Abstract
Aging has been shown to increase sensory thresholds for a variety of exteroceptive and proprioceptive stimuli. However, the influence of aging on interoceptive awareness has received relatively little empirical attention. Here we report an inverse association between aging and interoception, as indexed by the ability to sense the heartbeat at rest. In a group of 59 participants ranging in age from 22 to 63 years, age inversely predicted heartbeat detection ability, both within and across several measurement sessions. On average, age accounted for 30% of the variance in heartbeat detection accuracy. Other attribute variables including body mass index (BMI) and sex were not related to heartbeat detection ability. These findings provide clear empirical evidence that interoception, much like exteroception and proprioception, declines with age.
Introduction
Aging is a complex and multifaceted process that affects how organisms acquire and process sensory information from their environment. With respect to basic sensory processes, aging tends to increase sensory thresholds such that more potent or intense stimuli are required for information from the periphery to reach conscious awareness. This phenomenon occurs across many sensory modalities, affecting primarily the visual, auditory, olfactory, mechanosensory, thermosensory, and nociceptive systems (Skinner, Barrack, & Cook, 1984; Ivy, Petit, & Markus, 1992; Gescheider, Bolanowski, Hall, Hoffman, & Verrillo, 1994; Hurley, Rees, & Newham, 1998; Stevens & Choo, 1998; Gibson & Farrell, 2004; Kenshalo, 1986; Lin, Hsieh, Chao, Chang, & Hsieh, 2005; Shaffer & Harrison, 2007; Low Choy, Brauer, & Nitz, 2007; Shaffer & Harrison, 2007), and it has been observed across several mammalian species (Godde, Berkefeld, David-Jurgens, & Dinse, 2002; Verdu, Ceballos, Vilches, & Navarro, 2000; Wang, Davis, Zwick, Waxman, & Albers, 2006). Most of these sensory modalities have been classified as “exteroceptive” senses, as they relate to awareness and apprehension of stimuli that originate outside of the body, or as “proprioceptive”, as they relate to representation of the body in space. “Interoception” 1 corresponds essentially to sensations arising from within the body including the viscera (such as the feeling of the heartbeat, the breath and gastrointestinal sensations), to some extent the skin (for example, flushing of the skin and itching), as well as a host of chemical, endocrine and osmotic changes arising within the bloodstream (such as feelings related to thirst and hunger) (Cameron, 2001; Craig, 2002; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004; Damasio, 2003; Khalsa, Rudrauf, Sandesara, Olshansky, & Tranel, In press; Mayer, Naliboff, & Craig, 2006; Pollatos, Herbert, Kaufmann, Auer, & Schandry, 2007; Vaitl, 1996).
Against this background, an interesting question is whether aging also influences interoceptive awareness. From a neurophysiological standpoint, and in light of findings on exteroception and proprioception, it would seem logical to expect that aging might also reduce interoceptive awareness. However, there is limited empirical evidence on this issue. Clinical reports have suggested that several types of visceral pain seem to be reduced in older patients (Gibson & Helme, 2001), and in a few studies aging has been linked to increased thresholds for perception of esophageal pain (Lasch, Castell, & Castell, 1997) and gastric fullness/pain (Mertz, Fullerton, Naliboff, & Mayer, 1998), but not rectal sensation (Bannister, Abouzekry, & Read, 1987).
To begin to address this gap in our knowledge, we studied the effect of aging on one well-characterized interoceptive sensation, namely, the ability to sense the heartbeat at rest. Based on the general trends for other sensory functions, we predicted that increased age would be associated with decreased ability to detect the heartbeat.
Methods
The methods for this study are identical to those of a previously published study (Khalsa et al., 2008), and thus the current report is abbreviated where possible. This study was approved by the University of Iowa’s Institutional Review Board, and all participants provided informed consent prior to participation.
Participants
Fifty nine participants (23 men and 36 women) ranging in age from 22 to 63 years (M = 48, SD = 11) were included (Table 1). They had a mean BMI of 24.7 (SD = 5.6, range 16.6 – 44.2). During the initial screening process potential participants were asked whether they had ever been evaluated by a neurologist or psychiatrist, or diagnosed with a neurological or psychiatric disease. Any individual answering affirmative to this question was considered ineligible for participation in the study, and was excluded. Once recruited, participants filled out two standardized clinical mood inventories: the Beck Depression Inventory (Beck, 1993) and the Beck Anxiety Inventory. The results from these questionnaires indicated low levels of depression (mean: 7.5, SD = 7.0) and anxiety (mean: 5.8, SD = 8.1) in the participants.
Table 1.
