Abstract
Reversal of cardiac arrest requires reestablishment of aerobic metabolism by reperfusion with oxygenated blood of tissues that have been ischemic for variables periods of time. However, re-perfusion concomitantly activates a myriad of pathogenic mechanisms causing what is known as “reperfusion injury.” At the center of reperfusion injury are mitochondria, playing a critical role as effectors and targets of injury. Studies in animal models of ventricular fibrillation have shown that limiting myocardial cytosolic Na+ overload attenuates mitochondrial Ca2+ overload and maintains oxidative phosphorylation, which is the main bioenergetic function of mitochondria. This effect is associated with functional myocardial benefits such as preservation of myocardial compliance during chest compression and attenuation of myocardial dysfunction after return of spontaneous circulation. Additional studies in similar animal models of ventricular fibrillation have shown that mitochondrial injury leads to activation of the mitochondrial apoptotic pathway; characterized by the release of cytochrome c to the cytosol, reduction of caspase-9 levels, and activation of caspase-3 coincident with marked reduction in left ventricular function. Cytochrome c also “leaks” into the bloodstream attaining levels which are inversely proportional to survival. These data indicate that mitochondria play a key role during cardiac resuscitation by modulating energy metabolism and signaling apoptotic cascades and that targeting mitochondria could represent a promising strategy for cardiac resuscitation.
Introduction
Every year approximately 330 000 individuals in the United States (1) and 700 000 in Europe (2) suffer an episode of sudden cardiac arrest outside the hospital. Efforts to reestablish life are formidably challenging, requiring not only that cardiac activity be reestablished but that injury to vital organs be prevented, minimized, or reversed. Current resuscitation methods yield an average survival rate to hospital discharge with intact neurological function that approaches only 5%. Efficient Emergency Medical Services systems can initially reestablish cardiac activity in approximately 30% of victims (3–5) with over 30% dying before hospital admission (6). Of those admitted to a hospital, nearly 75% die before hospital discharge suffering variable degrees of myocardial dysfunction, neurological dysfunction, systemic inflammation, intercurrent illnesses, or a combination thereof (6–8). Thus, initial reestablishment of cardiac activity using current resuscitation techniques does not ensure ultimate survival. Novel resuscitation approaches are needed to increase the rate of initial resuscitation and subsequent survival with intact organ function.
Although resuscitation requires reperfusion of ischemic tissue with oxygenated blood to restore aerobic metabolism and organ function, reperfusion concomitantly activates multiple pathogenic mechanisms, collectively known as “reperfusion injury.” At the center of reperfusion injury are mitochondria, playing a critical role as effectors and targets of injury. Recent studies in our laboratory have centered on the effects that cardiac arrest and resuscitation have on mitochondria using various animal models of ventricular fibrillation (VF). The studies have been primarily focused on the myocardium, which represents a prime target of injury during cardiac arrest and resuscitation. The present article has been organized to i) provide a brief overview of mitochondrial anatomy and function, ii) discuss work along a line of research related to limiting cytosolic Na+ overload, which led to observations implicating mitochondria as the main functional target, and iii) examine recent evidence demonstrating activation of the mitochondrial apoptotic pathway during cardiac resuscitation.
Mitochondrial Anatomy and Function
Mitochondria have an outer and an inner membrane which delimit three submitochondrial compartments; namely, an intermembrane space, a matrix, and an intracristae space. The intermembrane space is located between the outer and the inner mitochondrial membrane. The matrix is enclosed by the inner mitochondrial membrane, which folds inwardly forming convoluted loops known as cristae. These cristae enclose a space known as the intracristae space which communicates with the intermembrane space through bottle-neck like junctions (9,10).
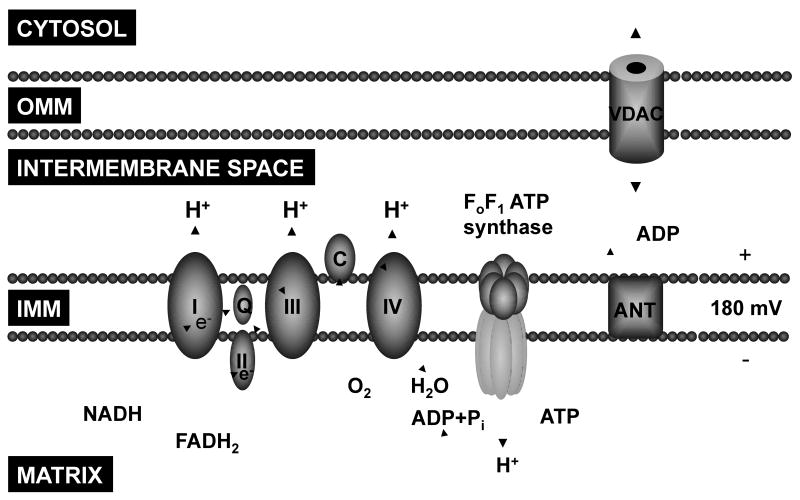
The primary function of mitochondria is the generation of ATP through oxidative phosphorylation. This process results from the oxidation of NADH and FADH2 and transfer of electrons through the electron transport chain (complex I, II, III, and IV) – located in the inner mitochondrial membrane – down their redox potentials. The energy released from the transfer of electrons is used to pump H+ into the intermembrane space creating a proton motive force which is then used by FoF1 ATP synthase to synthesize ATP from ADP and inorganic phosphate. ATP is then shuttled to the cytosol in exchange for ADP by the adenine nucleotide translocator (ANT) (Figure 1).
Figure 1.
Schematic rendition of key mitochondrial components involved in ATP synthesis via oxidative phosphorylation. OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane; I, II, III, and IV, respiratory chain complexes; Q, coenzyme Q; C, cytochrome c; ANT, adenine nucleotide translocator; VDAC, voltage-dependent anion channel.
In addition to the key role in energy metabolism, mitochondria can also signal cell death through the release of various pro-apoptotic proteins, including cytochrome c, apoptosis-inducing factor (11), Smac/DIABLO, endonuclease G, and a serine protease Omi/HtrA2 (12,13). Of these proteins, cytochrome c has been the most widely investigated.
Cytochrome c is a 14 kDa hemoprotein normally present in the intracristae space and intermembrane space attached to the inner mitochondrial membrane loosely bound to cardiolipin. Cytochrome c plays a key physiological role enabling electron transfer from complex III to complex IV (Figure 1). However, release of cytochrome c to the cytosol can activate the intrinsic apoptotic pathway through formation of an oligomeric complex known as the apoptosome which includes cytochrome c, dATP, the apoptotic protease activating factor-1 (Apaf-1), and procaspase-9 (14). The apoptosome activates caspase-9 which, in turn, activates downstream executioner caspases 3, 6, and 7 (15). Activation of these executioner caspases can lead to apoptotic cell death (16).
Cytochrome c release to the cytosol can occur under various pathological conditions including calcium overload (17), hypoxia (18), generation of reactive oxygen species (19), ultraviolet irradiation (20), serum deprivation (21), and growth factor withdrawal (21–3). Cytochrome c can also “leak” into the bloodstream; increased circulating levels have been reported in conditions associated with mitochondrial injury such as chemotherapy (24,25), acute myocardial infarction (26), the systemic inflammatory response syndrome (27), and influenza-associated encephalopathy (28,29).
