Abstract
BACKGROUND
Donor white blood cells (WBCs) present in transfusion products can lead to immune sequelae such as production of anti-HLA antibodies or GVHD in susceptible transfusion recipients. Eliminating the immunogenicity of blood products may prove to be of clinical benefit, particularly in patients requiring multiple transfusions in whom allosensitization is common. This study examines a method of pathogen reduction based on UV light illumination in the presence of riboflavin. In addition to pathogens, WBCs treated with this system are also affected and fail to stimulate proliferation of allogeneic PBMCs in vitro.
STUDY DESIGN AND METHODS
This study sought to determine the mechanisms regulating this loss of immunogenicity. Treated cells were examined for surface expression of a number of molecules involved in activation and adhesion, viability, cell-cell conjugation, and ability to stimulate immune responses in allogeneic PBMCs.
RESULTS
Compared with untreated controls, UV irradiated antigen presenting cells showed slightly reduced surface expression of HLA class II and costimulatory molecules and had more significant reductions in surface expression of a number of adhesion molecules. Furthermore, treated cells had a severe defect in cell-cell conjugation. The observed loss of immunogenicity was nearly complete, with UV irradiated cells stimulating barely measurable IFN-γ production and no detectable STAT-3, STAT-5, or CD3-ε phosphorylation in allospecific primed T cells.
CONCLUSION
These results suggest that defective cell-cell adhesion prevents UV irradiated cells from inducing T cell activation.
Keywords: Pathogen reduction, allogeneic stimulation, ultraviolet light, cell adhesion, T cell activation
INTRODUCTION
Donor white blood cells (WBCs) present in blood products carry the potential to cause a number of complications in recipients. Transfusion-associated graft vs. host disease (TA-GVHD) occurs when donor cells mount an immune response against the recipient, and consequently, at risk patients are often given prophylactically γ-irradiated or leukoreduced blood products. The recipient can also mount an immune response to the donor cells, a response that can be problematic if it impacts other host immune responses or enhances rejection of transplanted organs or platelets. Unfortunately, while cells given a sufficient dose of γ-irradiation are unable to mount a response against the host, they can still act as antigen presenting cells (APCs) as well as a source of antigen for host APCs, leaving the door open for recipient immune sensitization to donor antigens.
The Mirasol® pathogen reduction technology (Mirasol) treatment was developed to inactivate pathogens potentially present in blood products. This system involves exposure of blood products to UV light (primarily UVB) in the presence of riboflavin, causing irreversible damage to nucleic acids. It has been demonstrated that Mirasol can reduce the titers of a wide range of viruses, bacteria, and parasites by several logs.1–4 In addition to inactivating pathogens, this treatment has been shown to impact donor WBCs. Similar to treatment with γ-irradiation, Mirasol treatment prevents proliferation and ultimately kills WBCs, but unlike γ-irradiated cells, Mirasol treated cells lose their ability to act as APCs and stimulate an allogeneic response in vitro. In mixed lymphocyte reactions (MLRs), Mirasol treated PBMCs fail to stimulate proliferation of allogeneic PBMCs, whereas mitomycin C treated cells from the same donor induce a robust allogeneic response.5 There is some evidence for this effect in vivo, as UVB irradiation of platelets prior to infusion has been shown to reduce the development of alloantibodies and improve platelet count increments in lymphocytotoxic antibody-positive patients.6–8
UV light has been shown to have different immunoregulatory effects in vitro and in vivo depending on cell type, light wavelength, and energy dose. Exposure of the skin to UV has been well documented to induce both local and systemic immune suppression through changes in Langerhans cell function and induction of regulatory T cells and soluble immune modulators.9,10 Reintroduction of cells exposed ex vivo to UV has also been shown to have immunosuppressive properties. Extracorporeal photochemotherapy, in which PBMCs taken from the patient are treated with UVA in the presence of psoralen and then returned to the patient, has been used to induce selective immune suppression in patients suffering form GVHD, solid organ transplant rejection, and a number of autoimmune diseases such as systemic sclerosis, pemphigus vulgaris, rheumatoid arthritis, systemic lupus erythematosus, and atopic dermatitis.11–14
Analysis of UV exposure using in vitro systems has shown a wide range of immunomodulatory effects. Low doses of UV have been shown in various systems to block the ability of APCs to stimulate T cells with antigen or to act as accessory cells.15–17 UV exposure can induce changes in MHC expression (up, down, or not at all depending on locus, cell type, dose, and wavelength) and block induced expression of costimulatory molecules.18–21 Some adhesion molecules have been shown to be downregulated in response to UV. A reduction in surface LFA-3 has been observed in a UV-treated B cell line, and reductions in ICAM-1 have been observed in a number of systems.19,22–27 In addition to adhesion molecule downregulation, there is some evidence that UV exposure can lead to impaired cellular interaction, as reduced cluster sizes have been observed in cultures with increasing UV dose, and a UVB treated B cell line was shown to have less efficient conjugation with CTLs.21,23,25,28
In this study we sought to determine the mechanism responsible for loss of allogeneic responses to Mirasol treated WBCs. We examined surface expression of a number of molecules involved in activation and adhesion, and assessed viability, cell-cell conjugation, and ability to stimulate immune responses in allogeneic PBMCs.
MATERIALS AND METHODS
Processing and storage of PBMCs
PBMCs were isolated from the residual material of a leukoreduced platelet aphaeresis collection from normal donors. PBMCs were separated by Ficoll gradient, then washed and counted before use or freezing.
For long-term storage, cells were resuspended in freezing medium (90% fetal bovine serum (FBS), 10% DMSO) at a concentration of 5–20 million cells per ml in 1 ml aliquots. PBMCs were frozen in 1°C cyro “Mr. Frosty” freezing containers (VWR, West Chester, PA) to allow a controlled rate of freezing to −80°C for 24 hours prior to transfer into liquid nitrogen storage in the vapor phase at −135°C. Before use cells were rapidly thawed in a 37°C water bath until only a small ice chip remained, then transferred drop-wise into 10 ml of 37°C culture media, washed, and recounted.
Mirasol treatment
Freshly prepared PBMCs were spiked into plasma, with a portion set aside for use as an untreated control. The remainder was transferred into Mirasol illumination bags and either given the full Mirasol treatment or a modified treatment without riboflavin (UV only). For cells getting full treatment, 35 ml of 500 µM riboflavin solution was added by sterile docking prior to illumination. The illumination delivered a dose of 6.24 J/ml (approximately equal to 53,000 J/m2), with 68.2% of the energy in the spectral region of 280nm to 320 mm (predominantly UVB). Following treatment cells were washed, and frozen as described above.
MLR
Responder cells (normal PBMCs) were labeled with 10 µM CFSE (Sigma-Aldrich, St. Louis, MO) for 10 minutes at 37°C at a cell concentration of 50×106/ml. Stimulator cells were given either 3000 cGy of γ-irradiation or the Mirasol treatment to prevent proliferation. 5×104, 1×105, or 2×105 responder cells per well were co-cultured with 2×105 stimulator cells per well in 96-well round-bottom plates. At day seven cells were harvested, stained, and analyzed by flow cytometry.
To enrich for allo-specific responder cells to a given donor, normal PBMCs (responders) were co-cultured with γ-irradiated cells from the donor at a ratio of 1:2 responders:stimulators along with 50 U/ml IL-2 (Roche, Nutley, NJ) in culture flasks at 37°C. At day 6 cells were given fresh media, and at day 7 or 8, 20 U/ml IL-2. Lymphocyte separation medium (Cellgro, Mediatech, Inc, Lawrence, KS) was used to eliminate the majority of dead cells before use at days 12–14.
Flow cytometry surface staining
Surface expression of adhesion molecules was tested using the following stains from BD Pharmingen (San Diego, CA): Pacific blue-conjugated anti-CD3 (UCHT1), Alexa Fluor 700-conjugated anti-CD4 (RPA-T4), allophycocyanin- and Alexa Fluor 700-conjugated anti-CD8 (RPA-T8), allophycocyanin-H7-conjugated anti-HLA-DR (L243 (G46-6)), PE-conjugated anti-CD80 (L307.4), allophycocyanin-conjugated anti-CD86 (2331 (FUN-1)), allophycocyanin-conjugated anti-CD54 (HA58), PE-conjugated anti-CD102 (CBR-1C2/2.1), FITC-conjugated anti-CD50 (TÜ41), and FITC-conjugated anti-CD58 (1C3). LIVE/DEAD Fixable Dead Cell Stain Kits (Invitrogen, Carlsbad, CA) were used to determine viability of cells for some stains. Cells were labeled with antibodies for 30 minutes at 4°C, washed, then fixed with 1% paraformaldehyde (Sigma-Aldrich, St. Louis, MO). At least 10,000 cells from the subpopulation of interest were acquired (except where indicated) on an LSRII flow cytometer (BD Biosciences, San Jose, CA). Flow data analysis was performed with FlowJo software (TreeStar, San Carlos, CA).