Number of participants by age epoch and sex.
| Age epoch | Number | Males | Females |
|---|---|---|---|
| 22–30 | 7 | 4 | 3 |
| 31–40 | 5 | 3 | 2 |
| 41–50 | 15 | 2 | 13 |
| 51–60 | 26 | 12 | 14 |
| 61–63 | 6 | 2 | 4 |
| Total | 59 | 23 | 36 |
Tasks
Participants performed a baseline pulse detection task followed by a heartbeat detection task. During pulse detection, participants took their non-dominant wrist pulse and were required to judge whether a train of exteroceptive stimuli (50 ms tones) were simultaneous or non-simultaneous with pulse sensations. During heartbeat detection subjects were not allowed to take their pulse and were required to judge whether the same tones were simultaneous or non-simultaneous with perceived heartbeat sensations. The pulse detection task was used to ascertain whether participants were able to sustain a task oriented focus of attention and make simultaneity judgments about two different stimuli, thus serving as an appropriate control task for the current study (3 participants were excluded from further participation based on poor pulse detection performance, leaving 59 with usable heartbeat detection data).
Tone delivery
Tone delivery was triggered by each myocardial contraction, as indirectly measured from the R-wave of a lead II electrocardiogram (MP100 acquisition unit, Biopac Systems, Inc.). During “simultaneous” trials, tones were delivered at the same time as the subject’s own finger pulse, approximately 250–300 ms after the R-wave (corresponding to the R-wave to pulse interval, or RPI). During “non-simultaneous” trials, tones were delivered 400 ms after the RPI, approximately 650–700 ms after the R-wave. Thus tone delivery was temporally linked to each subject’s actual heartbeat during each trial. Note that tone delays of around 250 to 300 ms are perceived as “simultaneous” by accurate heartbeat detectors (Brener, Liu, & Ring, 1993; Jones, 1994; Ring & Brener, 1992; Schandry, Bestler, & Montoya, 1993); by contrast, longer delays in the range of 650–700 ms are perceived as “non-simultaneous” (Khalsa et al., 2008). Trial order was randomized within each task and across each visit. All participants performed the tasks in the supine position with their eyes closed, and were instructed to breathe normally.
Procedure
Participants completed the heartbeat detection task on two separate visits, spaced up to 2 weeks apart. During the first visit subjects completed one block of pulse detection followed by one block of heartbeat detection. During the second session participants repeated the block of heartbeat detection. All blocks consisted of 23 trials. Any subject not meeting the criterion for good pulse detection (≥ 16 out of 23 trials correct, p < .05 per binomial test) during the first visit was excused from the heartbeat detection portion of the study (3 participants were excluded based on poor pulse detection performance).
Accuracy measures
Accuracy scores were calculated using A′ = [1/2+((HR-FP)(1+HR-FP))/(4HR(1−FP))], a non-parametric signal detection analog of d′ used for signal detection conditions with low trial numbers (Grier, 1971). In this formula, HR = Hit Rate and FP = False positive. Following methods commonly utilized in heartbeat detection studies, A′ scores were normalized using the following formula: 2arcsin(sqrt A′) such that performance ranged from 0 to π (chance =π/2) (Brener et al, 1993; Jones, O’Leary, & Pipkin, 1984; Rouse, Jones, & Jones, 1988). Subjects were further classified as “good heartbeat detectors” if they displayed above chance performance during a block of testing, defined as ≥ 16 out of 23 trials correct, p < .05 per binomial test (Katkin, Wiens, & Ohman, 2001; Schneider, Ring, & Katkin, 1998; Wiens & Palmer, 2001). Pulse detection accuracy scores were calculated and normalized in the same manner as heartbeat detection accuracy.
Statistical analysis
Relationships between normalized heartbeat detection accuracy and demographic factors such as age, BMI, sex and normalized pulse detection were first examined using Pearson’s correlational coefficients. Age was the main focus of the study; the other demographic factors were selected because prior studies have suggested there could be significant correlations between these variables and heartbeat detection accuracy. For example, sex differences in heartbeat detection ability have sometimes been reported (Jones & Hollandsworth, 1981; Katkin, Blascovich, & Goldband, 1981; Whitehead, Drescher, & Heiman, 1977; but see Ring & Brener, 1992; Rouse, Jones, & Jones, 1988), and BMI has been reported to influence heartbeat detection ability (Rouse et al, 1988). Normalized pulse detection accuracy was included to assess for the potential influence of aging on non-specific cognitive (attentional, decision making) parameters required for heartbeat detection task performance. An intraclass correlational analysis examined the degree to which heartbeat detection accuracy across the two sessions was correlated. Next, we carried out multiple regression analyses with normalized heartbeat detection accuracy as the criterion variable, and entered in one step age, BMI, sex and normalized pulse detection accuracy as predictor variables. Regressions were first performed for each visit independently. Since heartbeat detection accuracy averaged across the two visits provides the most reliable trait measure of resting heartbeat detection ability, a regression was also performed for the average measure. Finally, a logistic regression analysis was performed, with heartbeat detection performance during each session (i.e., good heartbeat detector vs. non detector classification) as the criterion variable, and age, BMI, sex and normalized pulse detection accuracy as predictor variables. All t tests were one tailed.
Results
Table 2 shows the demographic data for good and poor heartbeat detectors during visit 1 and visit 2.
Table 2.
