Abstract
Purpose
Toxoplasmosis gondii (T. gondii) is a common world-wide parasite that presents in the eye with focal retinochoriditis and vitritis. Although it is rare, ocular toxoplamosis has been linked to primary intraocular (retinal) lymphoma, which is mostly a diffuse large B-cell lymphoma.
Methods
An elderly female patient was treated for recurrent ocular toxoplasmosis, and because of progressive vitritis, a diagnostic vitrectomy was performed. Shortly afterwards she developed multiple brain lesions. Pathological examinations of the vitreous specimen and cerebral tissues were conducted including tests for T. gondii, EBV, and CMV DNA.
Results
The patient initially responded to anti-toxoplasmosis treatment, but continued to have persistent vitritis. She was diagnosed with primary intraocular lymphoma and a repeated MRI revealed cerebral lesions. Brain biopsy confirmed lymphoma. T. gondii DNA was found in malignant vitreous cells, but was absent in the non-malignant vitreous cells and brain lymphoma cells. CMV and EBV genes were not found in any of the lymphoma cells.
Conclusion
T. gondii may have played a role in lymphoproliferation and PIOL development.
Keywords: B-cell lymphoma, intraocular lymphoma, primary intraocular (retinal) lymphoma, primary CNS lymphoma, toxoplasmosis, Toxoplasmosis gondii
Summary Statement
A patient was initially diagnosed and treated for ocular toxoplasmosis, but subsequently developed PIOL and eventual CNS lymphoma. Toxoplasmosis DNA was found within the PIOL cells, but absent in the vitreal non-malignant cells and brain lymphoma cells. We speculate chronic stimulation of the immunological response to toxoplasmosis could be oncogenic.
Ocular toxoplasmosis, caused by the parasitic protozoan Toxoplasmosis gondii (T. gondii), commonly presents clinically with a localized area of retinitis and vitritis. Primary intraocular lymphoma (PIOL, or primary retinal lymphoma) is a frequent masquerader of uveitis, which can result in delayed diagnosis and appropriate treatment. Two-thirds of PIOL patients have undiagnosed CNS involvement and the average survival time is 22.5 months.1 In this study, we describe a patient who was initially treated for ocular toxoplasmosis, subsequently developed PIOL with CNS involvement, and later died. Toxoplasmosis DNA was found within the malignant PIOL cells, but absent in the brain lymphoma cells and non-malignant vitreous cells.
Case Report
A 79 year-old female presented in December 2006 with a two month history of foggy vision more in the right than left eye, without systemic symptoms and was on no medications. Past history included uneventful bilateral cataract surgery in 1990. Ophthalmic examination revealed visual acuity of 20/25 in both eyes (OU), normal cornea and conjunctiva, trace cell and flare in the anterior chamber OU, and 1–2+ vitreal cells OU. The right eye (OD) had a focal area of retinitis with surrounding pigmentary changes in the superior equatorial retina and macular pigmentary changes without edema. The left eye (OS) was unremarkable except for myelinated nerve fibers near the superior arcade.
Laboratory analyses included toxoplasmosis IgG elevated at 10.7 (negative IgM), CMV IgG elevated at 2.26 (negative IgM), EB viral IgG > 5.00, EBV nuclear antigen IgG elevated at 1.93 (normal IgM), nonreactive FTA, and negative serologies for Lyme, bartonella henselae and quintana, and normal values for ANCA, ACE, lysozyme, CBC, Westegren sedimentation rate, and unremarkable CT of the chest. MRI with and without contrast material of the brain and orbits was normal. A bone marrow aspiration and biopsy did not find lymphoma.
Based on the elevated toxoplasmosis IgG titers, she was treated with trimethoprim 160 mg and sulfamethoxazole 800 mg (SEPTRA) twice a day for presumed ocular toxoplasmosis. The focal retinitis OD resolved with some decrease in the vitreous cells OU over several weeks; Septra was stopped in April 2007. In June 2007, the patient noticed increased floaters OD and restarted on Septra. In August 2007, she presented with vision of 20/25 OD, 20/20 OS, 1–2+ vitreal cells OU, and old inactive chorioretinal scarring in superior midperiphery OD. Septra was discontinued in October. Two months later (December 2007), the patient noticed blurry vision OD and restarted again on Septra. By February 2008, the vision was 20/40 OD, 20/25 OS; ophthalmic examination disclosed more vitreal cells OD than OS, but no active chorioretinal lesions OU. The patient had no systemic symptoms. A diagnostic vitrectomy OD was performed and a diagnosis of PIOL was made.