Intracellular staining
For intracellular IFN-γ stains, allo-specific enriched cells were stimulated at 37°C for 2 hours before addition of 10 µg/ml Brefeldin A (Sigma-Aldrich), then incubated overnight at 37°C. Cells were fixed with 100 µl of Fixation reagent A (Caltag, Burlingame, CA) for 10 minutes at room temperature and washed. The cells were then simultaneously permeabilized with 100 µl of Permeabilization reagent B (Caltag) and stained with allophycocyanin-conjugated anti-IFN-γ or isotype control, and Pacific blue-conjugated anti-CD3 with anti-FcR blocking antibody (Miltenyi Biotec, Auburn, CA) for 30 minutes at 4 °C, washed, and analyzed.
For intracellular staining of phosphorylated signaling molecules (PhosFlow), stimulator cells were pre-stained with Alexa Fluor 700-conjugated anti-CD4 and anti-CD8 so they could be gated out in the analysis. Allo-specific enriched cells were stimulated for two hours, then lysed and fixed with BD PhosFlow Lyse/Fix Buffer (BD Biosciences) for 10 minutes at 37°C, and frozen at −80°C overnight. Cells were thawed and washed in PBS with 2% FBS, and incubated for 30 minutes on ice in BD PhosFlow Perm Buffer III (BD Biosciences). Cells were then washed and stained in PBS with 2% FBS with Pacific blue-conjugated anti-CD3, allophycocyanin-conjugated anti-CD4, PE-conjugated anti-pSTAT3, and Alexa Fluor 647-conjugated anti-pSTAT5 for 30 minutes at 4°C, washed, and analyzed.
Conjugation Assays
Allo-specific enriched responder cells were labeled with PKH26 Red Fluorescent Cell Linker (Sigma-Aldrich) and stimulator cells were labeled with PKH67 Green Fluorescent Cell Linker (Sigma-Aldrich) according to manufacturer’s protocols. 5×105 labeled stimulators and 5×104 labeled responders per well were co-cultured in 96-well round-bottomed plates at 37°C, and after 2 hours, 4.5×105 unlabeled responders were added to some wells as a chase. Multiple wells were set up for each condition to allow harvesting without disruption of cells used for later time points. Cells were fixed in 1% paraformaldehyde (Sigma-Aldrich) at the given time, and analyzed by flow cytometry.
Multiplex bead-based analysis of phosphorylated proteins
Allo-specific enriched responder cells were stimulated with either γ-irradiated or Mirasol treated cells from the same donor with 106 each responders and stimulators per well in 96-well round-bottom plates. As a positive control, an additional stimulation condition was used with 2×106 PBMCs per well given anti-CD3 and anti-CD28 antibodies at a concentration of 5 µg/ml each. At given time intervals cells were washed in ice cold Tris Buffered Saline (Fisher Scientific, Pittsburgh, PA), lysed in Beadlyte Cell Signaling Universal Lysis Buffer (Millipore, Billerica, MA) with added Complete Protease Inhibitors (Roche) for 12 minutes at 4°C, filtered with Ultrafree-MC centrifugal filters (Millipore), flash frozen on dry ice, and stored at −80°C. Lysates were later thawed on ice and assayed with a Beadlyte 7-plex Human T Cell Receptor Signaling Kit – Phosphoprotein Kit (Millipore) according to the manufacturer’s protocol. Data were acquired on a Luminex-100 system and analyzed using Bio-Plex Manager software, v4.1 (Bio-Rad, Hercules, CA).
Statistical analysis
Two-tailed unpaired t-tests were used to determine the significance of differences between two given groups. For analysis containing more than two groups, one-way ANOVA with Tukey’s test for multiple comparisons was used.
RESULTS
Mirasol treated cells fail to stimulate allogeneic proliferation
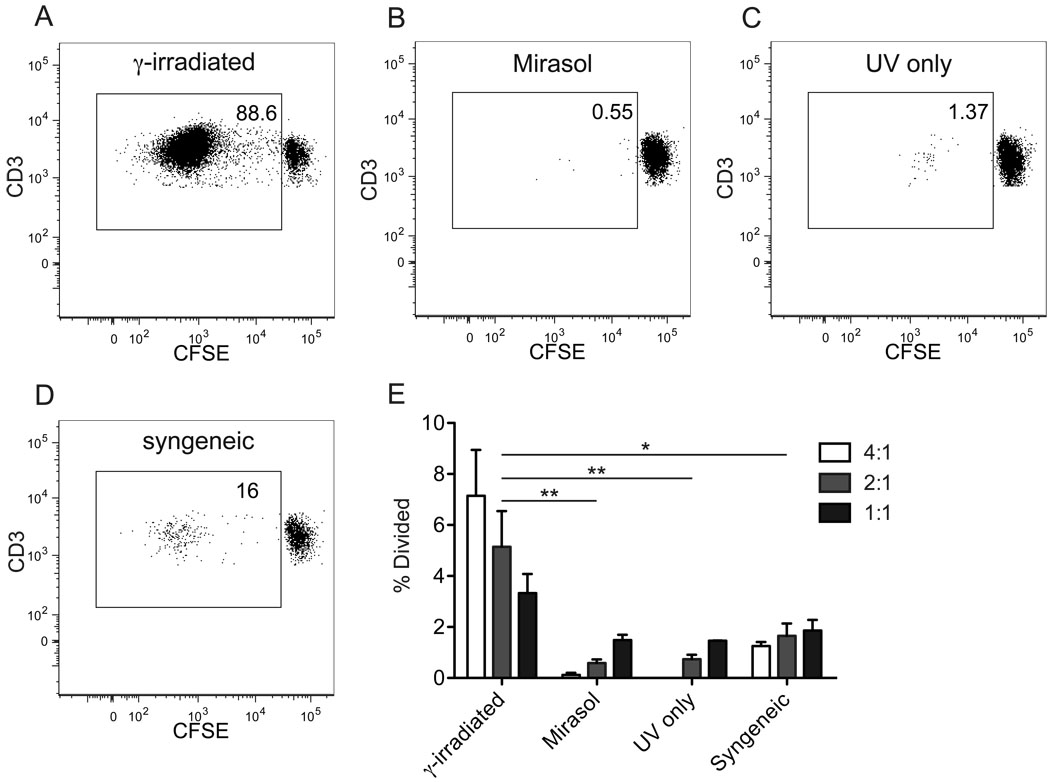
As shown in Figure 1, and as has been previously published, PBMCs treated with the Mirasol system fail to stimulate allogeneic proliferation in normal PBMCs.5 In our system PBMCs from three different donors were processed by first spiking into plasma, with portions of each left untreated or treated with Mirasol system. For one donor there was sufficient material to add a third group, which received a modified treatment without riboflavin prior to illumination (UV only). Cells were aliquoted and frozen after treatment and stored in liquid nitrogen until use. These cells were used in MLRs to stimulate normal allogeneic CFSE labeled PBMCs at varying ratios for one week in culture. γ-irradiated cells from all three donors stimulated robust proliferation of the labeled cells (Figure 1A), whereas the Mirasol treated cells (Figure 1B), UV only treated cells (Figure 1C), and γ-irradiated syngeneic cells (Figure 1D) did not. An estimation of the percent of the starting population that divided was calculated using FlowJo software based on the proportion of cells with diluted CFSE and the degree of CFSE dilution in these cells (Figure 1E). The γ-irradiated allogeneic response was optimal at a ratio of 4:1 stimulators:responders, with increased numbers of responders leading to a reduction in the proportion of cells responding, perhaps due to crowding. This trend was reversed in the background response observed in the Mirasol treated, the UV only treated, and the syngeneic control stimulations, suggesting that this background stimulation was the result of responder-responder interactions. This background proliferation may have been due to presentation of components of the fetal bovine serum added to the culture media.