Demographic data for good and poor heartbeat detectors.
| Good heartbeat detectors | Poor heartbeat detectors | |
|---|---|---|
| Visit 1 | ||
| Number (%) | 26 (44%) | 33 (56%) |
| Sex | 12 M: 14 F | 11 M: 22 F |
| Age (Mean +/− SD)a | 42.0 +/− 12.2 | 53.5 +/− 7.2 |
| BMI (Mean +/− SD)b | 23.7 +/− 4.8 | 25.4 +/− 6.1 |
| Visit 2 | ||
| Number (%) | 23 (39%) | 36 (61%) |
| Sex | 10 M: 13 F | 13 M: 23 F |
| Age (Mean +/− SD)c | 43.5 +/− 12.3 | 51.5 +/− 9.4 |
| BMI (Mean +/− SD)d | 24.3 +/− 4.6 | 24.9 +/− 6.1 |
t57 = 4.51, p < .00003;
t57= 1.15, p < .14;
t57 = 2.82, p < .004;
t57 = .40, p < .36.
Correlational analysis
Pearson’s correlations revealed significant inverse correlations between age and normalized heartbeat detection accuracy during visit 1 (r = −.49, p < .0001) and visit 2 (r = −.45, p < .0005). No other demographic factor was significantly correlated with heartbeat detection accuracy (all ps > .1). Heartbeat detection accuracy was moderately correlated across the two sessions (single measure intraclass correlation = .45, p < .0003, Cronbach’s alpha = .62).
Multiple regression analysis
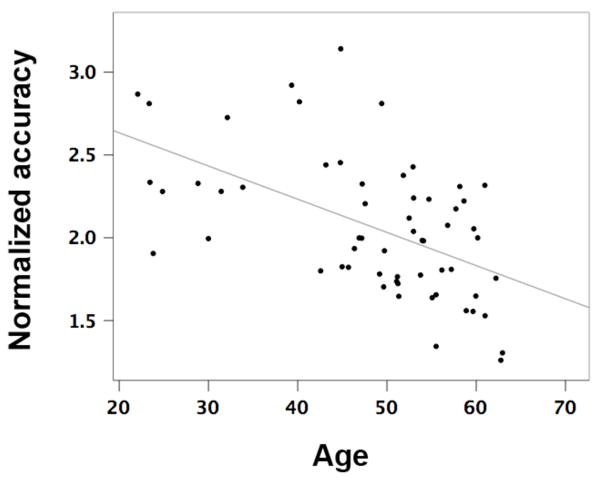
The multiple regression analysis indicated that of all the demographic predictors entered into the model, age was the only significant predictor of accuracy at the heartbeat detection task. This was true when the regression analysis was conducted on heartbeat detection accuracy measured during the first visit F(5,53) = 3.88, p < .006 (change in R2 = .27, β = −.46, t = −3.88, p < .00003), as well as during the second visit F(5,53) = 3.08 p < .017 (change in R2 = .23, β = −.45, t = −3.66, p < .0006). When the regression analysis was conducted on averaged heartbeat detection accuracy from both visits, age accounted for 30% of the variance in heartbeat detection accuracy F(5,53) = 4.51, p < .003 (β = −.53, t = −4.49, p < .00003) (figure 1).
Fig 1.
Average normalized resting heartbeat detection accuracy as a function of age (R2 = .30, p < .003).
Logistic regression analysis
The logistic regression analysis also indicated that age was the only demographic variable that significantly predicted whether an individual was classified as a good heartbeat detector. This was true for heartbeat detection performance measured during the first visit (Wald statistic = 10.12, p < .002) as well as during the second visit (Wald statistic = 5.80, p < .017) (table 3).
Table 3.
Logistic regression analysis of factors predicting likelihood of being classified as a good heartbeat detector during visit 1 and 2
| Predictor variable | B | S.E. | Wald | Df | Sig. | eB |
|---|---|---|---|---|---|---|
| Visit 1 | ||||||
| Age | −.122 | .038 | 10.12 | 1 | .001** | .885 |
| BMI | −.058 | .067 | .76 | 1 | .383 | .944 |
| Sex | −.540 | .673 | .64 | 1 | .423 | .583 |
| Pulse detection accuracy | −.221 | 1.216 | .03 | 1 | .856 | .802 |
| Visit 2 | ||||||
| Age | −.066 | .027 | 5.80 | 1 | .016* | .936 |
| BMI | −.011 | .057 | .04 | 1 | .846 | .989 |
| Sex | −.208 | .609 | .12 | 1 | .732 | .812 |
| Pulse detection accuracy | .011 | 1.191 | .00 | 1 | .993 | 1.011 |
p < .05;
p < .01
Analysis of sex-related effects
Since sex differences in heartbeat detection performance have sometimes been reported, we examined whether sex modulated the current results. The group was split into subgroups based on sex, men (n=23) and women (n=36), and relationships between normalized heartbeat detection accuracy and age, BMI, and normalized pulse detection were examined using Pearson’s correlational coefficients for each group. This analysis of possible sex differences was not intended a priori, but was conducted after the initial relationship between age and heartbeat detection ability was determined. Overall, the two groups did not differ with respect to age: mean age men = 47.1 +/− 12.5, mean age women = 49.2 +/− 10.5 (t57 = .71, p < .49). There were also no differences between men and women on heartbeat detection accuracy during the first (t57 = −1.3, p < .20) or second visit (t57 = .27, p < .80). Pearson’s correlations revealed significant inverse correlations between age and normalized heartbeat detection accuracy for both men and women during visit 1 (men: r = −.46, p < .026, women: r = −.50, p < .002) and visit 2 (men: r = −.44, p < .04, women: r = −.48, p < .003). No other demographic factor was significantly correlated with heartbeat detection accuracy in either the men or women subgroups (all ps > .30). We then examined whether sex differentially modulated the relationship between aging and heartbeat detection accuracy, using ANOVA with sex as the independent factor and age as a covariate. For visit 1 there was no significant main effect of sex F(1, 55) = .06, p < .82, and there was no significant interaction between sex and age F(1, 55) = .26, p < .62, while there was a significant effect of age F(1,55) = 16.3, p < .0003. Similarly, for visit 2 there was no significant main effect of sex F(1, 55) = .65, p < .44, and there was no significant interaction between sex and age F(1, 55) = .45, p < .52, while there was a significant effect of age F(1,55) = 14.2, p < .0005. Thus age was inversely associated with heartbeat detection ability in both men and women, and sex did not appear to modulate this relationship.