Cytosolic Na+ Overload and Mitochondrial Injury
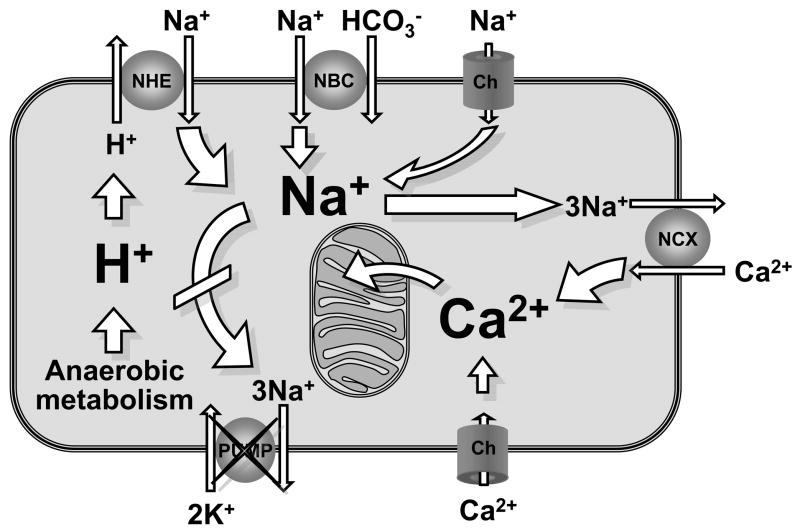
The combination of VF and lack of coronary blood flow prompts a shift to anaerobic metabolism leading to rapid development of intense and sustained intracellular acidosis. Intracellular acidosis activates the sarcolemmal sodium-hydrogen exchanger isoform-1 (NHE-1) initiating an electro-neutral Na+–H+ exchange that brings Na+ into the cell (30). Reperfusion with normal blood pH during cardiac resuscitation washes out H+ accumulated extracellularly during the preceding interval of no-flow leading to further intensification of the sarcolemmal Na+–H+ exchange and Na+ entry (30–32). Na+ may also enter through Na+ channels and the Na+-HCO3− co-transporter. Increased cytosolic Na+ influx is compounded by decreases in Na+-K+ ATPase activity (33), such that progressive and prominent increases in cytosolic Na+ occurs. The cytosolic Na+ excess, in turn, drives sarcolemmal Ca2+ influx through reverse mode operation of the sarcolemmal Na+–Ca2+ exchanger (NCX) with consequent cytosolic Ca2+ overload (34) (Figure 2).
Figure 2.
Schematic rendition of a cardiomyocyte during ischemia and reperfusion depicting Na+-induced cytosolic and mitochondrial Ca2+ overload. NHE, sodium-hydrogen exchanger iso-form-1; NBC, Na+-HCO3− cotransporter; NCX, Na+-Ca2+ exchanger; Ch, channel.
Cytosolic Ca2+ overload during ischemia and reperfusion has been identified as a primary effector of mitochondrial injury. Mitochondria can sequester large amounts of cytosolic Ca2+; a process which is regulated by the Ca2+ uniporter for influx and by the Na+–Ca2+ exchanger for efflux (35). However, as matrix Ca2+ levels progressively increase the mitochondrial Na+–Ca2+ exchanger becomes saturated and mitochondrial Ca2+ overload ensues (35). Mitochondrial Ca2+ overload can worsen cell injury in part by compromising its capability to sustain oxidative phosphorylation (36) and by promoting the release of pro-apoptotic factors (37).
The relevance of this mechanism of injury is highlighted by a large preclinical data demonstrating consistent attenuation of myocardial injury caused by ischemia and reperfusion when Na+ entry to the cell is limited as when NHE-1 activity is inhibited (30) or when Na+ channels are blocked (38,39).
Research over the last 7 years in our laboratory using various translational rat and pig models of cardiac arrest has shown consistent myocardial benefit associated with inhibition of NHE-1 activity during resuscitation from VF (32,40–49). These myocardial benefits include: 1) preservation of myocardial compliance and left ventricular wall thickness during VF enabling hemodynamically more effective chest compression, 2) reduction of reperfusion arrhythmias eliminating episodes of recurrent VF after return of spontaneous circulation, and 3) attenuation of myocardial injury leading to better post-resuscitation systolic and diastolic function with improved survival. Mechanistically, these benefits are associated with less cytosolic Na+ overload, less mitochondrial Ca2+ overload, and preservation of oxidative phosphorylation.
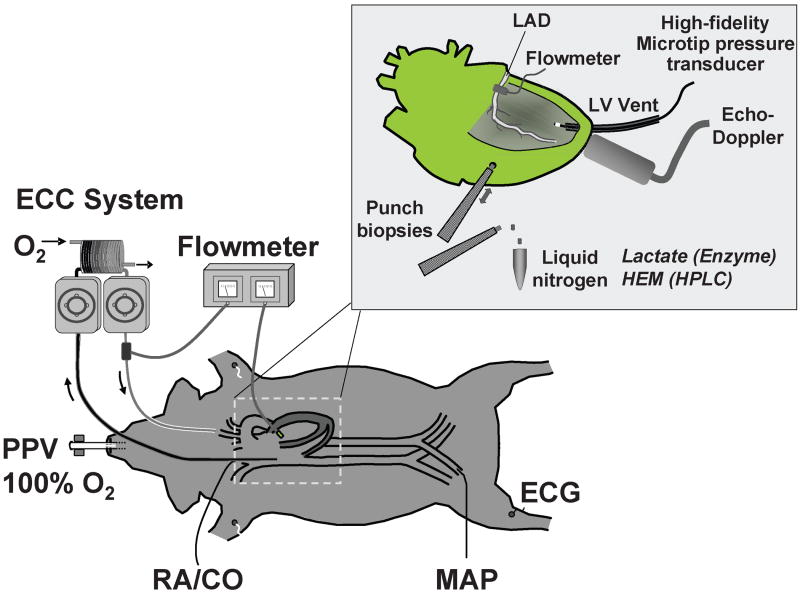
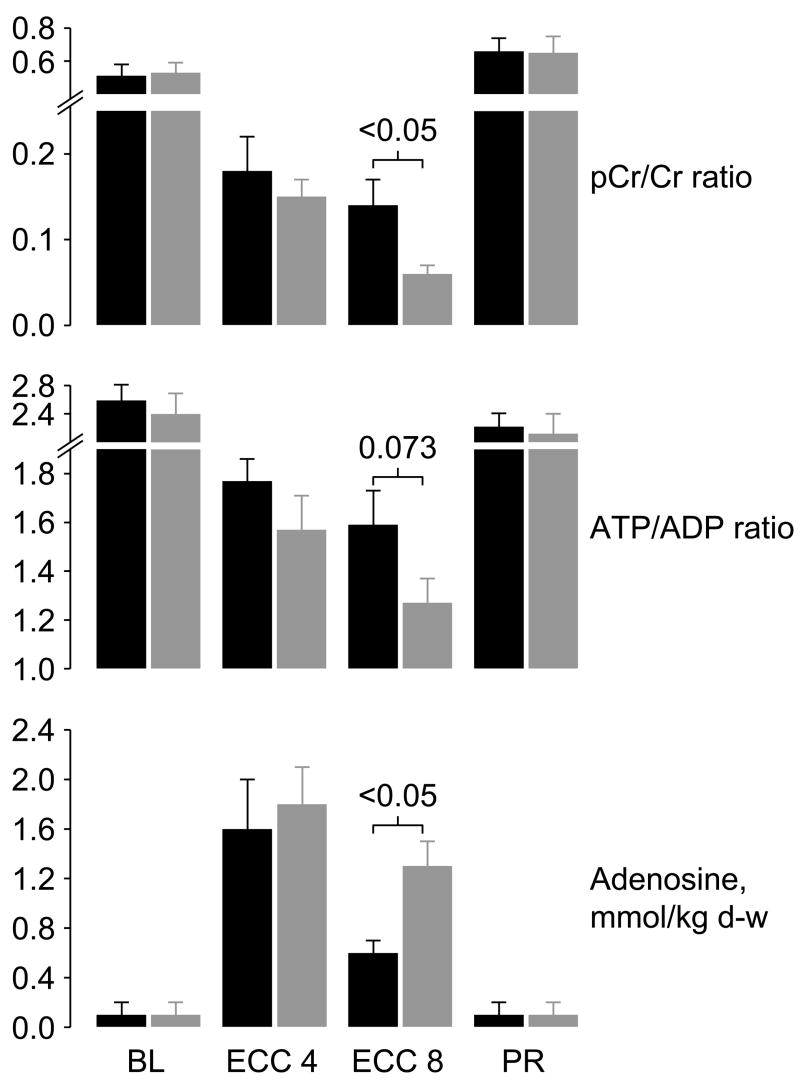
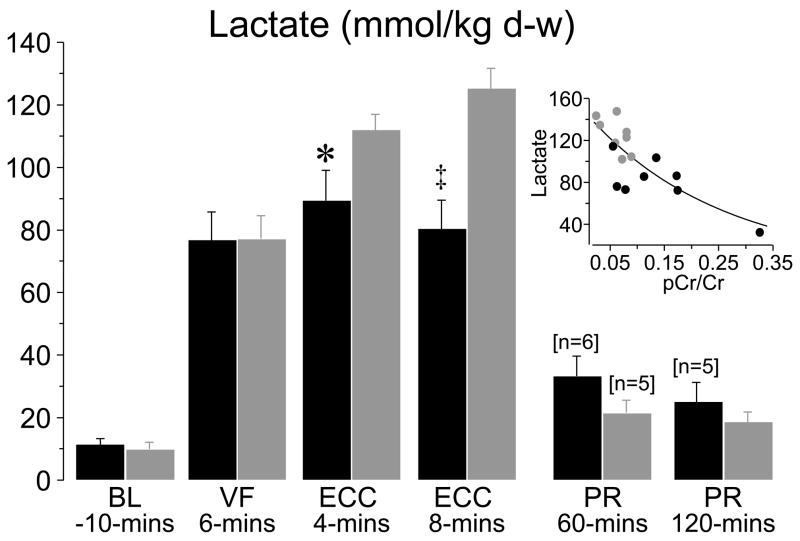
Two of these studies examined effects on mitochondria and are worth discussing in greater detail. In one study (49), an open-chest pig model of electrically-induced VF and extracorporeal circulation was developed to control coronary perfusion pressure while having direct access to the heart for functional and metabolic measurements (Figure 3). For this study, VF was induced by epicardial delivery of an alternating current and left untreated for 8 minutes. After this interval, extracorporeal circulation was started and the systemic blood flow adjusted to maintain a coronary perfusion pressure at 10 mmHg for 10 minutes before attempting defibrillation and restoration of spontaneous circulation. The target coronary perfusion pressure was chosen to mimic