Figure 1. Mirasol treated cells fail to stimulate allogeneic MLR.
PBMCs were labeled with CFSE and stimulated for one week with allogeneic PBMCs that were γ-irradiated (A), Mirasol treated (B), or given a modified treatment without riboflavin (UV only) (C). γ-irradiated syngeneic stimulator cells were used as a negative control (D). A–D are representative plots of live T cells from each of these groups at day 7. The % of the initial responder population that has divided by day 7 was back calculated in FlowJo and is shown for each stimulation (E). γ-irradiated and Mirasol treated cells from three different donors were used for each experiment and the experiment was run two times (each time with a different responder population) with the results plotted as mean and standard error. Stimulations were done in 96-well plates with a stimulator:responder ratio of 4:1 (white), 2:1 (light grey), or 1:1 (dark grey). Stimulators that did not get full Mirasol treatment were γ-irradiated to prevent proliferation. 10,000 T cells were collected for each sample where possible, for samples with little to no cell division, fewer cells were available and a minimum of 2,400 cells were collected for these groups. Differences between each treatment group were evaluated including the data from all three stimulator:responder ratios. * p < 0.05, ** p < 0.01.
The decreased responses to increased stimulator:responder ratios with treated stimulator cells could also suggest an inhibitory effect. The ability of Mirasol treated cells to inhibit proliferation, either through induction of tolerance (measured by priming with Mirasol treated cells and subsequent challenge with untreated cells from the same or different donor), or by direct inhibitory action (examined by addition of Mirasol treated cells to normal MLRs) was not detected using our in vitro system (data not shown).
To ensure that the freezing and storage process was not responsible for the differences observed due to a differential effect on the treated and untreated groups, an additional donor sample was processed and tested as described above, but without freezing and storage. As seen with the frozen cells, the γ-irradiated cells stimulated a proliferative response, whereas the Mirasol and UV only treated did not (data not shown).
UV treated cells die rapidly
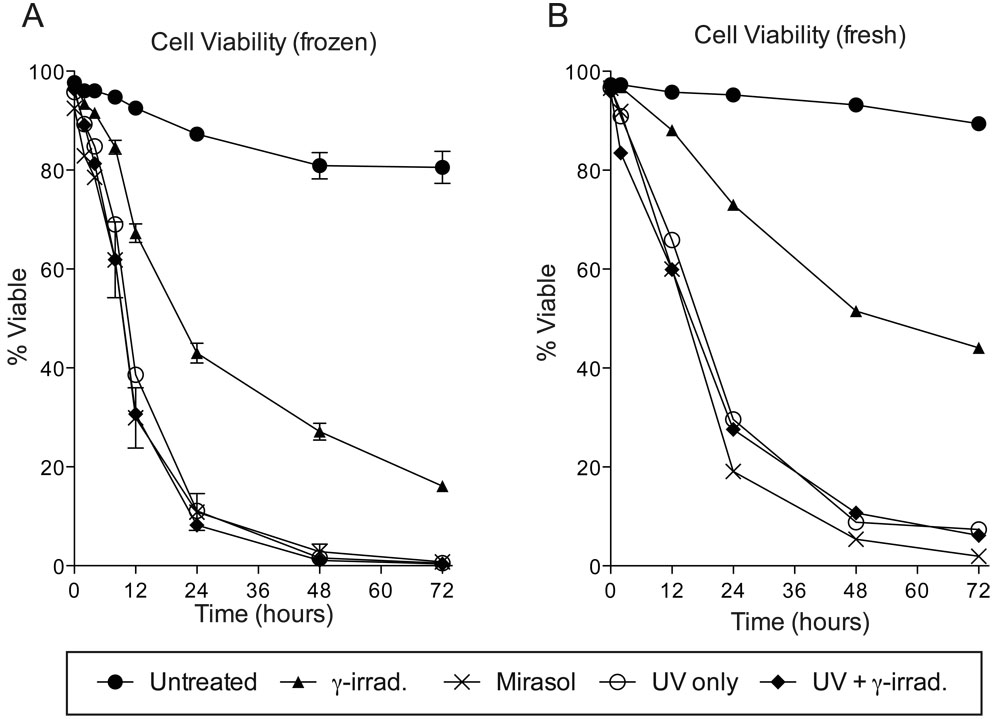
Both γ-irradiation and Mirasol treatment led to reduced viability. To assess the kinetics of cell death following these different treatments, the viability of cells given no treatment, γ-irradiation, Mirasol, or a modified UV only treatment (with or without γ-irradiation) was tracked over 72 hours by flow cytometry using aqua amine-reactive dye (Figure 2A). While all treated groups had reduced viability compared with untreated cells, all treatments involving UV led to faster death than what was observed with γ-irradiation alone. In the UV treated groups, almost all cells were dead by 48 hours, while approximately 16% of cells given γ-irradiation alone were still viable at 72 hours. Viability in the untreated and γ-irradiated groups was significantly different from t≥12 hours (p<0.01), while the Mirasol treated group was significantly different from both the untreated and γ-irradiated from t≥2 hours (p<0.01 and p<0.05, respectively). As only single samples were tracked for the UV only and UV with γ-irradiation treatments, quantitative comparisons could not be made for these two treatments. There was, however, considerable overlap between the survival curves for all three groups given UV (Mirasol, UV only, and UV with γ-irradiation), suggesting that the UV portion of the treatment was responsible for the accelerated rate of death. One set of fresh cells (not frozen) was processed with these different treatments and run for comparison, and these cells followed the same trends, though with slightly delayed death in the Mirasol and UV treated groups, and more substantially delayed death in the γ-irradiated group (Figure 2B).
Figure 2. Viability of Mirasol treated cells.
Cells that were untreated (n=3), γ-irradiated (n=3), Mirasol treated (n=2), or given a modified treatment without riboflavin (UV only) with or without γ-irradiation (n=1 for each), were cultured for 0, 2, 4, 8, 12, 24, 48, or 72 hours, then stained with aqua amine-reactive dye to determine viability. Means with standard error are displayed for groups with n>1 (A). One set of fresh cells (not frozen) was processed with each treatment and cultured for 0, 12, 24, 48, or 72 hours, then stained with aqua amine-reactive dye (B).
Mirasol treated APCs have reduced surface expression of HLA class II and costimulatory molecules
A possible explanation of the inability of Mirasol treated cells to stimulate allogeneic proliferation is failure to provide the necessary T cell receptor stimulation and costimulatory requirements for T cell activation. UV light exposure has been shown in other systems to impact the expression of these molecules in different ways depending on the dose and wavelength of the light, and cell type.18–21 To assess the impact of Mirasol treatment on availability of these signals, surface expression of HLA-DR, CD80, and CD86 was monitored over a 24 hour period by flow cytometry on thawed treated or untreated cells.