Discussion
As predicted, the findings from our study show that the ability to feel the resting heartbeat decreases with age. This relationship also appears reliable, as it was documented across two separate visits. Out of a number of different demographic factors, age was the only factor that significantly predicted heartbeat detection accuracy. Taken in a broader perspective, these findings suggest that interoception, much like exteroception and proprioception, declines with age.
It is not necessarily surprising that interoceptive awareness for heartbeat sensations would decline with age. There have been two other investigations of the effect of aging on heartbeat detection. One was a preliminary study that reported a trend toward significance of a decline in heartbeat detection ability with increasing age in men (Dickerson & Jones, 1990). The other report coincidentally noted a low level of heartbeat detection ability in a small sample of older participants (Jones, Jones, Cunningham, & Caldwell, 1985). However, the current study represents the first clear demonstration of the effect of aging on cardiac sensation.
One reason why the current finding may have escaped coverage for so long could be due to the fact that most studies of heartbeat detection have been conducted in limited age groups, principally utilizing undergraduate students as participants. In comparison, the current study utilized a sample with a much wider age range. In this context, it is also interesting to note that in the current study neither sex nor BMI exerted a significant influence on accuracy, as these factors have been found to predict heartbeat detection accuracy in previous studies of undergraduates (Rouse et al, 1988). This discrepancy may be explained by a disproportionate influence of aging in the current sample relative to that seen in prior studies limited to undergraduate student samples. It is also possible that sex and/or BMI exert nonlinear influences on cardiac sensation across the life span that the present analysis was not capable of detecting.
Several potential mechanisms could explain the effects of aging on awareness of heartbeat sensations. Firstly, decreased awareness of heartbeat sensations with aging might occur due to reduced central nervous system sensitivity. Although the brain structures mediating cardiac awareness have yet to be fully clarified (Jones, 1994; Craig, 2004; Khalsa et al, In press), sensory brain regions such as the insular and primary somatosensory cortices have been implicated (Cameron, Zubieta, Grunhaus, & Minoshima, 2000; Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004; Pollatos, Herbert, Kaufmann, Auer, & Schandry, 2007). Several recent studies have found decreased cortical thickness with aging in the primary somatosensory (Good et al., 2001; Salat et al., 2004; Sowell et al., 2003) and insular cortices (Good et al., 2001; Resnick, Pham, Kraut, Zonderman, & Davatzikos, 2003), and it is possible that decreased cortical thickness is responsible for the decreased cardiac awareness we observed. Importantly, these aging findings have been observed to occur in middle aged epochs (e.g., age 40 to 60) that were predominantly sampled in the current study (Salat et al., 2004; Sowell et al., 2003). Possible mechanisms underlying these age-related changes include reduced cortical thickness due to neuronal/glial loss, cellular atrophy, reduced neurotransmitter release, altered receptor morphology, or white matter atrophy. Future studies are needed to identify the specific etiological changes.
