally, our model can explain the occurrence of apneusis after blockade of post-synaptic NMDA-R with the dorsolateral pons (e.g. figure 3d). According to the present model, local blockade of ascending excitatory synaptic drive from the medullary pre-motor populations and from the I-driver populations would block the descending signal from pontine IE populations to medullary late-I populations. Thus the respiratory pattern generator is arrested in the inspiratory phase and shows apneusis. The present model would support our previous findings that the KF gates the generation of the post-I phase in the absence of BHR feedback (Dutschmann & Herbert 2006). In addition, the reciprocal excitatory coupling of post-I pre-motor populations and descending excitatory input to inhibitory post-I neurons allows the pons to directly switch to post-I, irrespective of ponto-medullary interactions. This can take place, for instance, during protective upper airway reflexes that involve glottal closure. This makes sense physiologically as protective reflexes have to prevent invasion of noxious substances into the lungs and therefore should be processed rapidly and independently from the state of the primary medullary rhythm and pattern-generating circuits (for review, see Dutschmann et al. 2004). An example of this is demonstrated by the observation that NMDA-R blockade within the KF in vagi intact animals caused no effect on the ongoing breathing pattern, while an evoked protective apnoeic response was attenuated (Dutschmann & Herbert 1998). Alternatively, the ascending drive from higher vocalization centres could target pontine post-I neurons of the KF (Smotherman et al. 2006; Zornik & Kelley 2008), with the aim of prolonging the expiratory interval via descending connectivity to medullary respiratory centres and, in parallel, providing the necessary motor drive for laryngeal adductor muscles for the motor act of vocalization.
The question of why the network mechanisms for mediating phase transition are distributed within ponto-medullary circuits while the essential circuits required for rhythm and pattern formation are located in the medulla oblongata is difficult to answer. We speculate that the network control for IE phase transition could be based in the pons because it is, evolutionarily speaking, a younger part of the brain. Therefore, the pontine-mediated phase transition is involved in ‘higher’ behavioural adaptations of breathing as they occur during sniffing and vocalization, for instance. These behavioural adaptations most certainly require the dynamic and plastic modulation of IE phase transition, not only in the context of lung ventilation.
7. Conclusions
The model introduced in the present review reflects possible ponto-medullary synaptic interactions that take place in the perfused brainstem preparation in the complete absence of BHR-related synaptic inputs and modulations from higher brain centres. Under in vivo conditions, both mechanisms involved in the control of IE transition are active. We believe that synaptic weights of the two parallel pathways can be adjusted in a state-dependent manner in order to produce a dynamic and plastic motor pattern of breathing.
Future challenges in respiration physiology involve breathing disorders associated with various neurological diseases. A recently published study on Mecp2−/y knockout mice, as model for the human Rett syndrome (Stettner et al. 2007), illustrates that synaptic instability of both the BHR pathway and the pontine pathways for phase transitions may cause unpredictable and pathological fluctuations that may contribute to the pathological respiratory phenotype of this animal model. Indeed, this is a good example, illustrating that synaptic malfunction in both pontine and BHR-associated pathways is associated with highly erratic breathing patterns as are seen in human patients (Weese-Mayer et al. 2006; Stettner et al. 2008) as well as in the associated animal models (Viemari et al. 2005; Stettner et al. 2007).
Acknowledgements
This work was supported by the Bernstein Centre for Computational Neurosciences (BCCN, 01GQ0432). We are grateful to Anna-Maria Bischoff for excellent technical support during the course of the experiments.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Brainstem neural networks vital for life’.
