Spherical bilayer lipid vesicles (liposomes) have been used extensively for drug delivery.1 Drug encapsulation by liposomes allows in vivo protection from degradative enzymes and thus an enhanced circulation time and bioavailability. Liposomes, immobilized on suitable implant surfaces, could conceivably act as capacious depots for pharmaceutically active compounds ensuring their controllable leakage into surrounding tissue. Furthermore, immobilized liposomal nanocontainers could provide viable fluidic solutions especially in niche areas where ultra-miniaturisation and biocompatibility are of critical importance.2 Surfaces onto which liposomes have been adsorbed2,3 include those provided by mica (native and silanized),4,5 glass,6 silica,7 gold,8 clay,9 liquid crystals,10 platinum,11 sulfonated polystyrene,12 self-assembled monolayers of 1-octadecanethiol,13 supported human serum albumin,14 and supported lipid bilayers.15 Owing to liposome-surface and liposome-liposome interactions, fusion and rupture events are common during adsorption particularly at higher coverage. Thus, when liposomes bind to a solid substrate, they tend to form a planar bilayer if the surface is hydrophilic and a planar monolayer if the surface is hydrophobic.16 Successful attempts to preclude this problem (e.g. via tethering)17 have been published, but still a need remains for simple systems that strongly adsorb liposomes while at the same time do not disrupt the liposome integrity. In this Communication we describe an integrated system which consists of soft nanocontainers (anionic liposomes) immobilized on “spherical polycationic brushes” (SPBs) defined as colloidal particles with grafted cationic macromolecules. SPBs have been recently used as effective building blocks for new functional materials.18,19 We demonstrate herein that immobilized liposomes retain their original size, shape and encapsulating power.
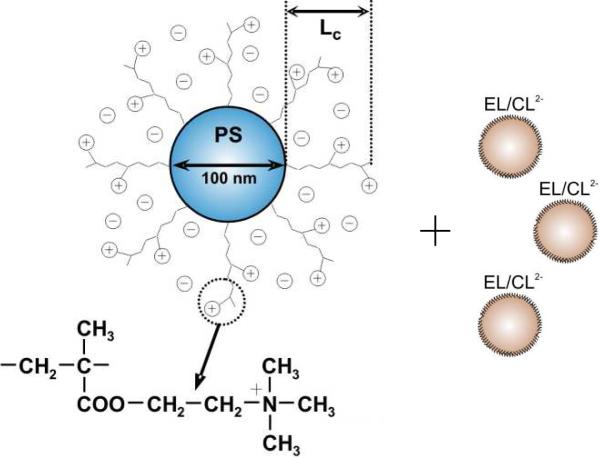
SPBs (Fig. 1) were prepared in three steps along the lines given previously.20 First, cationic polystyrene particles, ca. 100 nm in diameter, were synthesized by emulsion polymerization of styrene in the presence of a cationic surfactant, cetyltrimethylammonium bromide (CTAB, Fluka) and a cationic initiator, 2,2'-azobis(2-amidinopropane)dihydrochloride (V50, WAKO Chemicals). Next, the latex particles were covered by a very thin layer of the photoinitiator 2-[p-(2-hydroxy-2-methyl propiophenone)]ethylene glycolmethacrylate (HMEM, see Supporting Information). Lastly, polycationic chains were grown on the surface of latex particles by grafting a cationic monomer, (2-(acryloyloxy)ethyl) trimethylammonium chloride, under UV/VIS-radiation. According to dynamic light scattering (DLS), grafting increased the diameter of the particles to 230 nm, indicating a 65 nm thickness of the cationic corona (Lc in Fig. 1). In a typical experiment, an SPB suspension was mixed with a suspension of unilamellar anionic liposomes (ca.50 nm) prepared conventionally by sonication and composed of egg lecithin (EL) and a doubly-anionic phospholipid, cardiolipin (CL2-), in a molar ratio of 19:1 (Fig. 1).
Figure 1.
Schematic representation of a spherical polycationic brush (SPB) with a polystyrene (PS) core added to anionic liposomes composed of egg lecithin (EL) and cardiolipin (CL2-).