the low coronary perfusion pressure generated by closed-chest resuscitation. Two groups of 8 pigs each were randomized to receive the NHE-1 inhibitor zoniporide (3 mg/kg) or vehicle control as a right atrial bolus immediately before starting extracorporeal circulation. Like in a previous study using the NHE-1 inhibitor cariporide (42), zoniporide also prevented reductions in left ventricular compliance during the interval of VF and extracorporeal circulation, which in control pigs was characterized by progressive reductions in cavity size and progressive thickening of the left ventricular wall. These effects occurred without changes in coronary blood flow or coronary vascular resistance. Analysis of myocardial energy metabolites demonstrated that zoniporide prevented progressive loss of oxidative phosphorylation. This effect was evidenced by 1) a higher creatine phosphate to creatine ratio which is an indicator of oxidative phosphorylation and a sensitive marker of ischemia (50), 2) a numerically higher ATP/ADP ratio consistent with preservation of capability for ATP synthesis, and 3) attenuation of increases in myocardial adenosine suggesting that rephosphorylation of ADP was favored over breakdown into downstream products (Figure 4). These changes were accompanied by prominent amelioration of myocardial lactate increases, inversely related to increases in the creatine phosphate to creatine ratio suggesting coupling between these two energy related process (Figure 5). It is important to acknowledge, however, that additional mechanisms could have operated to mitigate the myocardial lactate increases including inhibition of phosphofructokinase (51) by the intracellular acidosis and lactate extrusion by the lactate/H+ co-transporter (52); two processes that could have been intensified by blocking H+ extrusion through the NHE-1 (Figure 2).
Figure 3.
Diagram depicting an open chest pig model of ventricular fibrillation and extracorporeal circulation. ECC, system for extracorporeal circulation; PPV, positive pressure ventilation; RA, right atrial pressure; CO, cardiac output; MAP, mean aortic pressure; ECG, electrocardiogram; LAD, left anterior descending coronary artery; LV, left ventricle; HEM (HPLC), high energy metabolites analyzed by high performance liquid chromatography. (Adapted from Ayoub I et al. Crit Care Med 2007;35:2329–36) (49).
Figure 4.
Creatine phosphate/creatine (PCr/Cr) ratios, adenosine triphosphate/adenosine diphosphate (ATP/ADP) ratios, and adenosine levels measured in left ventricular tissue in pigs randomized to receive either 3 mg/kg of zoniporide (black bars) or 0.9% NaCl (gray bars) into the right atrium after 8 minutes of untreated ventricular fibrillation, immediately before starting extracorporeal circulation (ECC). d-w, dry-weight. Measurements were obtained at baseline (BL), at minute 4 of ECC (ECC 4), at minute 8 of ECC (ECC 8), and at 60 minutes post-resuscitation (PR). Each group included 8 pigs each at baseline and during ECC and 6 pigs in the zoniporide group and 5 in the NaCl group at post-resuscitation. Values are mean ± SEM. Differences were tested by Student’s t-test. (Adapted from Ayoub I et al. Crit Care Med 2007;35:2329–36) (49).
Figure 5.
Myocardial lactate levels in left ventricular tissue in pigs randomized to receive either zoniporide (black symbols, n = 8) or NaCl (gray symbols, n = 8) before extracorporeal circulation. Numbers in brackets indicate when sample size decreased from the initial eight or from the preceding sample size. Insert demonstrates the relationship between myocardial lactate and the creatine phosphate to creatine ratio (pCr/Cr) at ECC 8 minutes. The regression line represents an exponential decay function (R2 = 0.63, p < 0.001). BL, baseline; VF, ventricular fibrillation; ECC, extracorporeal circulation; PR, postresuscitation; d-w, dry weight. Values are mean ± SEM; *p < 0.05, ‡p < 0.001 vs. NaCl by Student’s t-test. (Adapted from Ayoub I et al. Crit Care Med 2007;35:2329–36) (49).