References
- Andrews P. L., Horn C. C.2006Signals for nausea and emesis: implications for models of upper gastrointestinal diseases. Auton. Neurosci. 125, 100–115 (doi:10.1016/j.autneu.2006.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett D. J.1986Upper airway motor systems. In Handbook for physiology, section 3, the respiratory system, vol. II (eds Cherniack N. S., Widdicombe J. G.), pp. 223–245 Bethesda, MD: American Physiological Society [Google Scholar]
- Bianchi A. L., St-John W. M.1981Pontile axonal projections of medullary respiratory neurons. Respir. Physiol. 45, 167–183 (doi:10.1016/0034-5687(81)90058-X) [DOI] [PubMed] [Google Scholar]
- Bianchi A. L., St-John W. M.1982Medullary axonal projections of respiratory neurons of pontile pneumotaxic center. Respir. Physiol. 48, 357–373 (doi:10.1016/0034-5687(82)90039-1) [DOI] [PubMed] [Google Scholar]
- Bianchi A. L., Denavit-Saubie M., Champagnat J.1995Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol. Rev. 75, 1–45 [DOI] [PubMed] [Google Scholar]
- Bonham A. C.1995Neurotransmitters in the CNS control of breathing. Respir. Physiol. 101, 219–230 (doi:10.1016/0034-5687(95)00045-F) [DOI] [PubMed] [Google Scholar]
- Bonham A. C., Chen C. Y., Sekizawa S., Joad J. P.2006Plasticity in the nucleus tractus solitarius and its influence on lung and airway reflexes. J. Appl. Physiol. 101, 322–327 (doi:10.1152/japplphysiol.00143.2006) [DOI] [PubMed] [Google Scholar]
- Borday V., Foutz A. S., Nordholm L., Denavit-Saubié M.1998Respiratory effects of glutamate receptor antagonists in neonate and adult mammals. Eur. J. Pharmacol. 348, 235–246 (doi:10.1016/S0014-2999(98)00160-5) [DOI] [PubMed] [Google Scholar]
- Breuer J.1868Die Selbststeurung der Athmung durch den Nervus Vagus. Akad. Wiss. Wien (II) 58, 909–937 [Google Scholar]
- Chamberlin N. L., Saper C. B.1994Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J. Neurosci. 14, 6500–6510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin N. L., Saper C. B.1998A brainstem network mediating apneic reflexes in the rat. J. Neurosci. 18, 6048–6056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. I., Feldman J. L.1977Models of respiratory phase-switching. Fed. Proc. 36, 2367–2374 [PubMed] [Google Scholar]
- Cohen M. I., Shaw C. F.2004Role in the inspiratory off-switch of vagal inputs to rostral pontine inspiratory-modulated neurons. Respir. Physiol. Neurobiol. 143, 127–140 (doi:10.1016/j.resp.2004.07.017) [DOI] [PubMed] [Google Scholar]
- Cohen M. I., Huang W. X., Barnhardt R., See W. R.1993Timing of medullary late-inspiratory neuron discharges: vagal afferent effects indicate possible off-switch function. J. Neurophysiol. 69, 1784–1787 [DOI] [PubMed] [Google Scholar]
- Connelly C. A., Otto-Smith M. R., Feldman J. L.1992Blockade of NMDA receptor-channels by MK-801 alters breathing in adult rats. Brain Res. 596, 99–110 (doi:10.1016/0006-8993(92)91537-O) [DOI] [PubMed] [Google Scholar]
- Dick T. E., Bellingham M. C., Richter D. W.1994Pontine respiratory neurons in anesthetized cats. Brain Res. 636, 259–269 (doi:10.1016/0006-8993(94)91025-1) [DOI] [PubMed] [Google Scholar]
- Dick T. E., Shannon R., Lindsey B. G., Nuding S. C., Segers L. S., Baekey D. M., Morris K. F.2008Pontine respiratory-modulated activity before and after vagotomy in decerebrate cats. J. Physiol. 586, 4265–4282 (doi:10.1113/jphysiol.2008.152108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins E. G., Feldman J. L.1994Brainstem network controlling descending drive to phrenic motoneurons in rat. J. Comp. Neurol. 347, 64–86 (doi:10.1002/cne.903470106) [DOI] [PubMed] [Google Scholar]
- Dutschmann M., Herbert H.1998NMDA- and GABAA-receptors in the rat Kölliker–Fuse area control cardio-respiratory responses evoked by trigeminal ethmoidal nerve stimulation. J. Physiol. 510, 793–804 (doi:10.1111/j.1469-7793.1998.793bj.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M., Herbert H.2006The Kölliker–Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur. J. Neurosci. 24, 1071–1084 (doi:10.1111/j.1460-9568.2006.04981.x) [DOI] [PubMed] [Google Scholar]
- Dutschmann M., Paton J. F.2002Inhibitory synaptic mechanisms regulating upper airway patency. Respir. Physiol. Neurobiol. 131, 57–63 (doi:10.1016/S1569-9048(02)00037-X) [DOI] [PubMed] [Google Scholar]