Secondly, decreased awareness of heartbeat sensations with aging might occur due to decreased sensitivity of receptors in the periphery that transduce heartbeat sensations. But what are these receptors? It seems unlikely that afferent innervation from within the heart itself is involved, as cardiac transplant patients within the first few years after transplantion (i.e., before re-afferentation) display heartbeat detection rates well within the normal range (Barsky et al., 1998). It has also long been known that heartbeat sensations are localized to a number of different body regions at rest (Brener & Kluvitse, 1988; Jones, Jones, Rouse, Scott, & Caldwell, 1987; Ring & Brener, 1992), as well as during increased cardiovascular arousal, when most individuals clearly perceive heartbeat sensations (Khalsa et al, In press). These sensations are localized primarily to the chest, although additional body locations such as the neck, abdomen, head, arms and hands are common. Since several of these body locations (such as the neck, abdomen and head) share close proximity with major arteries (e.g. common carotid, abdominal aorta and external carotid arteries respectively), it has been suggested that arterial pulsations transmitted through the skin may provide one mechanism for perceiving heartbeat sensations (Jones et al, 1987; Khalsa et al, In press). This, in combination with the knowledge that individuals with a lower body mass index are better at detecting heartbeat sensations (Rouse et al, 1988), suggests that receptors in the skin may play a role in the apprehension of heartbeat sensations. Indeed, Knapp, Ring, & Brener (1997) found that fingertip sensory thresholds for vibrotactile stimuli presented at 250 Hz significantly predicted performance on a standard heartbeat detection task. Since this frequency range is considered to reliably activate Pacinian receptors, they went so far as to postulate that Pacinian receptors might play a role in mediating cardiac sensation. If the skin were truly shown to contribute to awareness of cardiac sensations, then one plausible explanatory mechanism for the effect of aging on cardiac sensation could be found in literature that has documented the decline in mechanical sensitivity that occurs with age within the Pacinian channel (Gescheider et al, 1994; Verrillo, Bolanowski, & Gescheider, 2002). Other changes typically associated with neurological disease may also reduce peripheral sensitivity to heartbeat sensation, such as demyelinization (often associated with inflammatory and/or degenerative disorders), and peripheral neuropathies (often associated with disorders such as diabetes mellitus and autoimmune disease) (Pauli, Hartl, Marquardt, Stalmann, & Strian, 1991). However, since the current study participants were screened for the presence of neurological disorders it is unlikely that these pathological processes played a role in the current findings.
Thirdly, decreased awareness of heartbeat sensations with aging could be influenced by a variety of other mechanisms. For example, heartbeat awareness is facilitated by physiological and cognitive stressors via increases in cardiac rate, contractility, blood pressure, and heart rate variability (Cameron & Minoshima, 2002; Eichler & Katkin, 1994; Khalsa et al, In press; Knapp-Kline & Kline, 2005; Pollatos, Herbert, Kaufmann, Auer, & Schandry, 2007; Ring, Liu, & Brener, 1994; Schandry, Bestler, & Montoya, 1993). Aging has been found to dampen sympathetic reflex regulation and sympathetic reactivity to mental and physical stressors (Laitinen, Niskanen, Geelen, Lansimies, & Hartikainen, 2004; Madden, Levy, & Stratton, 2006; Monahan, 2007). Consequently, older adults may be less aware of their heartbeats due to decreases in cardiac parameters such as heart rate or contractility (Poller, Nedelka, Radke, Ponicke, & Brodde, 1997). Aging has also been associated with decreased attentional capacity (Andres, Parmentier, & Escera, 2006; Mouloua & Parasuraman, 1995; Raz, 2000; West, Murphy, Armilio, Craik, & Stuss, 2002), and it is possible that the current findings could be influenced by task-specific decrements in attention with aging. We feel that this possibility is unlikely, as we used performance on a pulse detection task to screen for any individuals who were unable to successfully perform the requirements of the heartbeat detection task. Although this task does not provide a standard neuropsychological measure of attention, it identified whether participants were able to sustain a task oriented focus of attention and make simultaneity judgments about two different stimuli, and thus yielded a suitable control task for the current study. Future studies may determine the extent to which neuropsychological deficits in attention influence performance on interoceptive tasks.
The current findings yield additional tools for empirically testing certain tenets of the Somatic Marker Hypothesis (SMH) (Damasio, 1996), and for clarifying the link between aging, interoceptive awareness, and the influence of emotion on decision making. For example, according to the SMH both conscious (“in mind”) and non-conscious information derived from primary sensory cortices play a role in guiding decisions (Damasio, 1996). Does conscious awareness of bodily feedback influence decision making, and if so, towards what end? Are there functional consequences of decreased interoception with aging, as theories of emotion might predict? Do age-related declines in conscious bodily feedback reduce the influence of emotion on decision making? Could this underlie some of the reasoning and real life decision-making deficits observed in a subset of older adults (Denburg, Tranel, & Bechara, 2005)? Furthermore, could impaired interoception positively (or negatively) bias social and emotional behavioral changes as people age? Could some aging individuals benefit from interoceptive feedback training and/or augmentation, similar to the use of exteroceptive aids such as eyeglasses and hearing aids? Although these comments are speculative, we offer them in the hope that they might provoke further research on this topic.
There are several important limitations to our study. First, the sample size was relatively small and likely not representative of the entire population. For example, participants from across the entire life span were not sampled, particularly those from the youngest and oldest age groups (e.g., individuals younger than 18 and older than 65). Secondly, age epochs were unevenly sampled. As a result, by chance most individuals fell between the ages of 40 and 60 years, making it more difficult to generalize the present findings to the 22–40 age range. The small sample size of the current study also limits the strength of conclusions regarding subgroups within the population, such as possible sex-related differences in interoception. There were also unequal numbers of men and women dispersed across different age epochs. These limitations could be remedied through targeted enrollment of larger and equal numbers of men and women, focusing on equal distributions across the human life span. A final and more general consideration relates to the limitations imposed by cross sectional designs in aging research. Thus, while the current findings appear reliable, from the current design it cannot be guaranteed that aging was a causative factor. A longitudinal approach would help address this final limitation.