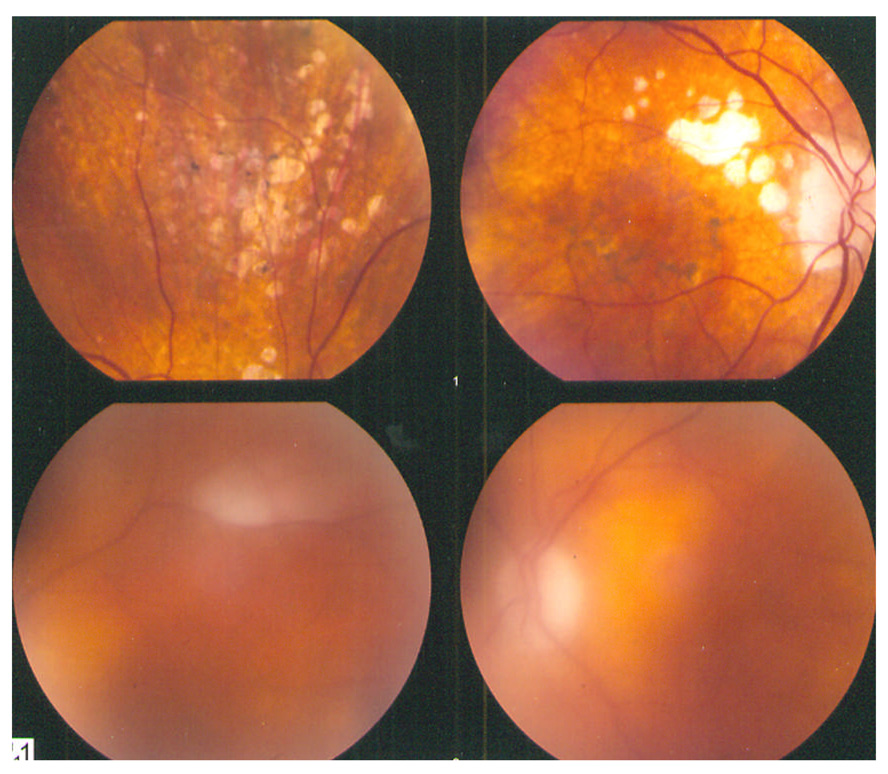
The patient was referred to a neurooncologist. A repeated brain MRI documented frontal lobe and basal ganglia lesions. A brain biopsy was performed and confirmed CNS B-cell lymphoma. In May 2008, the visual acuity was 20/25 OU, trace cells and flare in both anterior chambers, trace cells in the vitreous OD, 2+ cells in the OS, and macular pigmentary changes and an inactive chorioretinal lesion in the area of the prior retinitis in the superior mid-periphery OD (Figure 1: upper panel – OD; lower panel - OS). The patient did not respond to systemic chemotherapy and died shortly thereafter; no autopsy was performed.
Figure 1.
Fundus images (March 2008): OD – Inactive chorioretinal lesions and pigmentary changes in the superior mid-periphery. OS – a myelinated nerve fiber layer near the superior arcade.