- Dutschmann M., Wilson R. J. A., Paton J. F. R.2000Respiratory activity in neonatal rats. Auton. Neurosci. 84, 19–29 (doi:10.1016/S1566-0702(00)00177-6) [DOI] [PubMed] [Google Scholar]
- Dutschmann M., Mörschel M., Kron M., Herbert H.2004Development of adaptive behaviour of the respiratory network: implications for the pontine Kölliker–Fuse nucleus. Respir. Physiol. Neurobiol. 143, 155–165 (doi:10.1016/j.resp.2004.04.015) [DOI] [PubMed] [Google Scholar]
- Dutschmann M., Mörschel M., Reuter J., Zhang W., Gestreau C., Stettner G. M., Kron M.2008Postnatal emergence of synaptic plasticity associated with dynamic adaptation of the respiratory motor pattern. Respir. Physiol. Neurobiol. 164, 72–79 (doi:10.1016/j.resp.2008.06.013) [DOI] [PubMed] [Google Scholar]
- Ellenberger H. H., Feldman J. L.1990Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 513, 35–42 (doi:10.1016/0006-8993(90)91086-V) [DOI] [PubMed] [Google Scholar]
- Ezure K.2004Respiration-related afferents to parabrachial pontine regions. Respir. Physiol. Neurobiol. 143, 167–175 (doi:10.1016/j.resp.2004.03.017) [DOI] [PubMed] [Google Scholar]
- Ezure K., Tanaka I.2006Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuroscience 141, 1011–1023 (doi:10.1016/j.neuroscience.2006.04.020) [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Del Negro C. A.2006Looking for inspiration: new perspectives on respiratory rhythm. Nat. Rev. Neurosci. 7, 232–242 (doi:10.1038/nrn1871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. L., Gautier H.1976Interaction of pulmonary afferents and pneumotaxic center in control of respiratory pattern in cats. J. Neurophysiol. 39, 31–44 [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Cohen M. I., Wolotsky P.1976Powerful inhibition of pontine respiratory neurons by pulmonary afferent activity. Brain Res. 104, 341–346 (doi:10.1016/0006-8993(76)90629-6) [DOI] [PubMed] [Google Scholar]
- Fortuna M. G., West G. H., Stornetta R. L., Guyenet P. G.2008Bötzinger expiratory-augmenting neurons and the parafacial respiratory group. J. Neurosci. 28, 2506–2515 (doi:10.1523/JNEUROSCI.5595-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foutz A. S., Champagnat J., Denavit-Saubié M.1989Involvement of N-methyl-d-aspartate (NMDA) receptors in respiratory rhythmogenesis. Brain Res. 500, 199–208 (doi:10.1016/0006-8993(89)90314-4) [DOI] [PubMed] [Google Scholar]
- Fung M. L., Wang W., St-John W. M.1994Involvement of pontile NMDA receptors in inspiratory termination in rat. Respir. Physiol. 96, 177–188 (doi:10.1016/0034-5687(94)90125-2) [DOI] [PubMed] [Google Scholar]
- Gaytan S. P., Calero F., Nunez-Abades P. A., Morillo A. M., Pasaro R.1997Pontomedullary efferent projections of the ventral respiratory neuronal subsets of the rat. Brain Res. Bull. 42, 323–334 (doi:10.1016/S0361-9230(96)00292-4) [DOI] [PubMed] [Google Scholar]
- Guthmann A., Herbert H.1999Expression of N-methyl-d-aspartate receptor subunits in the rat parabrachial and Kölliker–Fuse nuclei and in selected pontomedullary brainstem nuclei. J. Comp. Neurol. 415, 501–517 (doi:10.1002/(SICI)1096-9861(19991227)415:4<501::AID-CNE6>3.0.CO;2-9) [PubMed] [Google Scholar]
- Haji A., Okazaki M., Takeda R.1999GABA(A) receptor-mediated inspiratory termination evoked by vagal stimulation in decerebrate cats. Neuropharmacology 38, 1261–1272 (doi:10.1016/S0028-3908(99)00057-X) [DOI] [PubMed] [Google Scholar]
- Haji A., Okazaki M., Yamazaki H., Takeda R.2002Physiological properties of late inspiratory neurons and their possible involvement in inspiratory off-switching in cats. J. Neurophysiol. 87, 1057–1067 [DOI] [PubMed] [Google Scholar]
- Harding R.1984Perinatal development of laryngeal function. J. Dev. Physiol. 6, 249–258 [PubMed] [Google Scholar]
- Hayashi F., Coles S. K., McCrimmon D. R.1996Respiratory neurons mediating the Breuer–Hering reflex prolongation of expiration in rat. J. Neurosci. 16, 6526–6536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert H., Moga M. M., Saper C. B.1990Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J. Comp. Neurol. 293, 540–580 (doi:10.1002/cne.902930404) [DOI] [PubMed] [Google Scholar]