Overall, these findings provide clear empirical evidence that interoception, much like exteroception and proprioception, declines with age.
Acknowledgments
Funding
Supported by NCCAM F31 AT003061 (SK), NIDA R01 DA022549 (DT) and NINDS P01 NS19632 (DT)
Footnotes
Note that although Sherrington originally classified sensory functions into the distinct categories of interoception, exteroception and proprioception (Sherrington, 1961), there is some disagreement as to whether the peripheral neural pathways mediating interoception follow this distinction (Craig, 2002; Dworkin, 2007).
References
- Andres P, Parmentier FB, Escera C. The effect of age on involuntary capture of attention by irrelevant sounds: a test of the frontal hypothesis of aging. Neuropsychologia. 2006;44(12):2564–8. doi: 10.1016/j.neuropsychologia.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Bannister JJ, Abouzekry L, Read NW. Effect of aging on anorectal function. Gut. 1987;28(3):353–7. doi: 10.1136/gut.28.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsky AJ, Ahern DK, Brener J, Surman OS, Ring C, Dec GW. Palpitations and cardiac awareness after heart transplantation. Psychosom Med. 1998;60(5):557–62. doi: 10.1097/00006842-199809000-00007. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck depression inventory. San Antonio: The Psychological Corporation; 1993. [Google Scholar]
- Brener J, Kluvitse C. Heartbeat detection: judgments of the simultaneity of external stimuli and heartbeats. Psychophysiology. 1988;25(5):554–61. doi: 10.1111/j.1469-8986.1988.tb01891.x. [DOI] [PubMed] [Google Scholar]
- Brener J, Liu X, Ring C. A method of constant stimuli for examining heartbeat detection: comparison with the Brener-Kluvitse and Whitehead methods. Psychophysiology. 1993;30(6):657–65. doi: 10.1111/j.1469-8986.1993.tb02091.x. [DOI] [PubMed] [Google Scholar]
- Cameron OG. Interoception: the inside story--a model for psychosomatic processes. Psychosom Med. 2001;63(5):697–710. doi: 10.1097/00006842-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Minoshima S. Regional brain activation due to pharmacologically induced adrenergic interoceptive stimulation in humans. Psychosom Med. 2002;64(6):851–61. doi: 10.1097/01.psy.0000038939.33335.32. [DOI] [PubMed] [Google Scholar]
- Cameron OG, Zubieta JK, Grunhaus L, Minoshima S. Effects of yohimbine on cerebral blood flow, symptoms, and physiological functions in humans. Psychosom Med. 2000;62(4):549–59. doi: 10.1097/00006842-200007000-00014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: why are some more aware than others? Trends Cogn Sci. 2004;8(6):239–41. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio A. Feelings of emotion and the self. Ann N Y Acad Sci. 2003;1001:253–61. doi: 10.1196/annals.1279.014. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1413–20. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Tranel D, Bechara A. The ability to decide advantageously declines prematurely in some normal older persons. Neuropsychologia. 2005;43(7):1099–106. doi: 10.1016/j.neuropsychologia.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Dickerson PC, Jones GE. The effect of age on cardiac awareness. Psychophysiology. 1990;27(4A):S25. [Google Scholar]
- Dworkin BR. Interoception. In: Cacciopo JT, Tassinary LG, Bernston GG, editors. Handbook of psychophysiology. Cambridge university press; 2007. pp. 482–506. [Google Scholar]
- Eichler S, Katkin ES. The relationship between cardiovascular reactivity and heartbeat detection. Psychophysiology. 1994;31(3):229–34. doi: 10.1111/j.1469-8986.1994.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Gescheider GA, Bolanowski SJ, Hall KL, Hoffman KE, Verrillo RT. The effects of aging on information-processing channels in the sense of touch: I. Absolute sensitivity. Somatosens Mot Res. 1994;11(4):345–57. doi: 10.3109/08990229409028878. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Farrell M. A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain. 2004;20(4):227–39. doi: 10.1097/00002508-200407000-00004. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Helme RD. Age-related differences in pain perception and report. Clin Geriatr Med. 2001;17(3):433–56. v–vi. doi: 10.1016/s0749-0690(05)70079-3. [DOI] [PubMed] [Google Scholar]
- Godde B, Berkefeld T, David-Jurgens M, Dinse HR. Age-related changes in primary somatosensory cortex of rats: evidence for parallel degenerative and plastic-adaptive processes. Neurosci Biobehav Rev. 2002;26(7):743–52. doi: 10.1016/s0149-7634(02)00061-1. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grier JB. Nonparametric indices for sensitivity and bias: computing formulas. Psychological Bulletin. 1971;75:424–29. doi: 10.1037/h0031246. [DOI] [PubMed] [Google Scholar]