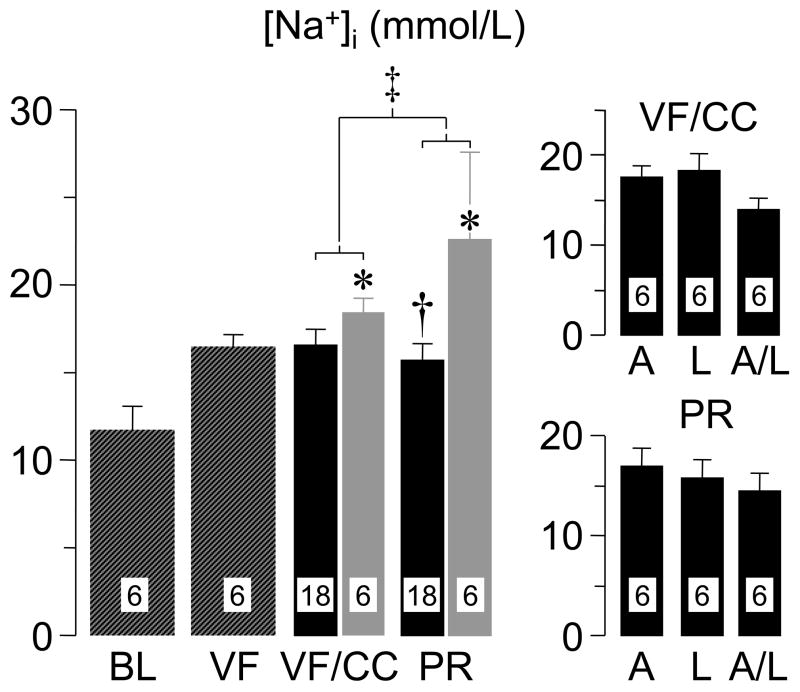
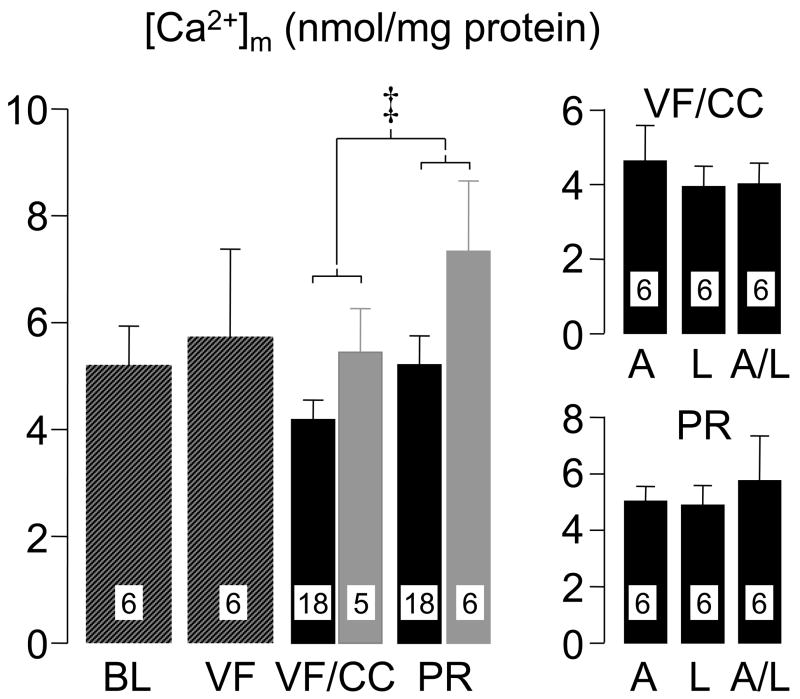
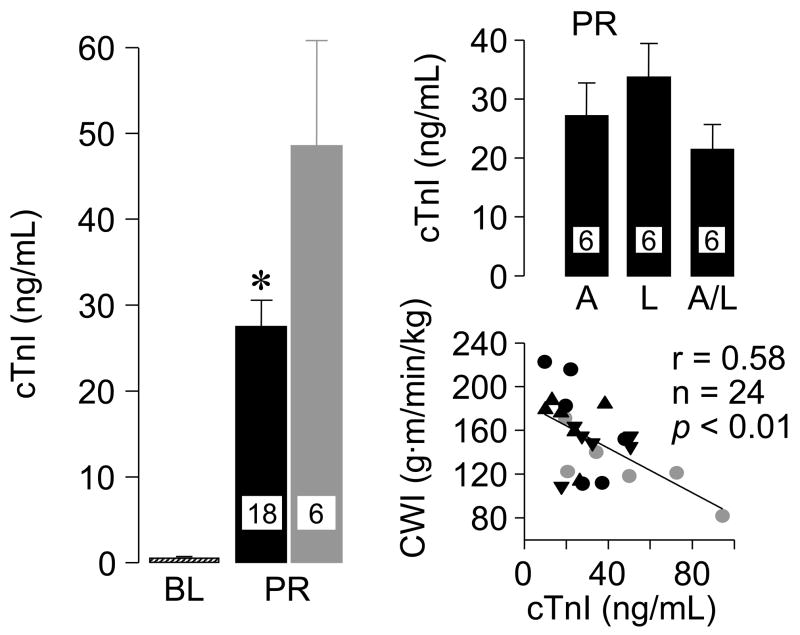
In another study (48), a rat model of VF and closed-chest resuscitation was used to examine the effects of various Na+-limiting interventions on intracellular Na+, mitochondrial Ca2+, cardiac function, and cardiospecific troponin I (cTnI) levels. For these studies, hearts were removed at specific time events; namely at baseline, during VF, during VF and chest compression, and after return of spontaneous circulation, with the rats from the last two time events randomized to receive a Na+-limiting intervention immediately before starting chest compression or vehicle control. The Na+-limiting interventions included a newly developed NHE-1 inhibitor AVE4454 (1 mg/kg), lidocaine (5 mg/kg), and the combination of AVE4454 and lidocaine. These rats were subjected to a 10 minute interval of untreated VF before attempting resuscitation. Limiting sarcolemmal Na+ entry attenuated increases in cytosolic Na+ and mitochondrial Ca2+ overload during chest compression and the post-resuscitation phase measured at 60 minutes after return of spontaneous circulation (Figures 6 and 7). Attenuation of cytosolic Na+ and mitochondrial Ca2+ increases was accompanied by lesser increases in cTnI and lesser post-resuscitation myocardial dysfunction (Figure 8).
Figure 6.
Intracellular Na+ ([Na+]i) in left ventricular tissue of rats at baseline (BL), at 15 minutes of untreated ventricular fibrillation (VF), at 15 minutes of VF accompanied by 5 minutes of chest compression (CC), and at 60 minutes post-resuscitation (PR). Hatched bars represent measurements without pharmacological treatment. Black bars represent rats treated with Na+ limiting interventions. Gray bars represent rats treated with vehicle control. The individual Na+-limiting interventions are shown on the right panels; A, selective sodium-hydrogen exchanger isoform-1 inhibitor AVE4454; L, lidocaine; and A/L, combination of AVE4454 and lidocaine. Numbers within bars denote number of hearts processed for the measurement. Values are mean ± SEM. *p < 0.05 vs BL by Kruskal-Wallis one-way ANOVA on ranks using Dunn’s Method for multiple comparisons; †p < 0.05 vs control by Student’s t-test in PR groups; ‡two-way ANOVA using time factor (VF/CC vs PR) and treatment factor (control vs Na+-limiting interventions) was significant for treatment factor (p = 0.013). (Adapted from Wang S et al. J Appl Physiol 2007;103:55–65) (48).
Figure 7.