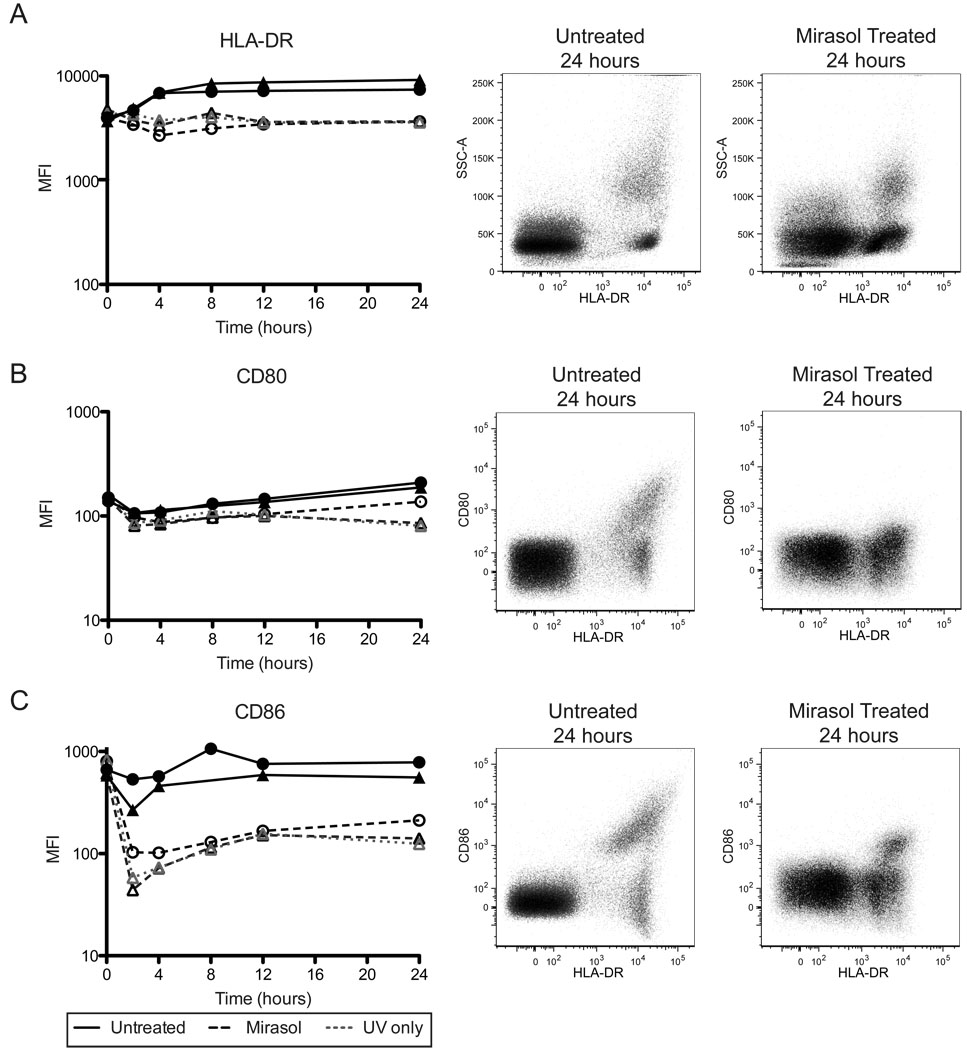
HLA-DR surface expression increased in the untreated APCs and decreased in both the Mirasol and UV only treated APCs, resulting in a 2 to 3-fold difference in median fluorescent intensity (MFI) between untreated and Mirasol treated by 24 hours (Figure 3A, p=0.0343). A subpopulation of HLA-DR+ cells increased expression of CD80 by 24 hours in the untreated cells, but this induced expression was lost with Mirasol or UV only treatment (Figure 3B). Evaluation of CD80 expression on the whole HLA-DR+ population diluted this loss, with no statistically significant difference in MFIs between Mirasol treated and untreated at 24 hours (p=0.0882). CD86 expression was stable in untreated APCs over 24 hours, with a subpopulation of HLA-DR+ cells expressing high levels of CD86. After Mirasol or UV only treatment a 5-fold reduction in CD86 expression among HLA-DR+ cells was observed as early as 2 hours, which rebounded slightly and was not statistically significant at 24 hours (Figure 3C, p=0.0532 at 24 hours for Mirasol treated versus untreated). The shifts observed in expression of HLA-DR, CD80, and CD86 were confirmed with fresh (unfrozen) cells from an additional donor, and results were consistent (data not shown).
Figure 3. Mirasol treated APCs have reduced surface HLA-DR and costimulatory molecule expression.
Cells that were untreated, Mirasol treated, or given a modified treatment without riboflavin (UV only), were cultured for 0, 2, 4, 8, 12, or 24 hours, then stained for surface expression of HLA-DR (A), CD80 (B), and CD86 (C). Left panels plot median fluorescent intensity for HLA-DR+ cells (APCs) over time. ▲ and ● are two different donors. The center panels are representative plots of an untreated sample at 24 hours, and the right panels are representative plots of Mirasol treated samples at 24 hours. Both the center and right panels are ungated populations.
Mirasol treated APCs have reduced surface expression of adhesion molecules
Adhesion molecules are important mediators of T cell activation, both through enabling stable cell-cell interactions and providing costimulatory signals. Different wavelengths and doses of UV have been shown to have varying affects on adhesion molecule expression in a number of cell types.19,22–25,27,29,30 To determine the impact of Mirasol treatment on adhesion molecule expression on APCs, surface expression of ICAM-1, ICAM-2, ICAM-3, and LFA-3 was assessed over a 24 hour period by flow cytometry on thawed treated or untreated cells.
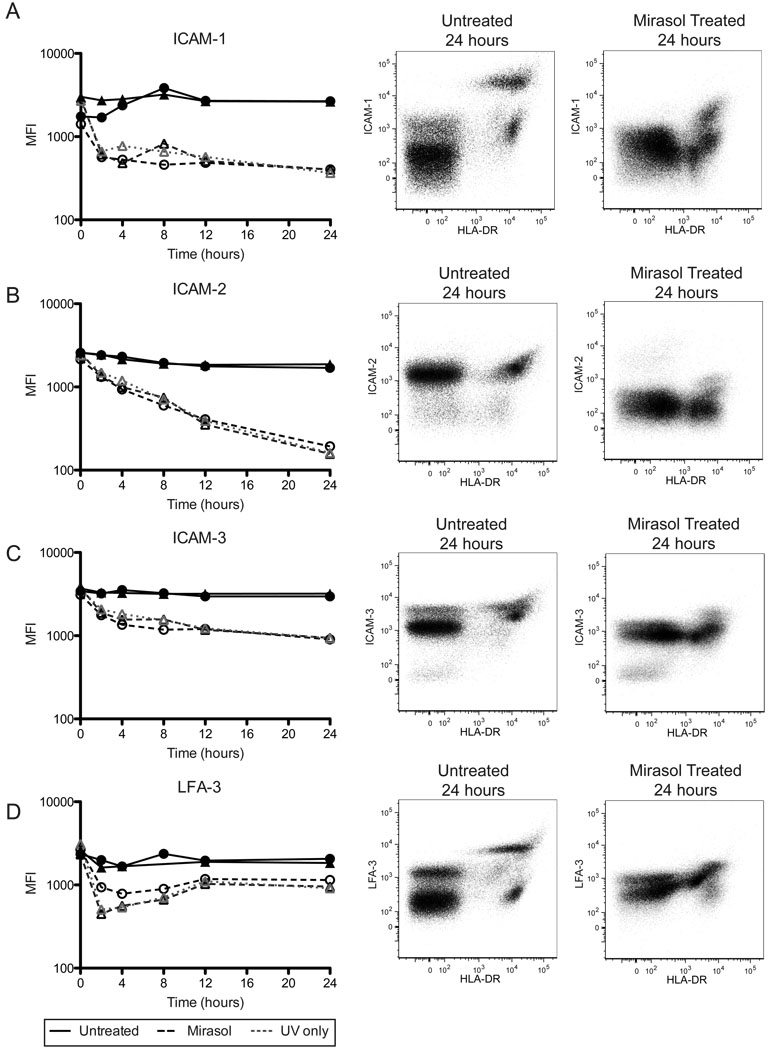
Surface expression of ICAM-1 (CD54) dropped rapidly over the first two hours in Mirasol or UV only treated APCs, then stabilized (Figure 4A, p< 0.0001 Mirasol vs. untreated cells at 24 hours). ICAM-2 (CD102) levels on the cell surface dropped steadily in Mirasol and UV only treated APCs to reach a log drop in MFI by 24 hours (Figure 4B, p=0.0031 Mirasol vs. untreated cells). The MFIs for treated cells at 24 hours were roughly equal to unstained cells (data not shown), suggesting little to no surface ICAM-2 on these cells. HLA-DR− PBMCs also expressed ICAM-2 at levels similar to HLA-DR+ cells, and surface expression on these non-APCs was similarly affected by treatment (Figure 4B, center and right panels).
Figure 4. Mirasol treated APCs have reduced surface adhesion molecule expression.
Cells that were untreated, Mirasol treated, or given a modified treatment without riboflavin (UV only), were cultured for 0, 2, 4, 8, 12, or 24 hours, then stained for surface expression of ICAM-1 (A), ICAM-2 (B), ICAM-3 (C), and LFA-3 (D). Left panels plot median fluorescent intensity for HLA-DR+ cells (APCs) over time. ▲ and ● are two different donors. The center panels are representative plots of an untreated sample at 24 hours, and the right panels are representative plots of Mirasol treated samples at 24 hours. Both the center and right panels are ungated populations.