- Hurley MV, Rees J, Newham DJ. Quadriceps function, proprioceptive acuity and functional performance in healthy young, middle-aged and elderly subjects. Age Ageing. 1998;27(1):55–62. doi: 10.1093/ageing/27.1.55. [DOI] [PubMed] [Google Scholar]
- Ivy GO, Petit TL, Markus EJ. A physiological framework for perceptual and cognitive changes in aging. 2. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- Jones GE. Perception of visceral sensations: a review of recent findings, methodologies, and future directions. Vol. 5. London: Jessica Kingsley Publishers; 1994. [Google Scholar]
- Jones GE, Hollandsworth JG. Heart rate discrimination before and after exercise-induced augmented cardiac activity. Psychophysiology. 1981;18(3):252–7. doi: 10.1111/j.1469-8986.1981.tb03029.x. [DOI] [PubMed] [Google Scholar]
- Jones GE, Jones KR, Cunningham RA, Caldwell JA. Cardiac awareness in infarct patients and normals. Psychophysiology. 1985;22(4):480–7. doi: 10.1111/j.1469-8986.1985.tb01635.x. [DOI] [PubMed] [Google Scholar]
- Jones GE, Jones KR, Rouse CH, Scott DM, Caldwell JA. The effect of body position on the perception of cardiac sensations: an experiment and theoretical implications. Psychophysiology. 1987;24(3):300–11. doi: 10.1111/j.1469-8986.1987.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Jones GE, O’Leary RT, Pipkin BL. Comparison of the Brener-Jones and Whitehead procedures for assessing cardiac awareness. Psychophysiology. 1984;21(2):143–8. doi: 10.1111/j.1469-8986.1984.tb00196.x. [DOI] [PubMed] [Google Scholar]
- Katkin ES, Blascovich J, Goldband S. Empirical assessment of visceral self-perception: individual and sex differences in the acquisition of heartbeat discrimination. J Pers Soc Psychol. 1981;40(6):1095–101. doi: 10.1037//0022-3514.40.6.1095. [DOI] [PubMed] [Google Scholar]
- Katkin ES, Wiens S, Ohman A. Nonconscious fear conditioning, visceral perception, and the development of gut feelings. Psychol Sci. 2001;12(5):366–70. doi: 10.1111/1467-9280.00368. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR., Sr Somesthetic sensitivity in young and elderly humans. J Gerontol. 1986;41(6):732–42. doi: 10.1093/geronj/41.6.732. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Damasio AR, Davidson RJ, Lutz A, Tranel D. Interoceptive awareness in experienced meditators. Psychophysiology. 2008;45(4):671–77. doi: 10.1111/j.1469-8986.2008.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SS, Rudrauf D, Sandesara C, Olshansky B, Tranel D. Bolus isoproterenol infusions provide a reliable method for assessing interoceptive awareness. International Journal of Psychophysiology. doi: 10.1016/j.ijpsycho.2008.08.010. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp K, Ring C, Brener J. Sensitivity to mechanical stimuli and the role of general sensory and perceptual processes in heartbeat detection. Psychophysiology. 1997;34(4):467–73. doi: 10.1111/j.1469-8986.1997.tb02391.x. [DOI] [PubMed] [Google Scholar]
- Knapp-Kline K, Kline JP. Heart rate, heart rate variability, and heartbeat detection with the method of constant stimuli: slow and steady wins the race. Biol Psychol. 2005;69(3):387–96. doi: 10.1016/j.biopsycho.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Laitinen T, Niskanen L, Geelen G, Lansimies E, Hartikainen J. Age dependency of cardiovascular autonomic responses to head-up tilt in healthy subjects. J Appl Physiol. 2004;96(6):2333–40. doi: 10.1152/japplphysiol.00444.2003. [DOI] [PubMed] [Google Scholar]
- Lasch H, Castell DO, Castell JA. Evidence for diminished visceral pain with aging: studies using graded intraesophageal balloon distension. Am J Physiol. 1997;272(1 Pt 1):G1–3. doi: 10.1152/ajpgi.1997.272.1.G1. [DOI] [PubMed] [Google Scholar]
- Lin YH, Hsieh SC, Chao CC, Chang YC, Hsieh ST. Influence of aging on thermal and vibratory thresholds of quantitative sensory testing. J Peripher Nerv Syst. 2005;10(3):269–81. doi: 10.1111/j.1085-9489.2005.10305.x. [DOI] [PubMed] [Google Scholar]
- Low Choy NL, Brauer SG, Nitz JC. Age-related changes in strength and somatosensation during midlife: rationale for targeted preventive intervention programs. Ann N Y Acad Sci. 2007;1114:180–93. doi: 10.1196/annals.1396.014. [DOI] [PubMed] [Google Scholar]