- Hering E.1868Die Selbststeurung der Athmung durch den Nervus Vagus. Akad. Wiss. Wien (II) 57, 672–677 [Google Scholar]
- Krolo M., Tonkovic-Capin V., Stucke A. G., Stuth E. A., Hopp F. A., Dean C., Zuperku E. J.2005Subtype composition and responses of respiratory neurons in the pre-Botzinger region to pulmonary afferent inputs in dogs. J. Neurophysiol. 93, 2674–2687 (doi:10.1152/jn.01206.2003) [DOI] [PubMed] [Google Scholar]
- Kron M., Reuter J., Gerhardt E., Manzke T., Zhang W., Dutschmann M.2008Emergence of BDNF induced postsynaptic potentiation of NMDA currents during the postnatal maturation of the Kölliker–Fuse nucleus of rat. J. Physiol. 586, 2331–2343 (doi:10.1113/jphysiol.2007.148916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L., Alheid G. F., Zuperku E. J., McCrimmon D. R.2006Central pathways of pulmonary and lower airway vagal afferents. J. Appl. Physiol. 101, 618–627 (doi:10.1152/japplphysiol.00252.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L., Karius D. R., Speck D. F.1994Role of N-methyl-d-aspartate receptors in the pontine pneumotaxic mechanism in the cat. J. Appl. Physiol. 76, 1138–1143 [DOI] [PubMed] [Google Scholar]
- Marckwald M.1887Die Athembewegungen und deren Innervation beim Kaninchen. Z. Biol. 23, 149–283 [Google Scholar]
- Monaghan D. T., Cotman C. W.1985Distribution of N-methyl-d-aspartate-sensitive l-[3H]glutamate-binding sites in rat brain. J. Neurosci. 5, 2909–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Abades P. A., Morillo A. M., Pasaro R.1993Brainstem connections of the rat ventral respiratory subgroups: afferent projections. J. Auton. Nerv. Syst. 42, 99–118 (doi:10.1016/0165-1838(93)90042-S) [DOI] [PubMed] [Google Scholar]
- Okazaki M., Takeda R., Yamazaki H., Haji A.2002Synaptic mechanisms of inspiratory off-switching evoked by pontine pneumotaxic stimulation in cats. Neurosci. Res. 44, 101–110 (doi:10.1016/S0168-0102(02)00091-3) [DOI] [PubMed] [Google Scholar]
- Oku Y., Dick T. E.1992Phase resetting of the respiratory cycle before and after unilateral pontine lesion in cat. J. Appl. Physiol. 72, 721–730 [DOI] [PubMed] [Google Scholar]
- Paton J. F. R.1996A working heart-brainstem preparation of the mouse. J. Neurosci. Methods 65, 63–68 (doi:10.1016/0165-0270(95)00147-6) [DOI] [PubMed] [Google Scholar]
- Paton J. F. R., Abdala A. P., Koizumi H., Smith J. C., St-John W. M.2006Respiratory rhythm generation during gasping depends on persistent sodium current. Nat. Neurosci. 9, 311–313 (doi:10.1038/nn1650) [DOI] [PubMed] [Google Scholar]
- Pierrefiche O., Foutz A. S., Champagnat J., Denavit-Saubié M.1992The bulbar network of respiratory neurons during apneusis induced by a blockade of NMDA receptors. Exp. Brain Res. 89, 623–639 (doi:10.1007/BF00229887) [DOI] [PubMed] [Google Scholar]
- Pierrefiche O., Haji A., Foutz A. S., Takeda R., Champagnat J., Denavit-Saubie M.1998Synaptic potentials in respiratory neurons during evoked phase switching after NMDA receptor blockade in the cat. J. Physiol. 508, 549–559 (doi:10.1111/j.1469-7793.1998.549bq.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon C. S., Young D. L.2006Nonassociative learning as gated neural integrator and differentiator in stimulus–response pathways. Behav. Brain Funct. 8, 29 (doi:10.1186/1744-9081-2-29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D. W.1982Generation and maintenance of the respiratory rhythm. J. Exp. Biol. 100, 93–107 [DOI] [PubMed] [Google Scholar]
- Richter D. W.1996Neural regulation of respiration: rhythmogenesis and afferent control. In Comprehensive human physiology, vol. II (eds Gregor R., Windhorst U.), pp. 2079–2095 Berlin, Germany: Springer Verlag [Google Scholar]
- Richter D. W., Spyer K. M.2001Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 24, 464–472 (doi:10.1016/S0166-2236(00)01867-1) [DOI] [PubMed] [Google Scholar]
- Rybak I. A., Paton J. F., Schwaber J. S.1997Modeling neural mechanisms for genesis of respiratory rhythm and pattern. II. Network models of the central respiratory pattern generator. J. Neurophysiol. 77, 2007–2026 [DOI] [PubMed] [Google Scholar]
- Rybak I. A., Shevtsova N. A., Paton J. F. R., Dick T. E., St-John W. M., Mörschel M., Dutschmann M.2004Modeling the pontomedullary respiratory network. Respir. Physiol. Neurobiol. 143, 307–319 (doi:10.1016/j.resp.2004.03.020) [DOI] [PubMed] [Google Scholar]