Surface expression of ICAM-3 (CD50) on Mirasol or UV only treated APCs dropped to levels roughly equivalent to HLA-DR− PBMCs, with most of the loss occurring in the first 4 hours (Figure 4C, p=0.0030 Mirasol vs. untreated cells at 24 hours). Expression of ICAM-3 on the HLA-DR− PBMCs did not appear to be as affected by treatment, with similar levels at 24 hours seen in the bulk of the treated and untreated cells. There was, however, a small HLA-DR− population expressing higher levels of ICAM-3 in the untreated group that was lost in the treated groups by four hours (Figure 4C, center and right panels and data not shown).
LFA-3 (CD58) surface expression was also impacted by treatment (Figure 4D). Untreated APCs had both high and low expressing populations at 24 hours, but in the Mirasol and UV only treated APCs these populations were harder to distinguish, with the high population expressing lower levels, and the low population expressing slightly increased levels. Overall, there was a decrease in MFI associated with Mirasol treatment compared with untreated (p=0.0254 at 24 hours). A similar, though less drastic shift was seen in the HLA-DR− populations (Figure 4D, center and right panels).
Changes in surface expression of these adhesion molecules were also examined in fresh treated and untreated cells from an additional donor. Data collected from this fresh sample followed the same trends seen with the frozen samples.
Mirasol treated cells are defective in cell-cell conjugation
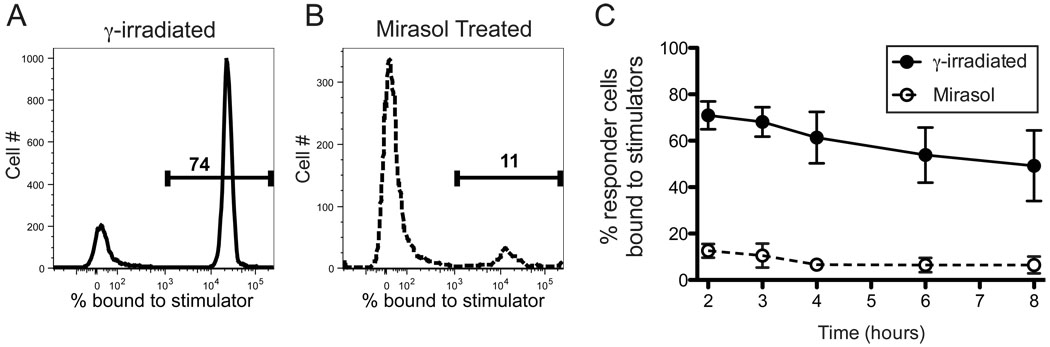
As shown in Figure 4, Mirasol treatment had a drastic effect on the expression of many of the key adhesion molecules involved in APC-T cell interactions, suggesting that an inability to form stable contacts with allo-reactive cells might be responsible for the defect observed in allo-specific proliferation. This is further supported by our observation that priming allogeneic cells with Mirasol treated cells did not enhance or depress subsequent challenge with treated or untreated cells from the same donor, suggesting that the responding cells may have been unaware of this initial exposure. To assess the impact of Mirasol treatment on the ability to form stable cell-cell contacts, Mirasol treated or γ-irradiated cells were labeled and co-cultured at high concentration in 96-well round-bottom plates with normal allogeneic PBMCs labeled with a different colored dye and tracked by flow cytometry over time. Unfortunately, because the frequency of the allo-reactive cells was low, and background was high, it was difficult to identify the conjugates. To address this we first enriched for allo-specific cells by priming the responder cells with γ-irradiated cells, then challenged these cells in the conjugation assay with Mirasol treated or γ-irradiated cells from this same donor. Co-cultures were set up at a ratio of 10:1 stimulators:responders, and samples were harvested at 2, 3, 4, 6, and 8 hours after setup. In parallel assays, a chase of unlabeled responders was added after 2 hours to address stability of interactions, and these samples were indistinguishable from those that received no chase (data not shown). Conjugation was quantified by gating on the labeled responder population and determining the percentage of these events that included a stimulator cell (Figure 5A–B). Mirasol treated cells displayed a severe defect in conjugation as compared with γ-irradiated cells, with 7 to 10-fold fewer stimulator bound responders at all time points (Figure 5C, p≤0.0001).
Figure 5. Cell conjugation is defective in Mirasol treated cells.
Normal PBMCs were primed with γ-irradiated cells to enrich for allo-specific cells. These responder cells were labeled with PKH red, and cultured with stimulators stained with PKH green. Stimulators were either γ-irradiated or Mirasol treated cells from the same donor as the priming cells. Representative sample plots (A–B) from t=2 hours show cells gated on red responder events, with the green stimulator events plotted as histograms. The markers denote the percentage of responders bound to stimulators. Four replicate samples were tested longitudinally from 2 – 8 hours (C). A minimum of 4,000 red events were collected for each sample. Error bars represent 95% confidence intervals.
Mirasol treated cells fail to effectively stimulate allogeneic cells
Although there was a severe defect in conjugation in Mirasol treated cells, a small number of double positive events were observed. This could be the result of background cross-linking during the cell fixation process, but it is also possible that this represented productive conjugation at a reduced level. If productive conjugates formed, it is possible that some level of activation could occur, even in the absence of proliferation. To explore this possibility, we assayed other indicators of T cell activation in allo-enriched PBMCs stimulated with Mirasol treated cells.
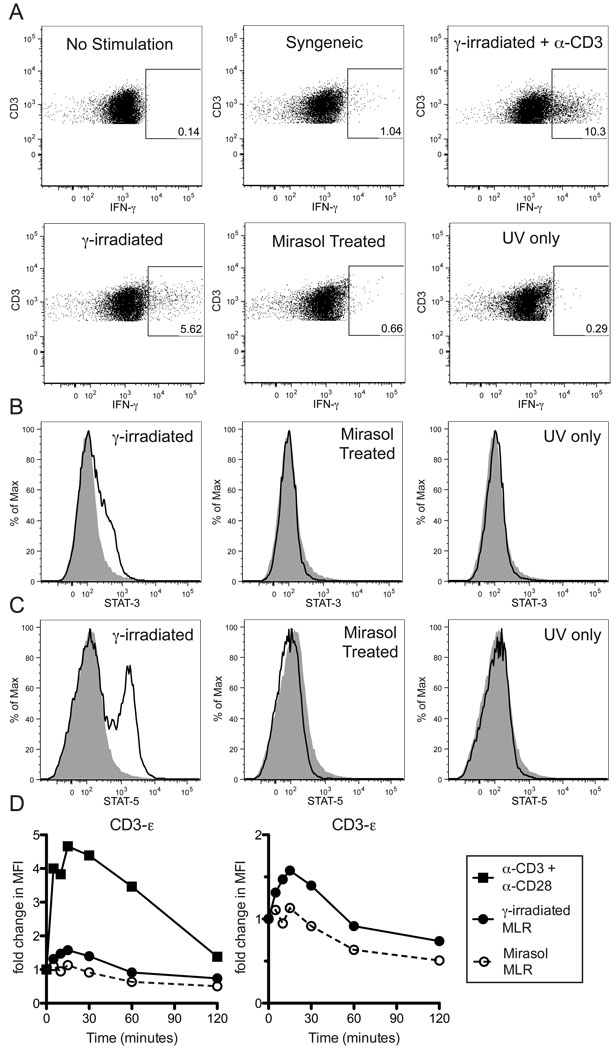
Allo-enriched PBMCs were restimulated with γ-irradiated, Mirasol treated, or UV only treated PBMCs, stained for intracellular IFN-γ and examined by flow cytometry. Allo-enriched PBMCs alone or restimulated with syngeneic γ-irradiated cells were included as negative controls, and allo-enriched PBMCs restimulated with γ-irradiated allogeneic PBMCs with anti-CD3 antibody were used as a positive control. Vigorous IFN-γ responses were detected using γ-irradiated allogeneic stimulator cells (mean 6.29% IFN-γ+), compared to barely detectable responses using the Mirasol and UV only treated allogeneic stimulator cells (0.60% and 0.20% IFN-γ+, respectively, p<0.05 compared to γ-irradiated, Figure 6A). The control cells stimulated with syngeneic γ-irradiated cells consistently had low-level IFN-γ responses that exceeded what was observed with Mirasol and UV only treated stimulator cells, or with responders alone, though the differences between unstimulated, syngeneic stimulated, Mirasol stimulated, and UV only treated stimulated were not statistically significant. The background stimulation by syngeneic cells may be due to presentation of components of the fetal bovine serum used in the media along with increased cell concentration compared with responders alone.