- Madden KM, Levy WC, Stratton JR. Normal aging impairs upregulation of the beta-adrenergic but not the alpha-adrenergic response: aging and adrenergic upregulation. J Cardiovasc Pharmacol. 2006;48(4):153–9. doi: 10.1097/01.fjc.0000246405.89380.48. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131(6):1925–42. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Mertz H, Fullerton S, Naliboff B, Mayer EA. Symptoms and visceral perception in severe functional and organic dyspepsia. Gut. 1998;42(6):814–22. doi: 10.1136/gut.42.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R3–R12. doi: 10.1152/ajpregu.00031.2007. [DOI] [PubMed] [Google Scholar]
- Mouloua M, Parasuraman R. Aging and cognitive vigilance: effects of spatial uncertainty and event rate. Exp Aging Res. 1995;21(1):17–32. doi: 10.1080/03610739508254265. [DOI] [PubMed] [Google Scholar]
- Pauli P, Hartl L, Marquardt C, Stalmann H, Strian F. Heartbeat and arrhythmia perception in diabetic autonomic neuropathy. Psychol Med. 1991;21(2):413–21. doi: 10.1017/s0033291700020523. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Herbert BM, Kaufmann C, Auer DP, Schandry R. Interoceptive awareness, anxiety and cardiovascular reactivity to isometric exercise. Int J Psychophysiol. 2007;65(2):167–73. doi: 10.1016/j.ijpsycho.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Poller U, Nedelka G, Radke J, Ponicke K, Brodde OE. Age-dependent changes in cardiac muscarinic receptor function in healthy volunteers. J Am Coll Cardiol. 1997;29:187–93. doi: 10.1016/s0735-1097(96)00437-8. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. 2. Mahwah, NJ: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23(8):3295–301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring C, Brener J. The temporal locations of heartbeat sensations. Psychophysiology. 1992;29(5):535–45. doi: 10.1111/j.1469-8986.1992.tb02027.x. [DOI] [PubMed] [Google Scholar]
- Ring C, Liu X, Brener J. Cardiac stimulus intensity and heartbeat detection: effects of tilt-induced changes in stroke volume. Psychophysiology. 1994;31(6):553–64. doi: 10.1111/j.1469-8986.1994.tb02348.x. [DOI] [PubMed] [Google Scholar]
- Rouse CH, Jones GE, Jones KR. The effect of body composition and gender on cardiac awareness. Psychophysiology. 1988;25(4):400–7. doi: 10.1111/j.1469-8986.1988.tb01876.x. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Shaffer SW, Harrison AL. Aging of the somatosensory system: a translational perspective. Phys Ther. 2007;87(2):193–207. doi: 10.2522/ptj.20060083. [DOI] [PubMed] [Google Scholar]
- Schandry R, Bestler M, Montoya P. On the relation between cardiodynamics and heartbeat perception. Psychophysiology. 1993;30(5):467–74. doi: 10.1111/j.1469-8986.1993.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Schneider TR, Ring C, Katkin ES. A test of the validity of the method of constant stimuli as an index of heartbeat detection. Psychophysiology. 1998;35(1):86–9. [PubMed] [Google Scholar]
- Shaffer SW, Harrison AL. Aging of the somatosensory system: a translational perspective. Phys Ther. 2007;87(2):193–207. doi: 10.2522/ptj.20060083. [DOI] [PubMed] [Google Scholar]
- Sherrington C. The integrative action of the nervous system. 2. New Haven: Yale university press; 1961. [Google Scholar]
- Skinner HB, Barrack RL, Cook SD. Age-related decline in proprioception. Clin Orthop Relat Res. 1984;184:208–11. [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stevens JC, Choo KK. Temperature sensitivity of the body surface over the life span. Somatosens Mot Res. 1998;15(1):13–28. doi: 10.1080/08990229870925. [DOI] [PubMed] [Google Scholar]
- Vaitl D. Interoception. Biol Psychol. 1996;42(1–2):1–27. doi: 10.1016/0301-0511(95)05144-9. [DOI] [PubMed] [Google Scholar]
- Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5(4):191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- Verrillo RT, Bolanowski SJ, Gescheider GA. Effect of aging on the subjective magnitude of vibration. Somatosens Mot Res. 2002;19(3):238–44. doi: 10.1080/0899022021000009161. [DOI] [PubMed] [Google Scholar]
- Wang S, Davis BM, Zwick M, Waxman SG, Albers KM. Reduced thermal sensitivity and Nav1.8 and TRPV1 channel expression in sensory neurons of aged mice. Neurobiol Aging. 2006;27(6):895–903. doi: 10.1016/j.neurobiolaging.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Murphy KJ, Armilio ML, Craik FI, Stuss DT. Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain Cogn. 2002;49(3):402–19. doi: 10.1006/brcg.2001.1507. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Drescher VM, Heiman P. Relation of Heart Rate Control to Heartbeat Perception. Biofeedback Self Regul. 1977;2(4):371–92. [PubMed] [Google Scholar]
- Wiens S, Palmer SN. Quadratic trend analysis and heartbeat detection. Biol Psychol. 2001;58(2):159–75. doi: 10.1016/s0301-0511(01)00110-7. [DOI] [PubMed] [Google Scholar]