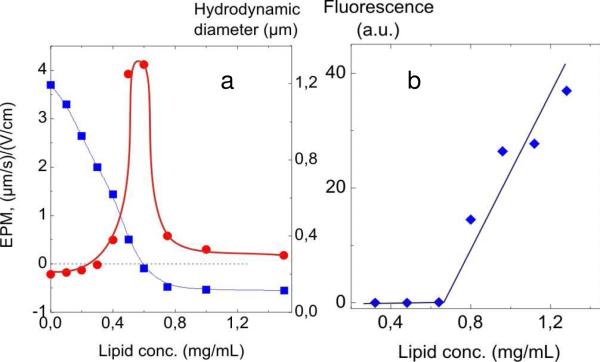
Injection of the EL/CL2- liposomes into an SPB suspension caused the charge on the cationic SBPs to decrease as registered by laser electrophoresis (Fig. 2a). When sufficient anionic liposomes were added, the electrophoretic mobility (EPM) of the liposome/SPB complex declined to zero, indicative of total charge neutralization. Further addition of liposomes created a mild negative charge on the complex. The diameter of the complex, measured by DLS, increased sharply to 1300 nm at the charge-neutralization point where the EPM = 0 (Fig. 2a). Further liposome addition caused a decrease in size to about 300 nm which is 70 nm larger than that of the initial SPB species. In summary, both electrophoresis and DLS data demonstrate conclusively the presence of liposome/SPB complexation.
Figure 2.
(a) Effect of EL/CL2- liposomes on the EPM (left-hand axis) and on the hydrodynamic diameter (right-hand axis) of the SPB particles; (b) Dependence of relative fluorescence of supernatants after separation of SPB/liposome complexes vs. liposome concentration. SPB concentration 0.38 mg/mL; [SPB+] = 10-4 M; 10-2 M borate buffer, pH 9.
In order to determine the number of liposomes which ultimately bind to a single SPB particle, we performed fluorescence measurements of EL/CL2- liposomes with 0.5 wt% of N-fluoroscein-iso-thiocyanyldipalmitoylphosphatidylethanol amine, a fluorescent lipid, incorporated into the bilayer during the liposome preparation. A series of mixtures with varied concentration of labeled EL/Cl2- liposomes (0.3–1.3 mg/mL) and a constant SPB concentration of 1.5 mg/mL was prepared, incubated for 30 min, and centrifuged; the fluorescent intensities of the clear supernatants were then measured. Fig. 2b shows negligible fluorescence in the supernatants up to 0.65 mg/mL lipid after which the fluorescent rises steeply owing to unbound lipid. These fluorescence “titrations” indicate that a maximum of ~36 liposomes are able to bind to each SPB particle at the conditions close to the neutralization point.
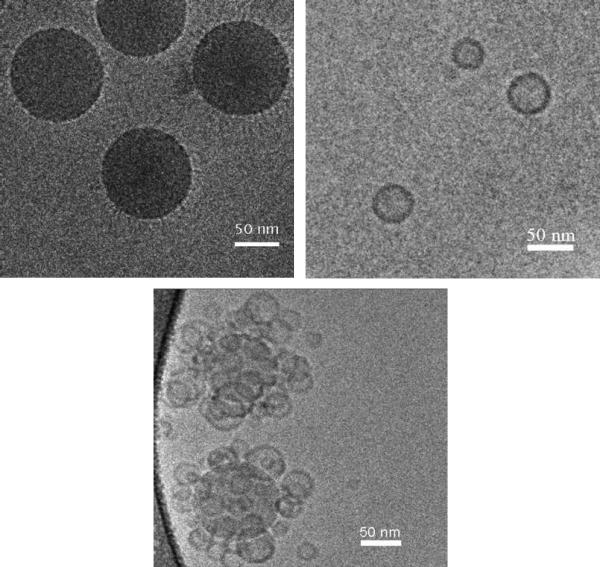
The resulting SPB/EL/CL2- liposomes supra-aggregates were visualized with the aid of cryo-transmission electron microscopy (cryo-TEM) which preserves the morphology of soft objects.21 Fig. 3A shows a cryo-TEM micrograph of SPB particles by themselves in an aqueous buffer. Polycationic chains projecting from the polystyrene core are visible. A cryo-TEM of the EL/CL2- liposomes by themselves (Fig. 3B) gives sizes in agreement with the DLS measuremesnts. Fig. 3C is a cryo-TEM of two SPB/liposome complexes, taken immediately after their formation, in which multiple liposomes cover each SPB particle. Clearly, the liposomes retain their normal shape upon complexation with the cationic brushes. A cryo-TEM, taken two hours after complex formation (not shown), was identical to Fig. 3C and indicative of complex stability over this time period.
Figure 3.
Cryogenic transmission electron microscopy images of 1.5 mg/mL SPB suspension (a), 0.1 mg/mL EL/CL2- liposomes suspension (b), and SPB/liposome complex, prepared by mixing of 1.5 mg/mL SPB suspension and 0.1 mg/mL liposome suspension (c).