Mitochondrial Ca2+ ([Ca2+]m) in left ventricular tissue of rats. For interpretation of bars and abbreviations refer to legend for Figure 6. ‡Two-way ANOVA using time factor (VF/CC vs PR) and treatment factor (control vs Na+-limiting interventions) was significant for both, time factor (p = 0.045) and treatment factor (p = 0.021). (Adapted from Wang S et al. J Appl Physiol 2007;103:55–65) (48).
Figure 8.
Cardiospecific troponin I (cTnI) in plasma at baseline (BL) and at 60 minutes post-resuscitation (PR) in rats subjected to ventricular fibrillation and resuscitation. Black symbols represent rats treated with a Na+-limiting intervention (AVE4454 circles, lidocaine inverted triangle, and AVE4454 and lidocaine combined upright triangles). Gray symbols represent control rats. Values are mean ± SEM. *P< 0.05 vs. control by Student’s t-test. The scatterplot depicts the correlation between cTnI and cardiac work index (CWI) at 60 min post-resuscitation. (Adapted from Wang S et al. J Appl Physiol 2007;103:55–65) (48).
Activation of the Mitochondrial Apoptotic Pathway
The preceding studies demonstrating a critical involvement of mitochondria during cardiac arrest and resuscitation prompted work designed to examine whether such injury could be accompanied by activation of the mitochondrial apoptotic pathway. These studies were conducted in a rat model of VF and closed-chest resuscitation in which the heart was removed at predefined intervals.
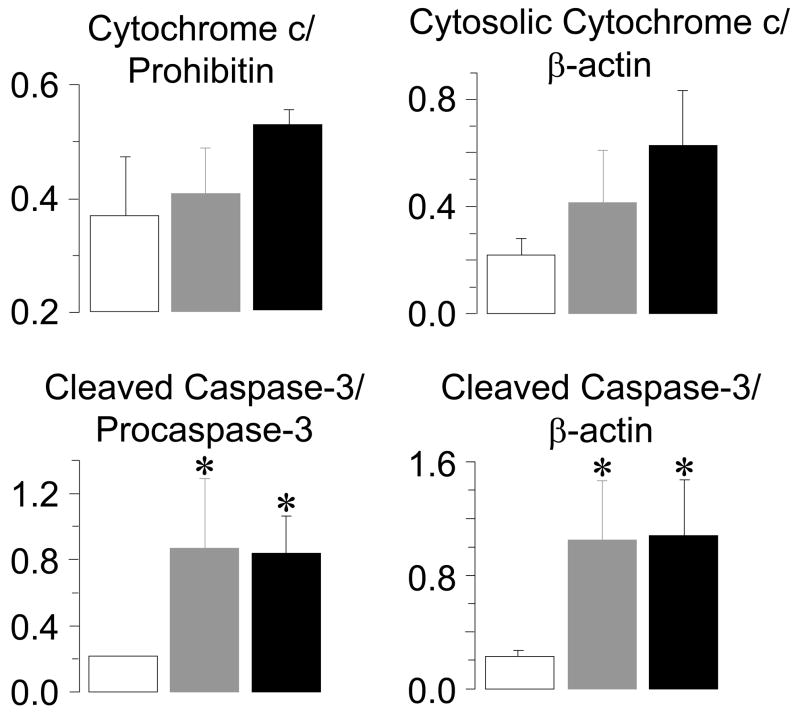
VF was electrically induced and left untreated for 4 or 8 minutes after which resuscitation was attempted by providing 8 additional minutes of chest compression followed by biphasic waveform defibrillation (53). A sham group served as control. Hearts were harvested at 4 hours after resuscitation, time at which the left ventricular stroke work index was only 23% of sham hearts. Analysis of left ventricular tissue demonstrated cytochrome c release to the cytosol, decreased procaspase-9 and cleaved caspase-9 levels, increased 17-kDa caspase-3 fragments, and increased caspase-3 activity consistent with activation of the mitochondrial apoptotic pathway (Figure 9). In more recent studies – published in abstract form (54) – we observed that activation of the mitochondrial apoptotic pathway was not accompanied by internucleosomal DNA fragmentation which is the hallmark of apoptotic cell death. This observation was made using a ligation-mediated polymerase chain reaction, which is a highly sensitive and specific reaction for identifying DNA fragments with blunt, 5′ phosphorylated, ends characteristic of endonucleolytic cleavage (55). We further demonstrated that caspase-3 inhibition initiated before induction of VF failed to prevent post-resuscitation myocardial dysfunction even though caspase-3 activity was reduced to baseline levels. These were intriguing observation questioning the significance cas-pase-3 activation during cardiac resuscitation, at least within the time frame of the measurements; namely, the initial 4 hours post-resuscitation. Thus, the release of cytochrome c in left ventricular mitochondria with subsequent activation of the mitochondrial apoptotic pathway is at the time of this writing an interesting observation in need of further work to determine its pathophysiological significance.
Figure 9.
Densitometry of left ventricular immunoblots demonstrating numerical increases in mitochondrial cytochrome c relative to prohibitin and cytosolic cytochrome c relative to β-actin and statistically significant increases in 17 kDa cleaved caspase-3 fragments in the cytosolic fraction relative to pro-caspase-3 and β-actin at 240 minutes post-resuscitation. Rats were randomized to untreated VF lasting 4 minutes (gray bars, n = 4), 8 minutes (black bars, n = 4), or to sham intervention (open bars, n = 4). Values are mean ± SEM. *p < 0.05 vs sham by one-way ANOVA and Dunn’s test for multiple comparisons. (Adapted from Radhakrishnan J et al. Am J Physiol Heart Circ Physiol 2007; 292:767–75) (53).
However, despite these observations it would be safe to postulate that release of cytochrome c represents – at the very least – a marker of mitochondrial injury. Furthermore, because cardiac arrest and resuscitation affects the whole body, it is highly likely that similar mitochondrial injury occurs ubiquitously throughout the body. Given these considerations and previous studies showing increased levels of plasma cytochrome c in conditions associated with mitochondrial injury, we examined whether cardiac arrest and resuscitation causes release of cytochrome c to the bloodstream.
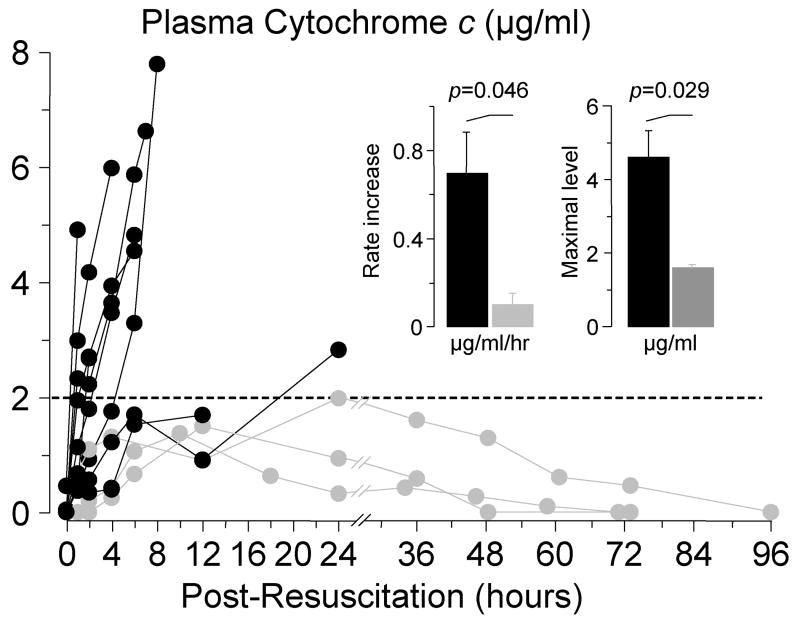
Thus, plasma cytochrome c was serially measured in rats successfully resuscitated from an 8-minute interval of untreated VF after 8 minutes of closed-chest resuscitation. Cytochrome c levels were measured until the levels had normalized or the rat had died. In survivors, plasma cytochrome c gradually increased to levels that did not exceed 2 μg/ml, returning to baseline within 48 to 96 hours. In non-survivor rats, however, cytochrome c rapidly increased to levels that substantially exceeded those observed in survivor rats, without reversal before demise from cardiovascular dysfunction (53) (Figure 10). These observations further strengthened the idea that mitochondria are injured during resuscitation from cardiac arrest.