Pathology Findings
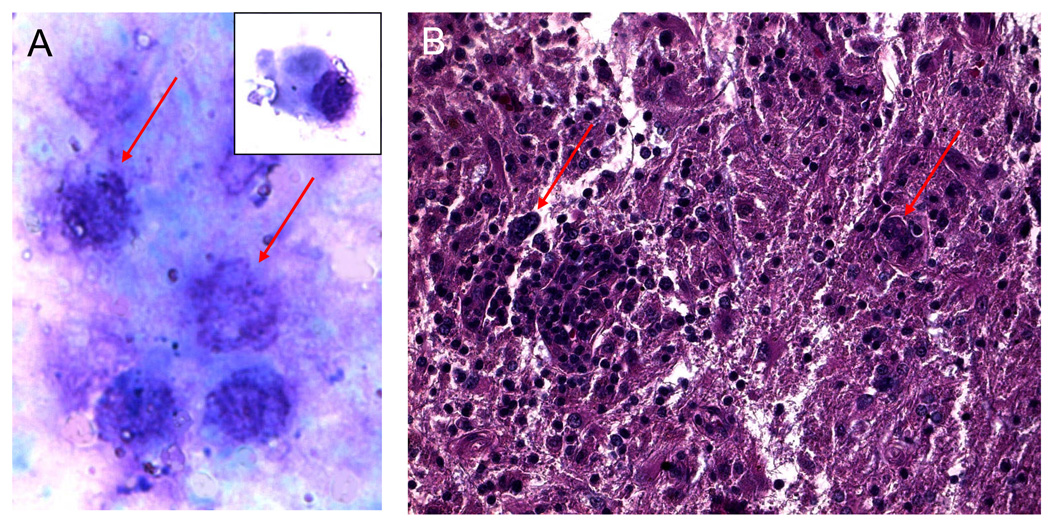
Cytological analysis of the vitrectomy specimen revealed several large atypical cells, with large nuclei, multiple nucleole, and scanty basophilic cytoplasm (Figure 2A). The brain biopsy disclosed infiltration by many enlarged atypical CD20+ cells with prominent nuclei and multiple neucleoli, consistent with large B-cell lymphoma (Figure 2B).
Figure 2.
A. Cytology of vitreous cells revealing reactive lymphocytes, plasma cell (insert), and large maligant lymphoma cells identifiable by their prominent nuclei, multiple nucleoli, and scanty basophilic cytoplasm. (Giemsa, original magnification, ×640)
B. Histology of brain biopsy showing large atypical lymphoid cells (arrows) infiltrated the CNS. (hematoxylin & eosin, original magnification, ×200)
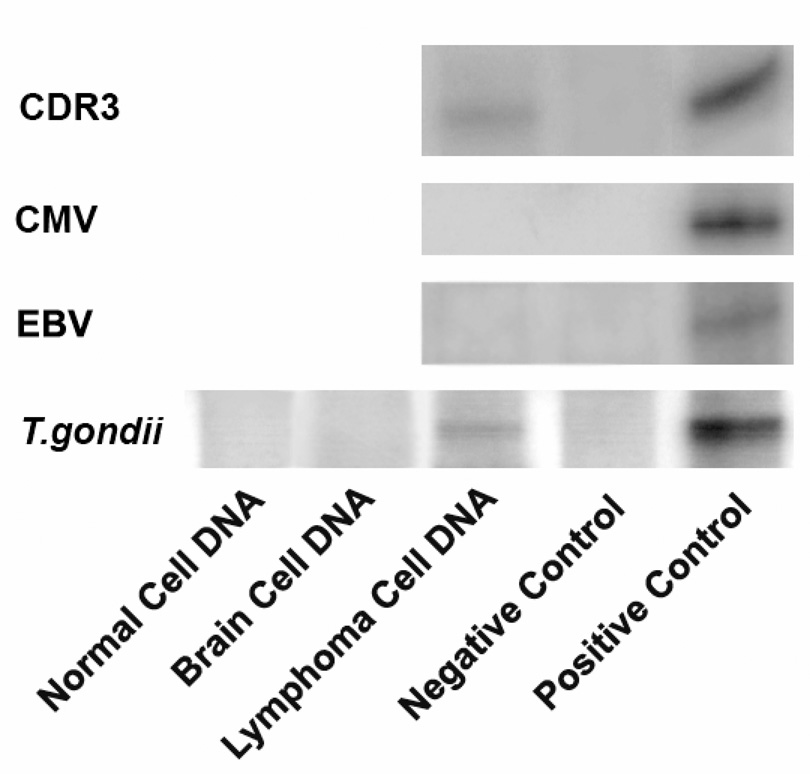
IgH gene rearrangement was detected in the vitreous atypical cells using microdissection and polymerase chain reaction (PCR) technique (Figure 3).2 Additionally, interleukin (IL)-10 to IL-6 ratio was greater than 1.0 in the vitreous.
Figure 3.