Figure 6. Mirasol treated are cells unable to activate untreated T cells.
Normal PBMCs were primed with γ-irradiated cells to enrich for allo-specific cells. These primed cells were labeled with CFSE, then restimulated overnight with γ-irradiated, Mirasol treated, or UV only treated allogeneic cells from the same donor as the priming cells. Additional groups that were either unstimulated, or stimulated with syngeneic cells or γ-irradiated allogeneic cells + α-CD3 antibodies were included as controls. CFSE+CD3+ gates were used to identify responder T cells (A). Gates are drawn around IFN-γ+ T cells based on unstimulated control and confirmed with isotype control stains. A minimum of 8,000 T cells were collected for each sample. This experiment was repeated three times with representative data displayed. In B–C the primed cells were restimulated with labeled cells (from the same donor as the priming cells) that were either γ-irradiated, Mirasol treated, or UV only treated for two hours, then stained intracellularly for STAT-3, STAT-5, and CD3. Stains of responder T cells for STAT-3 (B) and STAT-5 (C) are plotted with the unstimulated control stain shown in grey for comparison. In D, normal PBMCs were stimulated with anti-CD3 + anti-CD28 antibodies ■, and primed cells were restimulated with γ-irradiated cells ●, or Mirasol treated cells ○ (from the same donor as the priming cells), for 0, 5, 10, 15, 30, 60, or 120 minutes, lysed, and examined for the phosphorylation of CD3-ε using a multiplex bead-based kit. Fold change over t=0 is plotted over time. The left panel shows all three stimulation conditions, the right panel shows only the two MLR stimulations. Experiments in B–D were repeated twice with representative data displayed.
To look at early indicators of T cell activation, phosphorylation of STAT-3 and STAT-5 was assessed by PhosFlow. Allo-enriched PBMCs were restimulated with γ-irradiated, Mirasol treated, or UV only treated PBMCs and compared with unstimulated cells for the presence of phosphorylated STAT-3 and 5. Levels of both phosphorylated STAT-3 (Figure 6B) and phosphorylated STAT-5 (Figure 6C) were increased in allo-enriched cells stimulated with γ-irradiated cells, but no increase was seen in allo-enriched cells stimulated with Mirasol or UV only treated PBMCs.
To look for signs of activation further upstream, phosphorylation of the CD3-ε chain was accessed. Allo-enriched PBMCs were restimulated with γ-irradiated or Mirasol treated cells. As a positive control, normal PBMCs were stimulated with anti-CD3 and anti-CD28 antibodies. Cell lysates were assayed using a multiplex bead-based kit, and MFIs were normalized to baseline readings. Increased phosphorylation of the CD3-ε chain was observed in allo-enriched cells stimulated with γ-irradiated cells that peaked at 15 minutes (Figure 6D). This response was weaker than what was observed with antibody stimulation, but followed similar kinetics and was repeatable. In contrast, stimulation of allo-enriched cells with Mirasol treated cells produced no increase in CD3-ε chain phosphorylation.
DISCUSSION
In this study we have demonstrated a number of defects in Mirasol treated APCs that may contribute to the observed loss of immunogenicity. Treated APCs have reduced surface expression of a number of surface receptors critical for T cell activation and adhesion, including HLA-DR, CD80, CD86, ICAM-1, ICAM-2, ICAM-3 and LFA-3. The loss of surface adhesion molecule expression in treated cells corresponded with severe defects in cell-cell conjugation. Treated cells also died rapidly, with less than 20% viability by 24 hours, and close to 0% by 72 hours, a faster rate than what was observed after γ-irradiation. In addition to confirming that Mirasol treated cells are unable to stimulate allogeneic proliferation, we found them able to stimulate only very weak IFN-γ production when using primed allo-specific cells. This weak response did not result in further T cell activation, as evidenced by lack of phosphorylation of STAT-3 and STAT-5, and even extended as far upstream as a lack of detectable CD3-ε phosphorylation. These data, along with our failure to detect any signs of tolerance induction or inhibition in vitro, suggest that Mirasol treated cells fail to stimulate allogeneic stimulation due to an inability to productively interact with cells around them.
Our results show that Mirasol inactivation of donor WBCs is independent of riboflavin addition, as cells given UV alone also failed to stimulate proliferation and had equivalent loss of viability and surface receptor expression. Though not needed for WBC inactivation, the addition of riboflavin does play an important role in ensuring irreversible nucleotide damage in certain pathogens and also appears to be important in maintaining platelet quality during storage following UV exposure.31–33
While we believe the defects observed in cell conjugation are primarily the result of decreased surface adhesion molecule expression, other factors may contribute. Short wave UV light has been shown to polymerize lipids in artificial lipid bilayers leading to reduced membrane fluidity.34,35 Electron microscopy images of UVB irradiated mouse T cell hybridoma cells show a loss of membrane microvilli and damage to organelle structure, suggesting gross physical disruption of the cell.28 This, in combination with potential protein cross-linking, may make it difficult for the remaining surface adhesion receptors to move within the membrane to sites of contact with other cells to stabilize interactions. The increased rate of cell death (as measured by failure to exclude dye) could also contribute to the reduced immunogenicity of the treated cells. This does not provide a very satisfactory explanation, however, as “killed” cells, whether mitomycin C treated or irradiated, are typically used in T cell stimulations, and lipid bilayers loaded with ICAM-1 and class II MHC36 or simple antibody cross-linking of CD3 and CD28 is sufficient to stimulate proliferation T cells37.
The mechanisms involved in regulating surface expression of adhesion molecules following UV exposure have yet to be identified, but possible explanations extend beyond DNA damage, as UV can initiate a number of cellular changes. UV exposure has been shown to alter membrane potential, produce reactive oxygen species, and initiate signal cascades starting with activation of Src tyrosine kinases, involving H-Ras and Raf-1, and resulting in NF-κB and AP-1 activation.38–40 This observed NF-κB and AP-1 activation has been shown to be independent of DNA damage but dependent on the cell membrane.41,42 Effects on the cell membrane may be mediated in part by direct action on membrane lipids, as described above.
The inactivation of WBCs by Mirasol may have important clinical implications. Currently, leukoreduction and γ-irradiation are used to minimize the risks associated with transfer of foreign WBCs into immunocompromised hosts. In addition, an alternate pathogen reduction method using UVA treatment with added psoralens has been shown to inhibit leukocyte proliferation and IL-8 production in vitro and effectively block TA-GVHD in mice.43,44 Leukoreduction reduces the number of WBCs by 2–3 logs45, which reduces but does not eliminate the risk of TA-GVHD, as cases have been reported in recipients of leukoreduced units.46–48 The WBCs remaining after leukoreduction appear to be capable of expansion in the host, as recipients of leukoreduced blood are equally likely to develop transfusion-associated microchimerism as those receiving non-reduced blood.49 γ-irradiation at doses of 2500–3000 cGy effectively blocks proliferation of donor WBCs as measured by limiting dilution analysis and has been effective in prevention of TA-GVHD.50,51 Even so, these γ-irradiated WBCs can survive for a while without division. We have found lingering viable T cells in culture 2 weeks after γ-irradiation with 3000 cGy (unpublished observations), and in a murine model of transfusion, transferred cells γ-irradiated with 2500 cGy lasted just as long in the recipient as untreated cells.52
Neither leukoreduction nor γ-irradiation removes the risk of allo-sensitization, and the low-level presence of viable cells may have additional immunomodulatory effects. We have shown here that Mirasol treated WBCs die rapidly, much faster than after γ-irradiation with 3000 cGy, with most cells dead at 24 hours and almost complete cell death by 72 hours. Furthermore, we have demonstrated that the Mirasol treated cells are not capable of stimulating or even binding to allo-specific primed cells. The lack of functional APCs in treated products could be useful for patients receiving multiple transfusions, transplant recipients, or others for whom allo-sensitization is a concern. Earlier studies evaluating the effect of UVB irradiating platelets prior to transfusion have shown a reduction, but not elimination of allosensitization.6,8 The failure to completely eliminate allosensitization may be due to the red cell transfusions these patients received in addition to the platelets. The red cell units were filtered, but still contained low levels of untreated white blood cells (up to 5×106/unit).