Figure 10.
Serial measurements of plasma cytochrome c by reverse-phase high performance liquid chromatography in rats successfully resuscitated after 8 minutes of untreated ventricular fibrillation. Measurements were made until cytochrome c levels had returned to baseline or the rat had died. Gray symbols represent survivors (n = 3); black symbols represent non-survivors (n = 9). (Adapted from Radhakrishnan J et al. Am J Physiol Heart Circ Physiol 2007; 292:767–75) (53).
The observation that plasma cytochrome c attained levels inversely proportional to survival invited discussion on the potential mechanisms of cytochrome c release hypothesizing that understanding of such mechanisms could point to specific targets for therapeutic intervention.
Two main mechanisms have been proposed to explain cytochrome c release from mitochondria; namely, opening of the mitochondrial permeability transition pore (mPTP) and selective permeabilization of the outer mitochondrial membrane (OMM).
mPTP opening
This mechanism of cytochrome c release involves opening of the mPTP allowing molecules of up to 1500 Da to enter the mitochondrial matrix along with water and solutes leading to mitochondrial swelling, unfolding of inner mitochondrial membrane cristae, and disruption of the OMM ultimately causing cytochrome c release to the cytosol (56,57). Pathophysiological conditions that can open the mPTP include Ca2+ overload, production of reactive oxygen species (ROS), depletion of ATP and ADP, and increases in inorganic phosphate (56); all of which are characteristically present during ischemia and reperfusion. Although the exact composition of the mPTP has not been completely resolved, it seems to involve the apposition of transmembrane proteins from the inner and the outer mitochondrial membrane, including ANT, the voltage dependent anion channel, the peripheral benzodiazepine receptor, a hexokinase, the mitochondrial creatine kinase, and cyclophilin-D (56). mPTP opening causes collapse of the electrochemical gradient across the inner mitochondrial membrane leading to uncoupling of oxidative phosphorylation.
OMM permeabilization
Cytochrome c can also be released without mPTP opening through selective permeabilization of the OMM. This effect is modulated by proteins of the B-cell lymphoma-2 (Bcl-2) family through oligomerization and formation of channel-like structures in the OMM (67). Among the various family members, Bcl-2–associated X protein (Bax), Bcl-2 homologous antagonist/killer (Bak), and truncated BH3 interacting domain death agonist (Bid) are considered pro-apoptotic and favor formation of the channel-like structures in the OMM (68). Bcl-2, Bcl-xL, and Bcl-w are considered anti-apoptotic and oppose this effect. Bcl-2 has been shown to inhibit OMM permeabilization by antagonizing Bax and/or Bak conformational changes, membrane insertion, and oligomerization (69).
Release of cytochrome c is further facilitated during ischemia and reperfusion by peroxidation of cardiolipin consequent to mitochondrial Ca2+ overload and ROS production (70,71). Cardiolipin is the principal lipid constituent of the inner mitochondrial membrane and to which a fraction of cytochrome c is bound. Peroxidation of cardiolipin decreases its binding affinity for cytochrome c facilitating its release from mitochondria (71).
Conclusions
The quest for interventions that could prevent or mitigate reperfusion injury has prompted an intense scientific pursuit for decades. This pursuit has broadened our understanding of the underlying pathogenic processes; yet, the development of clinical interventions targeting reperfusion injury has remained an elusive goal. Identification of functional intracellular effectors may lead to the recognition of more robust targets for therapeutic intervention. The growing evidence identifying mitochondria as effectors and targets of reperfusion injury is bringing renewed hope that novel and more effective interventions could be developed for resuscitation from cardiac arrest.
Acknowledgments
Dr. Gazmuri has received funding from the National Institutes of Health, the Kossman Foundation, Sanofi-Aventis, and the VA Merit Review. The remaining authors have not disclosed any potential conflicts of interest.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Sans S, Kesteloot H, Kromhout D. The burden of cardiovascular diseases mortality in Europe. Task Force of the European Society of Cardiology on Cardiovascular Mortality and Morbidity Statistics in Europe. Eur Heart J. 1997;18:1231–1248. [PubMed] [Google Scholar]
- 3.Brown CG, Martin DR, Pepe PE, Stueven H, Cummins RO, Gonzalez E, Jastremski M the Multicenter High-Dose Epinephrine Study Group. A comparison of standard-dose and high-dose epinephrine in cardiac arrest outside the hospital. N Engl J Med. 1992;327:1051–1055. doi: 10.1056/NEJM199210083271503. [DOI] [PubMed] [Google Scholar]
- 4.Kellermann AL, Hackman BB, Somes G. Predicting the outcome of unsuccessful prehospital advanced cardiac life support. JAMA. 1993;270:1433–1436. [PubMed] [Google Scholar]
- 5.Lombardi G, Gallagher J, Gennis P. Outcome of out-of-hospital cardiac arrest in New York City. The pre-hospital arrest survival evaluation (PHASE) study. JAMA. 1994;271:678–683. [PubMed] [Google Scholar]
- 6.Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, Cariou A, Rozen-berg A, Carli P, Weber S, Dhainaut JF. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 7.Checchia PA, Sehra R, Moynihan J, Daher N, Tang W, Weil MH. Myocardial injury in children following resuscitation after cardiac arrest. Resuscitation. 2003;57:131–137. doi: 10.1016/s0300-9572(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 8.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 9.Frezza C, Cipolat S, Martins de BO, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De SB, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Mannella CA. The relevance of mitochondrial membrane topology to mitochondrial function. Biochim Biophys Acta. 2006;1762:140–147. doi: 10.1016/j.bbadis.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Kern KB, Sanders AB, Raife J, Milander MM, Otto CW, Ewy GA. A study of chest compression rates during cardiopulmonary resuscitation in humans. The importance of rate-directed chest compressions. Arch Intern Med. 1992;152:145–149. [PubMed] [Google Scholar]
- 12.Cai J, Yang J, Jones DP. Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta. 1998;1366:139–149. doi: 10.1016/s0005-2728(98)00109-1. [DOI] [PubMed] [Google Scholar]