Polymerase chain reaction (PCR) amplification of DNA and chemiluminescent Southern blot from patient's non-malignant and lymphoma cells in the vitreous. Non-malignant and lymphoma cells were separated by microdissection.2 Rearrangement of the third complementarity determining region (CDR3) of the variable diversity joining site of heavy chain immunoglobulin (IgH) gene was used to detect IgH monoclonality. Detection of T. gondii DNA was carried out with T. gondii B1 gene-specific primers as reported.3 No EBV and CMV genes were detected.
Lymphoma and non-neoplastic cells were separated by microdissection to also analyze for T. gondii DNA, Epstein-Barr virus (EBV) and cytomegalovirus (CMV) as previously described.3 T. gondii DNA was detected in ocular lymphoma cells, but absent in non-neoplastic vitreous cells and CNS lymphoma cells (Figure 3). CMV and EBV were negative in both the lymphoma and non-neoplastic cells.
Discussion
The patient described in this report was treated medically for ocular toxoplasmosis because of active retinitis, uveitis, and a positive toxoplasmosis IgG titer. The initial evaluation for lymphoma including brain MRI imaging showed no lesions, however approximately one year later revealed multiple frontal and basal ganglia lesions.
Our initial impression was recurrent bilateral toxoplasmosis. Although we observed no active retinal lesions of toxoplasmosis OS, there are reported cases of ocular toxoplasmosis vitritis without apparent retinal lesions.3 However, the vitreal cells never completely resolved and eventually increased OU, we performed a vitrectomy OD, that found PIOL cells. A repeat MRI showed lesions and a brain biopsy confirmed B cell lymphoma. In retrospect, the focal retinitis and overlying vitreal cells OD may have represented early signs of PIOL or alternatively a combination of PIOL and an active toxoplasmosis lesion.
We previously reported the presence of T. gondii DNA in the malignant lymphoma vitreous cells of two patients with PIOL, but absent in their non-neoplastic vitreous cells.4 Due to this previous association and after the current pathological analysis revealed the patient had PIOL, we assessed the malignant vitreous cells for T. gondii DNA. A positive finding as well as a high serum T. gondii titer led us to speculate whether toxoplasmosis may have played a role in PIOL development, especially after T. gondii DNA was found to be absent in the non-malignant vitreous cells.
Many infectious and parasitic agents are known to be mutagenic in humans, however relatively few cases of T. gondii associated carcinogenesis have been published.5 Toxoplasma cysts have been reported among pituitary adenoma tumor cells and an Australian population study found a possible association of T. gondii infection with meningioma.6,7 C. parvum, a parasitic protozoa of the same family and order as T. gondii, was shown to cause adenocarinoma in the gut of immunocompromised mice.8 Although a pathological mechanism for toxoplasmosis carcinogenesis has not been proposed, T. gondii infection may cause chronic antigen stimulation of the immune system. This immunological response resulting from T. gondii infection has been shown to be genotoxic in mice, causing DNA damage by the generation of nitric oxide, γ-interferon, tumor necrosis factor-α (TNF-α), and reactive oxygen species (ROS).9 These cellular mechanisms are likely active during ocular toxoplasmosis inflammation and particularly in infected lymphoma cells.
Whether this genotoxicity could cause significant mutagenesis to induce a malignant lymphadenopathy is unknown, but the presence of T. gondii DNA in the ocular malignant cells of our patient and absence in non-malignant vitreous cells is intriguing. It is possible that genotoxicity from the immunological response to ocular T. gondii infection led to PIOL development. Therefore, the lymphoma cells may not actually contain T. gondii, but still test positive for T. gondii DNA because of their co-localization with the infected tissue. Consequently, the patient’s CNS lymphoma and cerebral lesions if metastasized from the PIOL cells in the eye would be negative for T. gondii as found in this case.
Nonetheless, an alternative hypothesis is that the proliferative lymphoma cells had a greater susceptibility to uptake T. gondii. T. gondii attachment to host cell receptors has been reported to be greatest during the mid-synthesis-(S) phase of the cell cycle.10 As a result, increased cell division and replication cycles could lead to increased T. gondii entry. However, cholesterol in the host cell membrane has been shown to play a necessary role in T. gondii entry into the host cell.11 Cholesterol has previously been reported to be at lower levels in malignant lymphoma cells than healthy lymphocytes.12
To determine whether the PIOL and CNS lymphomas were metastasis of each other, a gene sequencing test of IgH clonality could be performed, as previously reported in a case of “oculocerebral” lymphoma.13 However, an insufficient number of PIOL cells in the vitrectomy sample hampered the success of gene sequencing in the present case. While it is possible that toxoplasmosis is not a direct etiology in this PIOL case, the thorough patient history and molecular diagnosis of this case follows results of our previous association between the two pathologies,3 an association of which one should be aware.