Further work in vivo is still required to fully understand the immunogenicity of Mirasol treated blood products, as additional indirect mechanisms may come into play in the host. Dead and dying cells or cellular fragments from treated blood could be taken up by host APCs and presented to host T cells, which could result in indirect allo-sensitization. Alternatively, antigen from treated cells presented in this context could initiate immune tolerance or suppression, as is seen in extracorporeal photochemotherapy. We did not see any evidence of direct tolerance induction or immune suppression by Mirasol treated cells in vitro, but this does not rule out indirect initiation of these pathways in vivo by transferred cells. A third possibility, however, is that the damage to membrane structure and loss of surface adhesion molecule expression from the UVB component of the Mirasol treatment will lead to impaired uptake by host APCs along with the observed defect in direct cell-cell interactions. If this is the case, treatment could make donor WBCs essentially invisible to the host immune system, with important implications for allosensitization in transfusion recipients.
ACKNOWLEDGEMENTS
Brian Custer was consulted on the appropriateness of statistical analyses used. Dale Hirschkorn provided assistance with flow cytometry. Kirk Weimer provided assistance with Mirasol illuminator calibration. This study was supported by research funding from CaridianBCT Biotechnologies to P.J.N. and NIH grant HL-083388.
Footnotes
Conflict of Interest: Rachael P. Jackman and John W. Heitman have no conflict of interest. Susanne Marschner and Raymond P. Goodrich are employed by CaridianBCT Biotechnologies, Philip J. Norris has a consulting relationship with and received research funding for this project from CaridianBCT Biotechnologies.
REFERENCES
- 1.Goodrich RP, Edrich RA, Li J, Seghatchian J. The Mirasol PRT system for pathogen reduction of platelets and plasma: an overview of current status and future trends. Transfus Apher Sci. 2006;35:5–17. doi: 10.1016/j.transci.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Cardo LJ, Rentas FJ, Ketchum L, Salata J, Harman R, Melvin W, Weina PJ, Mendez J, Reddy H, Goodrich R. Pathogen inactivation of Leishmania donovani infantum in plasma and platelet concentrates using riboflavin and ultraviolet light. Vox Sang. 2006;90:85–91. doi: 10.1111/j.1423-0410.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 3.Cardo LJ, Salata J, Mendez J, Reddy H, Goodrich R. Pathogen inactivation of Trypanosoma cruzi in plasma and platelet concentrates using riboflavin and ultraviolet light. Transfus Apher Sci. 2007;37:131–137. doi: 10.1016/j.transci.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Rentas F, Harman R, Gomez C, Salata J, Childs J, Silva T, Lippert L, Montgomery J, Richards A, Chan C, Jiang J, Reddy H, Li J, Goodrich R. Inactivation of Orientia tsutsugamushi in red blood cells, plasma, and platelets with riboflavin and light, as demonstrated in an animal model. Transfusion. 2007;47:240–247. doi: 10.1111/j.1537-2995.2007.01094.x. [DOI] [PubMed] [Google Scholar]
- 5.Fast LD, Dileone G, Li J, Goodrich R. Functional inactivation of white blood cells by Mirasol treatment. Transfusion. 2006;46:642–648. doi: 10.1111/j.1537-2995.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 6.Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. The Trial to Reduce Alloimmunization to Platelets Study Group. N Engl J Med. 1997;337:1861–1869. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 7.Blundell EL, Pamphilon DH, Fraser ID, Menitove JE, Greenwalt TJ, Snyder EL, Repucci AJ, Hedberg SL, Anderson JK, Buchholz DH, Kagen LR, Aster RH. A prospective, randomized study of the use of platelet concentrates irradiated with ultraviolet-B light in patients with hematologic malignancy. Transfusion. 1996;36:296–302. doi: 10.1046/j.1537-2995.1996.36496226140.x. [DOI] [PubMed] [Google Scholar]
- 8.Slichter SJ, Davis K, Enright H, Braine H, Gernsheimer T, Kao KJ, Kickler T, Lee E, McFarland J, McCullough J, Rodey G, Schiffer CA, Woodson R. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105:4106–4114. doi: 10.1182/blood-2003-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 10.Leitenberger J, Jacobe HT, Cruz PD., Jr Photoimmunology--illuminating the immune system through photobiology. Semin Immunopathol. 2007;29:65–70. doi: 10.1007/s00281-007-0063-6. [DOI] [PubMed] [Google Scholar]
- 11.Babic AM. Extracorporeal photopheresis: Lighting the way to immunomodulation. Am J Hematol. 2008;83:589–591. doi: 10.1002/ajh.21166. [DOI] [PubMed] [Google Scholar]
- 12.Knobler R. Extracorporeal photochemotherapy--present and future. Vox Sang. 2000;78 Suppl 2:197–201. [PubMed] [Google Scholar]
- 13.Oliven A, Shechter Y. Extracorporeal photopheresis: a review. Blood Rev. 2001;15:103–108. doi: 10.1054/blre.2001.0155. [DOI] [PubMed] [Google Scholar]
- 14.Russo GG, Mullen C. Cutaneous and noncutaneous disorders treated with extracorporeal photopheresis. Int J Dermatol. 2001;40:89–100. doi: 10.1046/j.1365-4362.2001.01182.x. [DOI] [PubMed] [Google Scholar]
- 15.Jakway JP, Shevach EM. Stimulation of T-cell activation by UV-treated, antigen-pulsed macrophages: evidence for a requirement for antigen processing and interleukin 1 secretion. Cell Immunol. 1983;80:151–162. doi: 10.1016/0008-8749(83)90102-8. [DOI] [PubMed] [Google Scholar]
- 16.Lindahl-Kiessling K, Safwenberg J. Inability of UV-irradiated lymphocytes to stimulate allogeneic cells in mixed lymphocyte culture. Int Arch Allergy Appl Immunol. 1971;41:670–678. doi: 10.1159/000230559. [DOI] [PubMed] [Google Scholar]
- 17.Rich EA, Elmets CA, Fujiwara H, Wallis RS, Ellner JJ. Deleterious effect of ultraviolet-B radiation on accessory function of human blood adherent mononuclear cells. Clin Exp Immunol. 1987;70:116–126. [PMC free article] [PubMed] [Google Scholar]
- 18.Deeg HJ, Sigaroudinia M. Ultraviolet B-induced loss of HLA class II antigen expression on lymphocytes is dose, time, and locus dependent. Exp Hematol. 1990;18:916–919. [PubMed] [Google Scholar]
- 19.Fujihara M, Takahashi TA, Azuma M, Ogiso C, Maekawa TL, Yagita H, Okumura K, Sekiguchi S. Decreased inducible expression of CD80 and CD86 in human monocytes after ultraviolet-B irradiation: its involvement in inactivation of allogenecity. Blood. 1996;87:2386–2393. [PubMed] [Google Scholar]
- 20.Hanlon DJ, Berger CL, Edelson RL. Photoactivated 8-methoxypsoralen treatment causes a peptide-dependent increase in antigen display by transformed lymphocytes. Int J Cancer. 1998;78:70–75. doi: 10.1002/(sici)1097-0215(19980925)78:1<70::aid-ijc12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Young JW, Baggers J, Soergel SA. High-dose UV-B radiation alters human dendritic cell costimulatory activity but does not allow dendritic cells to tolerize T lymphocytes to alloantigen in vitro. Blood. 1993;81:2987–2997. [PubMed] [Google Scholar]
- 22.De Luca DJ, Trefzer U, Tubesing KA, Elmets CA. ICAM-1 mRNA levels and relative transcription rates are decreased by UV irradiation of monocytes. Photochem Photobiol. 1997;65:609–615. doi: 10.1111/j.1751-1097.1997.tb01901.x. [DOI] [PubMed] [Google Scholar]
- 23.Kobata T, Ikeda H, Ohnishi Y, Urushibara N, Nakata SO, Takahashi TA, Sekiguchi S. Ultraviolet irradiation inhibits killer-target cell interaction. Vox Sang. 1993;65:25–31. doi: 10.1111/j.1423-0410.1993.tb04520.x. [DOI] [PubMed] [Google Scholar]