- 13.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 14.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 15.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 16.Zou H, Li Y, Liu X, Wang X. An APAF-1. cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 17.Petrosillo G, Ruggiero FM, Pistolese M, Paradies G. Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (MPT)-dependent and MPT-independent mechanisms: role of cardiolipin. J Biol Chem. 2004;279:53103–53108. doi: 10.1074/jbc.M407500200. [DOI] [PubMed] [Google Scholar]
- 18.de Moissac D, Gurevich RM, Zheng H, Singal PK, Kirshenbaum LA. Caspase activation and mitochondrial cytochrome C release during hypoxia-mediated apoptosis of adult ventricular myocytes. J Mol Cell Cardiol. 2000;32:53–63. doi: 10.1006/jmcc.1999.1057. [DOI] [PubMed] [Google Scholar]
- 19.von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation. 1999;99:2934–2941. doi: 10.1161/01.cir.99.22.2934. [DOI] [PubMed] [Google Scholar]
- 20.Gao W, Pu Y, Luo KQ, Chang DC. Temporal relationship between cytochrome c release and mitochondrial swelling during UV-induced apoptosis in living HeLa cells. J Cell Sci. 2001;114:2855–2862. doi: 10.1242/jcs.114.15.2855. [DOI] [PubMed] [Google Scholar]
- 21.Bialik S, Cryns VL, Drincic A, Miyata S, Wollowick AL, Srinivasan A, Kitsis RN. The mitochondrial apoptotic pathway is activated by serum and glucose deprivation in cardiac myocytes. Circ Res. 1999;85:403–414. doi: 10.1161/01.res.85.5.403. [DOI] [PubMed] [Google Scholar]
- 22.Charles I, Khalyfa A, Kumar DM, Krishnamoorthy RR, Roque RS, Cooper N, Agarwal N. Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Invest Ophthalmol Vis Sci. 2005;46:1330–1338. doi: 10.1167/iovs.04-0363. [DOI] [PubMed] [Google Scholar]
- 23.Martinou I, Desagher S, Eskes R, Antonsson B, Andre E, Fakan S, Martinou JC. The release of cytochrome c from mitochondria during apoptosis of NGF-deprived sympathetic neurons is a reversible event. J Cell Biol. 1999;144:883–889. doi: 10.1083/jcb.144.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barczyk K, Kreuter M, Pryjma J, Booy EP, Maddika S, Ghavami S, Berdel WE, Roth J, Los M. Serum cytochrome c indicates in vivo apoptosis and can serve as a prognostic marker during cancer therapy. Int J Cancer. 2005;116:167–173. doi: 10.1002/ijc.21037. [DOI] [PubMed] [Google Scholar]
- 25.Renz A, Berdel WE, Kreuter M, Belka C, Schulze-Osthoff K, Los M. Rapid extracellular release of cytochrome c is specific for apoptosis and marks cell death in vivo. Blood. 2001;98:1542–1548. doi: 10.1182/blood.v98.5.1542. [DOI] [PubMed] [Google Scholar]
- 26.Alleyne T, Joseph J, Sampson V. Cytochrome-c detection: a diagnostic marker for myocardial infarction. Appl Biochem Biotechnol. 2001;90:97–105. doi: 10.1385/abab:90:2:97. [DOI] [PubMed] [Google Scholar]
- 27.Adachi N, Hirota M, Hamaguchi M, Okamoto K, Watanabe K, Endo F. Serum cytochrome c level as a prognostic indicator in patients with systemic inflammatory response syndrome. Clin Chim Acta. 2004;342:127–136. doi: 10.1016/j.cccn.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Hosoya M, Nunoi H, Aoyama M, Kawasaki Y, Suzuki H. Cytochrome c and tumor necrosis factor-alpha values in serum and cerebrospinal fluid of patients with influenza-associated encephalopathy. Pediatr Infect Dis J. 2005;24:467–470. doi: 10.1097/01.inf.0000160995.07461.b8. [DOI] [PubMed] [Google Scholar]
- 29.Hosoya M, Kawasaki Y, Katayose M, Sakuma H, Watanabe M, Igarashi E, Aoyama M, Nunoi H, Suzuki H. Prognostic predictive values of serum cytochrome c, cytokines, and other laboratory measurements in acute encephalopathy with multiple organ failure. Arch Dis Child. 2006;91:469–472. doi: 10.1136/adc.2005.078436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karmazyn M, Sawyer M, Fliegel L. The na(+)/h(+) exchanger: a target for cardiac therapeutic intervention. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:323–335. doi: 10.2174/1568006054553417. [DOI] [PubMed] [Google Scholar]
- 31.Imahashi K, Kusuoka H, Hashimoto K, Yoshioka J, Yamaguchi H, Nishimura T. Intracellular sodium accumulation during ischemia as the substrate for reperfusion injury. Circ Res. 1999;84:1401–406. doi: 10.1161/01.res.84.12.1401. [DOI] [PubMed] [Google Scholar]
- 32.Gazmuri RJ, Hoffner E, Kalcheim J, Ho H, Patel M, Ayoub IM, Epstein M, Kingston S, Han Y. Myocardial protection during ventricular fibrillation by reduction of proton-driven sarcolemmal sodium influx. J Lab Clin Med. 2001;137:43–55. doi: 10.1067/mlc.2001.111693. [DOI] [PubMed] [Google Scholar]
- 33.Avkiran M, Ibuki C, Shimada Y, Haddock PS. Effects of acidic reperfusion on arrhythmias and Na(+)-K(+)-ATPase activity in regionally ischemic rat hearts. Am J Physiol. 1996;270:H957–964. doi: 10.1152/ajpheart.1996.270.3.H957. [DOI] [PubMed] [Google Scholar]
- 34.An J, Varadarajan SG, Camara A, Chen Q, Novalija E, Gross GJ, Stowe DF. Blocking Na(+)/H(+) exchange reduces [Na(+)](i) and [Ca(2+)](i) load after ischemia and improves function in intact hearts. Am J Physiol. 2001;281:H2398–2409. doi: 10.1152/ajpheart.2001.281.6.H2398. [DOI] [PubMed] [Google Scholar]
- 35.Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium. 2000;28:285–296. doi: 10.1054/ceca.2000.0168. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto S, Matsui K, Ohashi N. Protective effect of Na+/H+ exchange inhibitor, SM-20550, on impaired mitochondrial respiratory function and mitochondrial Ca2+ overload in ischemic/reperfused rat hearts. J Cardiovasc Pharmacol. 2002;39:569–575. doi: 10.1097/00005344-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Borutaite V, Brown GC. Mitochondria in apoptosis of ischemic heart. FEBS Lett. 2003;541:1–5. doi: 10.1016/s0014-5793(03)00278-3. [DOI] [PubMed] [Google Scholar]
- 38.Nasser FN, Walls JT, Edwards WD, Harrison CE., Jr Lidocaine-induced reduction in size of experimental myocardial infarction. Am J Cardiol. 1980;46:967–975. doi: 10.1016/0002-9149(80)90353-7. [DOI] [PubMed] [Google Scholar]
- 39.Hinokiyama K, Hatori N, Ochi M, Maehara T, Tanaka S. Myocardial protective effect of lidocaine during experimental off-pump coronary artery bypass grafting. Ann Thorac Cardiovasc Surg. 2003;9:36–42. [PubMed] [Google Scholar]
- 40.Gazmuri RJ, Ayoub IM, Hoffner E, Kolarova JD. Successful ventricular defibrillation by the selective sodium-hydrogen exchanger isoform-1 inhibitor cariporide. Circulation. 2001;104:234–239. doi: 10.1161/01.cir.104.2.234. [DOI] [PubMed] [Google Scholar]
- 41.Gazmuri RJ, Ayoub IM, Kolarova JD, Karmazyn M. Myocardial protection during ventricular fibrillation by inhibition of the sodium-hydrogen exchanger isoform-1. Crit Care Med. 2002;30:S166–S171. doi: 10.1097/00003246-200204001-00010. [DOI] [PubMed] [Google Scholar]