- Rybak I. A., et al. 2008Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J. Neurophysiol. 100, 1770–1799 (doi:10.1152/jn.90416.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer P., Pierrefiche O., Foutz A. S., Denavit-Saubié M.1990Effects of N-methyl-d-aspartate (NMDA) receptor blockade on breathing pattern in newborn cat. Brain Res. Dev. Brain Res. 56, 290–293 (doi:10.1016/0165-3806(90)90095-G) [DOI] [PubMed] [Google Scholar]
- Segers L. S., Shannon R., Lindsey B. G.1985Interactions between rostral pontine and ventral medullary respiratory neurons. J. Neurophysiol. 54, 318–334 [DOI] [PubMed] [Google Scholar]
- Segers L. S., Nuding S. C., Dick T. E., Shannon R., Baekey D. M., Solomon I. C., Morris K. F., Lindsey B. G.2008Functional connectivity in the pontomedullary respiratory network. J. Neurophysiol. 100, 1749–1769 (doi:10.1152/jn.90414.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck G. C., Harper R. M.1980Pneumotaxic area neuronal discharge during sleep–waking states in the cat. Exp. Neurol. 67, 79–102 (doi:10.1016/0014-4886(80)90162-4) [DOI] [PubMed] [Google Scholar]
- Siniaia M. S., Young D. L., Poon C. S.2000Habituation and desensitization of the Hering–Breuer reflex in rat. J. Physiol. 523, 479–491 (doi:10.1111/j.1469-7793.2000.t01-1-00479.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Morrison D. E., Ellenberger H. H., Otto M. R., Feldman J. L.1989Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J. Comp. Neurol. 281, 69–96 (doi:10.1002/cne.902810107) [DOI] [PubMed] [Google Scholar]
- Smith J. C., Abdala A. P., Koizumi H., Rybak I. A., Paton J. F. R.2007Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J. Neurophysiol. 98, 3370–3387 (doi:10.1152/jn.00985.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman M., Kobayasi K., Ma J., Zhang S., Metzner W.2008A mechanism for vocal–respiratory coupling in the mammalian parabrachial nucleus. J. Neurosci. 26, 4860–4869 (doi:10.1523/JNEUROSCI.4607-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G., Poon C. S.2004Functional and structural models of pontine modulation of mechanoreceptor and chemoreceptor reflexes. Respir. Physiol. Neurobiol. 143, 281–292 (doi:10.1016/j.resp.2004.05.009) [DOI] [PubMed] [Google Scholar]
- Stella G.1938On the mechanism of production and the physiological significance of apneusis. J. Physiol. 93, 10–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner G. M., Huppke P., Brendel C., Richter D. W., Gärtner J., Dutschmann M.2007Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2−/y knockout mice. J. Physiol. 579, 863–876 (doi:10.1113/jphysiol.2006.119966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettner G. M., Huppke P., Gärtner J., Richter D. W., Dutschmann M.2008Disturbances of breathing in Rett syndrome: results from patients and animal models. Adv. Exp. Med. Biol. 605, 503–507 (doi:10.1007/978-0-387-73693-8_88) [DOI] [PubMed] [Google Scholar]
- St-John W. M.1987Influence of pulmonary inflations on discharge of pontile respiratory neurons. J. Appl. Physiol. 63, 2231–2239 [DOI] [PubMed] [Google Scholar]
- St-John W. M.1998Neurogenesis of patterns of automatic ventilatory activity. Prog. Neurobiol. 56, 97–117 (doi:10.1016/S0301-0082(98)00031-8) [DOI] [PubMed] [Google Scholar]
- Viemari J. C., et al. 2005Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J. Neurosci. 25, 11521–11530 (doi:10.1523/JNEUROSCI.4373-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer D. E., Lieske S. P., Boothby C. M., Kenny A. S., Bennett H. L., Silvestri J. M., Ramirez J. M.2006Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr. Res. 60, 443–449 (doi:10.1203/01.pdr.0000238302.84552.d0) [DOI] [PubMed] [Google Scholar]
- Zoccal D. B., Simms A. E., Bonagamba L. G., Braga V. A., Pickering A. E., Paton J. F., Machado B. H.2008Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J. Physiol. 586, 3253–3265 (doi:10.1113/jphysiol.2008.154187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornik E., Kelley D. B.2008Regulation of respiratory and vocal motor pools in the isolated brain of Xenopus laevis. J. Neurosci. 28, 612–621 (doi:10.1523/JNEUROSCI.4754-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]