- 24.Krutmann J, Czech W, Parlow F, Trefzer U, Kapp A, Schopf E, Luger TA. Ultraviolet radiation effects on human keratinocyte ICAM-1 expression: UV-induced inhibition of cytokine-induced ICAM-1 mRNA expression is transient, differentially restored for IFN gamma versus TNF alpha, and followed by ICAM-1 induction via a TNF alpha-like pathway. J Invest Dermatol. 1992;98:923–928. doi: 10.1111/1523-1747.ep12460737. [DOI] [PubMed] [Google Scholar]
- 25.Krutmann J, Khan IU, Wallis RS, Zhang F, Rich EA, Ellner JJ, Elmets CA. Cell membrane is a major locus for ultraviolet B-induced alterations in accessory cells. J Clin Invest. 1990;85:1529–1536. doi: 10.1172/JCI114600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krutmann J, Trefzer U. Modulation of the expression of intercellular adhesion molecule-1 (ICAM-1) in human keratinocytes by ultraviolet (UV) radiation. Springer Semin Immunopathol. 1992;13:333–344. doi: 10.1007/BF00200532. [DOI] [PubMed] [Google Scholar]
- 27.Meineke V, Moede T, Gilbertz KP, Mayerhofer A, Ring J, Kohn FM, Van Beuningen D. Protein kinase inhibitors modulate time-dependent effects of UV and ionizing irradiation on ICAM-1 expression on human hepatoma cells. Int J Radiat Biol. 2002;78:577–583. doi: 10.1080/09553000110113056. [DOI] [PubMed] [Google Scholar]
- 28.Maekawa TL, Takahashi TA, Fujihara M, Akasaka J, Fujikawa S, Minami M, Sekiguchi S. Effects of ultraviolet B irradiation on cell-cell interaction; implication of morphological changes and actin filaments in irradiated cells. Clin Exp Immunol. 1996;105:389–396. doi: 10.1046/j.1365-2249.1996.d01-760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Legitimo A, Consolini R, Di Stefano R, Bencivelli W, Mosca F. Psoralen and UVA light: an in vitro investigation of multiple immunological mechanisms underlying the immunosuppression induction in allograft rejection. Blood Cells Mol Dis. 2002;29:24–34. doi: 10.1006/bcmd.2002.0533. [DOI] [PubMed] [Google Scholar]
- 30.Tronnier M, Alexander M, Wolff HH. Adhesion molecule expression in normal skin and melanocytic lesions. Role of UV-irradiation and architectural characteristics in nevi. J Cutan Pathol. 1997;24:278–285. doi: 10.1111/j.1600-0560.1997.tb00792.x. [DOI] [PubMed] [Google Scholar]
- 31.Kumar V, Lockerbie O, Keil SD, Ruane PH, Platz MS, Martin CB, Ravanat JL, Cadet J, Goodrich RP. Riboflavin and UV-light based pathogen reduction: extent and consequence of DNA damage at the molecular level. Photochem Photobiol. 2004;80:15–21. doi: 10.1562/2003-12-23-RA-036.1. [DOI] [PubMed] [Google Scholar]
- 32.Martin CB, Wilfong E, Ruane P, Goodrich R, Platz M. An action spectrum of the riboflavin-photosensitized inactivation of Lambda phage. Photochem Photobiol. 2005;81:474–480. doi: 10.1562/2004-08-25-RA-292. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Lockerbie O, de Korte D, Rice J, McLean R, Goodrich RP. Evaluation of platelet mitochondria integrity after treatment with Mirasol pathogen reduction technology. Transfusion. 2005;45:920–926. doi: 10.1111/j.1537-2995.2005.04381.x. [DOI] [PubMed] [Google Scholar]
- 34.Morigaki K, Kiyosue K, Taguchi T. Micropatterned composite membranes of polymerized and fluid lipid bilayers. Langmuir. 2004;20:7729–7735. doi: 10.1021/la049340e. [DOI] [PubMed] [Google Scholar]
- 35.Okazaki T, Inaba T, Tatsu Y, Tero R, Urisu T, Morigaki K. Polymerized lipid bilayers on a solid substrate: morphologies and obstruction of lateral diffusion. Langmuir. 2009;25:345–351. doi: 10.1021/la802670t. [DOI] [PubMed] [Google Scholar]
- 36.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 37.June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990;11:211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 38.Devary Y, Gottlieb RA, Lau LF, Karin M. Rapid and preferential activation of the c-jun gene during the mammalian UV response. Mol Cell Biol. 1991;11:2804–2811. doi: 10.1128/mcb.11.5.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devary Y, Gottlieb RA, Smeal T, Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992;71:1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- 40.Ichiki H, Sakurada H, Kamo N, Takahashi TA, Sekiguchi S. Generation of active oxygens, cell deformation and membrane potential changes upon UV-B irradiation in human blood cells. Biol Pharm Bull. 1994;17:1065–1069. doi: 10.1248/bpb.17.1065. [DOI] [PubMed] [Google Scholar]
- 41.Devary Y, Rosette C, DiDonato JA, Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993;261:1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- 42.Simon MM, Aragane Y, Schwarz A, Luger TA, Schwarz T. UVB light induces nuclear factor kappa B (NF kappa B) activity independently from chromosomal DNA damage in cell-free cytosolic extracts. J Invest Dermatol. 1994;102:422–427. doi: 10.1111/1523-1747.ep12372194. [DOI] [PubMed] [Google Scholar]
- 43.Grass JA, Hei DJ, Metchette K, Cimino GD, Wiesehahn GP, Corash L, Lin L. Inactivation of leukocytes in platelet concentrates by photochemical treatment with psoralen plus UVA. Blood. 1998;91:2180–2188. [PubMed] [Google Scholar]
- 44.Grass JA, Wafa T, Reames A, Wages D, Corash L, Ferrara JL, Lin L. Prevention of transfusion-associated graft-versus-host disease by photochemical treatment. Blood. 1999;93:3140–3147. [PubMed] [Google Scholar]
- 45.Rebulla P, Bertolini F, Parravicini A, Sirchia G. Leukocyte-poor blood components: a purer and safer transfusion product for recipients? Transfus Med Rev. 1990;4:19–23. doi: 10.1016/s0887-7963(90)70238-6. [DOI] [PubMed] [Google Scholar]
- 46.Akahoshi M, Takanashi M, Masuda M, Yamashita H, Hidano A, Hasegawa K, Kasajima T, Shimizu M, Motoji T, Oshimi K, et al. A case of transfusion-associated graft-versus-host disease not prevented by white cell-reduction filters. Transfusion. 1992;32:169–172. doi: 10.1046/j.1537-2995.1992.32292180149.x. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi H, Nishiuchi T, Tamura H, Takeda K. Transfusion-associated graft-versus-host disease caused by leukocyte-filtered stored blood. Anesthesiology. 1993;79:1419–1421. doi: 10.1097/00000542-199312000-00034. [DOI] [PubMed] [Google Scholar]
- 48.Williamson LM, Stainsby D, Jones H, Love E, Chapman CE, Navarrete C, Lucas G, Beatty C, Casbard A, Cohen H. The impact of universal leukodepletion of the blood supply on hemovigilance reports of posttransfusion purpura and transfusion-associated graft-versus-host disease. Transfusion. 2007;47:1455–1467. doi: 10.1111/j.1537-2995.2007.01281.x. [DOI] [PubMed] [Google Scholar]
- 49.Reed W, Lee TH, Norris PJ, Utter GH, Busch MP. Transfusion-associated microchimerism: a new complication of blood transfusions in severely injured patients. Semin Hematol. 2007;44:24–31. doi: 10.1053/j.seminhematol.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Luban NL, Drothler D, Moroff G, Quinones R. Irradiation of platelet components: inhibition of lymphocyte proliferation assessed by limiting-dilution analysis. Transfusion. 2000;40:348–352. doi: 10.1046/j.1537-2995.2000.40030348.x. [DOI] [PubMed] [Google Scholar]
- 51.Pelszynski MM, Moroff G, Luban NL, Taylor BJ, Quinones RR. Effect of gamma irradiation of red blood cell units on T-cell inactivation as assessed by limiting dilution analysis: implications for preventing transfusion-associated graft-versus-host disease. Blood. 1994;83:1683–1689. [PubMed] [Google Scholar]
- 52.Lee TH, Reed W, Mangawang-Montalvo L, Watson J, Busch MP. Donor WBCs can persist and transiently mediate immunologic function in a murine transfusion model: effects of irradiation, storage, and histocompatibility. Transfusion. 2001;41:637–642. doi: 10.1046/j.1537-2995.2001.41050637.x. [DOI] [PubMed] [Google Scholar]