Acknowledgments
The National Eye Institute Intramural Research Program provided the funding of the study.
References
- 1.Whitcup SM, de Smet MD, Rubin BI, et al. Intraocular lymphoma. Clinical and histopathologic diagnosis. Ophthalmology. 1993;100:1399–1406. doi: 10.1016/s0161-6420(93)31469-7. [DOI] [PubMed] [Google Scholar]
- 2.Shen DF, Zhuang Z, Le Hoang P, et al. Utility of microdissection and polymerase chain reaction for the detection of immunoglobulin gene rearrangement and translocation in primary intraocular lymphoma. Ophthalmology. 1998;105:1664–1669. doi: 10.1016/S0161-6420(98)99036-4. [DOI] [PubMed] [Google Scholar]
- 3.Shen DF, Herbort CP, Tuaillon NT, et al. Detection of toxoplasma Gondii in primary intraocular B-cell lymphoma. Modern Pathology. 2001;14:995–999. doi: 10.1038/modpathol.3880424. [DOI] [PubMed] [Google Scholar]
- 4.Holland GN, Muccioli C, Silveira C, et al. Intraocular inflammatory reactions without focal necrotizing retinochoroiditis in patients with acquired systemic toxoplasmosis. Am J Ophthalmol. 1999 Oct;128(4):413–420. doi: 10.1016/s0002-9394(99)00300-1. #3. [DOI] [PubMed] [Google Scholar]
- 5.Khurana SM, Dubey L, Malla N. Association of parasitic infections and cancers. Indian J.Med.Microbiol. 2005;23(2):74–79. doi: 10.4103/0255-0857.16044. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Qi L, Cheng H, Huang G. Two case reports of pituitary adenoma associated with Toxoplasma gondii infection. J.Clin.Pathol. 2002;55(12):965–966. doi: 10.1136/jcp.55.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan P, Hurley SF, Johnson AM. Tumors of the brain and presence of antibodies to Toxoplasma gondii. Int.J.Epidemiol. 1993;22(3):412–419. doi: 10.1093/ije/22.3.412. [DOI] [PubMed] [Google Scholar]
- 8.Certad G, Ngouanesevanh T, Guyot K, et al. Cryptosporidium parvum, a potential cause of colic adenocarcinoma. Infect.Agent.Cancer. 2007;2:22. doi: 10.1186/1750-9378-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribeiro DA, Pereira PC, Machado JM, et al. Does toxoplasmosis cause DNA damage? An evaluation in isogenic mice under normal diet or dietary restriction. Mutat.Res. 2004;559(1–2):169–176. doi: 10.1016/j.mrgentox.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Grimwood J, Mineo JR, Kasper LH. Attachment of Toxoplasma gondii to Host Cell is Host Cell Cycle Dependent. Infect. Immun. 1996;64:4099–4104. doi: 10.1128/iai.64.10.4099-4104.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coppens I, Joiner KA. Host but Not Parasite Cholesterol Controls Toxoplasma Cell Entry by Modulating Organelle Discharge. Mol. Bio. Cell. 2003;14:3804–3820. doi: 10.1091/mbc.E02-12-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinitzky M, Inbar M. Difference in Microviscosity Induced by Different Cholesterol Levels in the Surface Membrane Lipid Layer of Normal Lymphocytes and Malignant Lymphoma Cells. J. Mol. Biol. 1974;85:603–615. doi: 10.1016/0022-2836(74)90318-0. [DOI] [PubMed] [Google Scholar]
- 13.Coupland SE, Hummel M, Stein H, et al. Demonstration of identical clonal derivation in a case of “oculocerebral” lymphoma. Br. J. Ophthalmol. 2005;89:238–239. doi: 10.1136/bjo.2004.047001. [DOI] [PMC free article] [PubMed] [Google Scholar]