- 42.Ayoub IM, Kolarova JD, Yi Z, Trevedi A, Deshmukh H, Lubell DL, Franz MR, Maldonado FA, Gazmuri RJ. Sodium-hydrogen exchange inhibition during ventricular fibrillation: Beneficial effects on ischemic contracture, action potential duration, reperfusion arrhythmias, myocardial function, and resuscitability. Circulation. 2003;107:1804–1809. doi: 10.1161/01.CIR.0000058704.45646.0D. [DOI] [PubMed] [Google Scholar]
- 43.Gazmuri RJ, Ayoub IM, Kolarova J. Myocardial protection during resuscitation from cardiac arrest. Curr Opin Crit Care. 2003;9:199–204. doi: 10.1097/00075198-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Ayoub IM, Kolarova J, Kantola RL, Sanders R, Gazmuri RJ. Cariporide minimizes adverse myocardial effects of epinephrine during resuscitation from ventricular fibrillation. Crit Care Med. 2005;33:2599–2605. doi: 10.1097/01.ccm.0000186773.88576.83. [DOI] [PubMed] [Google Scholar]
- 45.Kolarova J, Yi Z, Ayoub IM, Gazmuri RJ. Cariporide potentiates the effects of epinephrine and vasopressin by nonvascular mechanisms during closed-chest resuscitation. Chest. 2005;127:1327–1334. doi: 10.1378/chest.127.4.1327. [DOI] [PubMed] [Google Scholar]
- 46.Kolarova JD, Ayoub IM, Gazmuri RJ. Cariporide enables hemodynamically more effective chest compression by leftward shift of its flow-depth relationship. Am J Physiol Heart Circ Physiol. 2005;288:H2904–H2911. doi: 10.1152/ajpheart.01181.2004. [DOI] [PubMed] [Google Scholar]
- 47.Gazmuri RJ, Ayoub IM. The case for sodium-hydrogen exchanger isoform-1 inhibition during cardiac resuscitation remains strong. Crit Care Med. 2006;34:1580–1582. doi: 10.1097/01.CCM.0000216687.86553.EC. [DOI] [PubMed] [Google Scholar]
- 48.Wang S, Radhakrishnan J, Ayoub IM, Kolarova JD, Taglieri DM, Gazmuri RJ. Limiting sarcolemmal Na+ entry during resuscitation from VF prevents excess mitochondrial Ca2+ accumulation and attenuates myocardial injury. J Appl Physiol. 2007;103:55–65. doi: 10.1152/japplphysiol.01167.2006. [DOI] [PubMed] [Google Scholar]
- 49.Ayoub IM, Kolarova J, Kantola R, Radhakrishnan J, Gazmuri RJ. Zoniporide preserves left ventricular compliance during ventricular fibrillation and minimizes post-resuscitation myocardial dysfunction through benefits on energy metabolism. Crit Care Med. 2007;35:2329–2336. doi: 10.1097/01.ccm.0000280569.87413.74. [DOI] [PubMed] [Google Scholar]
- 50.Ye J, Clark MG, Colquhoun EQ. Creatine phosphate as the preferred early indicator of ischemia in muscular tissues. J Surg Res. 1996;61:227–236. doi: 10.1006/jsre.1996.0109. [DOI] [PubMed] [Google Scholar]
- 51.Busa WB. Mechanisms and consequences of pH-mediated cell regulation. Annu Rev Physiol. 1986;48:389–402. doi: 10.1146/annurev.ph.48.030186.002133. [DOI] [PubMed] [Google Scholar]
- 52.Lin H, Suleiman MS. Cariporide enhances lactate clearance upon reperfusion but does not alter lactate accumulation during global ischaemia. Pflugers Arch. 2003;447:8–13. doi: 10.1007/s00424-003-1134-8. [DOI] [PubMed] [Google Scholar]
- 53.Radhakrishnan J, Wang S, Ayoub IM, Kolarova JD, Levine RF, Gazmuri RJ. Circulating levels of cytochrome c after resuscitation from cardiac arrest: a marker of mitochondrial injury and predictor of survival. Am J Physiol Heart Circ Physiol. 2007;292:H767–H775. doi: 10.1152/ajpheart.00468.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radhakrishnan J, Ayoub IM, Wang S, Kolarova JD, Taglieri DM, Gazmuri RJ. Activation of the mitochondrial apoptotic pathway without evidence of apoptotic cell death after resuscitation from ventricular fibrillation. Circulation. 2006;114:II-1194. abstract. [Google Scholar]
- 55.Staley K, Blaschke AJ, Chun J. Apoptotic DNA fragmentation is detected by a semiquantitative ligation-mediated PCR of blunt DNA ends. Cell Death Differ. 1997;4:66–75. doi: 10.1038/sj.cdd.4400207. [DOI] [PubMed] [Google Scholar]
- 56.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 57.Halestrap AP. The mitochondrial permeability transition: its molecular mechanism and role in reperfusion injury. Biochem Soc Symp. 1999;66:181–203. doi: 10.1042/bss0660181. [DOI] [PubMed] [Google Scholar]
- 58.Crompton M, Costi A, Hayat L. Evidence for the presence of a reversible Ca2+-dependent pore activated by oxidative stress in heart mitochondria. Biochem J. 1987;245:915–918. doi: 10.1042/bj2450915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crompton M, Costi A. A heart mitochondrial Ca2(+)-dependent pore of possible relevance to re-perfusion-induced injury. Evidence that ADP facilitates pore interconversion between the closed and open states. Biochem J. 1990;266:33–39. doi: 10.1042/bj2660033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nazareth W, Yafei N, Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol. 1991;23:1351–1354. doi: 10.1016/0022-2828(91)90181-k. [DOI] [PubMed] [Google Scholar]
- 61.Griffiths EJ, Halestrap AP. Protection by Cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 62.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307(Pt 1):93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bernardi P. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. J Biol Chem. 1992;267:8834–8839. [PubMed] [Google Scholar]
- 64.Kristian T, Bernardi P, Siesjo BK. Acidosis promotes the permeability transition in energized mitochondria: implications for reperfusion injury. J Neurotrauma. 2001;18:1059–1074. doi: 10.1089/08977150152693755. [DOI] [PubMed] [Google Scholar]
- 65.Halestrap AP. Calcium-dependent opening of a non-specific pore in the mitochondrial inner membrane is inhibited at pH values below 7. Implications for the protective effect of low pH against chemical and hypoxic cell damage. Biochem J. 1991;278(Pt 3):715–719. doi: 10.1042/bj2780715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akao M, O’Rourke B, Teshima Y, Seharaseyon J, Marban E. Mechanistically distinct steps in the mitochondrial death pathway triggered by oxidative stress in cardiac myocytes. Circ Res. 2003;92:186–194. doi: 10.1161/01.res.0000051861.21316.e9. [DOI] [PubMed] [Google Scholar]
- 67.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 68.Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G, Korsmeyer SJ. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc Natl Acad Sci U S A. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mikhailov V, Mikhailova M, Pulkrabek DJ, Dong Z, Venkatachalam MA, Saikumar P. Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J Biol Chem. 2001;276:18361–18374. doi: 10.1074/jbc.M100655200. [DOI] [PubMed] [Google Scholar]
- 70.Lesnefsky EJ, Slabe TJ, Stoll MS, Minkler PE, Hoppel CL. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H2770–H2778. doi: 10.1152/ajpheart.2001.280.6.H2770. [DOI] [PubMed] [Google Scholar]
- 71.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]