As mentioned above, a key question relates to the integrity of the liposomes when complexed with the brushes, i.e. whether the liposomal membrane remains intact or whether pores and other defects appear in the membrane. To address this question, we prepared a suspension of EL/Cl2- liposomes filled with a 1 M NaCl solution. Formation of defects, if such occurred, would result in leakage of salt from the liposomes into the surrounding solution where the salt can be detected by conductometry. Initially, a control experiment was carried out in which a NaCl-loaded liposome suspension was treated with a Triton X-100 solution; as expected, we observed NaCl leakage and a sharp rise in conductivity. But when NaCl-loaded liposomes were mixed with an SPB suspension, only a negligible increase in conductivity was detected. Thus, the anionic EL/CL2- liposomes maintain their ability to encapsulate when bound to the grafted polystyrene.
For comparison, we added NaCl-loaded EL/ Cl2- liposomes to a suspension of latex particles lacking the grafted polycationic chains. Cationic polystyrene core lattices, obtained in the first step of the above-described synthetic procedure, were used for the experiment. With the aid of electrophoresis, the anionic liposomes were found to adsorb onto these cationic particles; however the interaction was accompanied by a NaCl leakage and a consequent rise in conductivity. The result demonstrates a principal difference in the fate of anionic liposomes bound to the two types of cationic colloidal particles: a disruption of liposomes when directly adsorbed onto a rigid cationic surface but a conservation of liposome integrity when adsorbed onto a surface bearing mobile cationic chains.
In summary, evidence is provided for electrostatic adsorption of anionic liposomes onto the surface of spherical particles bearing grafted linear cationic macromolecules The size, shape, and encapsulation ability of liposomes remain unchanged upon adsorption onto SPBs. The supramolecular aggregates, representing an assembly of self-assemblies, have potential applications in the drug delivery field.
Supplementary Material
Acknowledgments
The work was supported by the Russian Foundation for Fundamental Research (08-03-0074 and 09-03-12336), the German Science Foundation (SFB 481), the Fogarty International Research Cooperation Award (TW05555) and the National Institutes of Health (FMM).
Footnotes
Supporting Information Available: Synthesis of liposomes and SPBs; structure of HMEM. This material is available free of charge through the Internet at http://pubs.acs.org.
References
- 1.Gregoriadis G. Trends in Biotechnology. 1995;13:527–537. doi: 10.1016/S0167-7799(00)89017-4. [DOI] [PubMed] [Google Scholar]
- 2.Christensen SM, Stamou D. Soft Matter. 2007;3:828–836. doi: 10.1039/b702849k. [DOI] [PubMed] [Google Scholar]
- 3.Richter RP, Berat R, Brisson AR. Langmuir. 2006;22:3497–3505. doi: 10.1021/la052687c. [DOI] [PubMed] [Google Scholar]
- 4.Klacar S, Dimitrievski K, Kasemo B. J. Phys. Chem B. 2009;113:5681–5685. doi: 10.1021/jp810874h. [DOI] [PubMed] [Google Scholar]
- 5.Teschke O, de Souza EF. Langmuir. 2002;18:6513–6520. [Google Scholar]
- 6.Benes M, Billy D, Benda A, Speijer H, Hof M, Hermens W. Th. Langmuir. 2004;20:10129–10137. doi: 10.1021/la048811u. [DOI] [PubMed] [Google Scholar]
- 7.Nordlund G, Loenneborg R, Brezinski R. Langmuir. 2009;25:4601–4606. doi: 10.1021/la8036296. [DOI] [PubMed] [Google Scholar]
- 8.Cho N-J, Wang G, Edvardson M, Glenn JS, Hook F, Frank CW. Anal. Chem. 2009;81:4752–4761. doi: 10.1021/ac900242s. [DOI] [PubMed] [Google Scholar]
- 9.Undabeytia T, Nir S, Gomara MJ. Langmuir. 2004;20:6605–6610. doi: 10.1021/la0494472. [DOI] [PubMed] [Google Scholar]
- 10.Brake JM, Daschner MK, Abbott NL. Langmuir. 2005;21:2218–2228. doi: 10.1021/la0482397. [DOI] [PubMed] [Google Scholar]
- 11.Reimhult E, Holoeck F, Kasemo B. Langmuir. 2003;19:1681–1691. [Google Scholar]
- 12.Pereira EMA, Petri DFS, l Carmona-Ribeiro AM. J. Phys. Chem. B. 2002;106:8762–8767. [Google Scholar]
- 13.Ha TH, Kim K. Langmuir. 2001;17:1999–2007. [Google Scholar]
- 14.Randino A, Cambria A, Sarpietro MG, Satriano C. J. Coll. Inter. Sci. 2000;231:66–73. doi: 10.1006/jcis.2000.7083. [DOI] [PubMed] [Google Scholar]
- 15.Seantier B, Kaseno B. Langmuir. 2009;25:5767–5722. doi: 10.1021/la804172f. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Hong L, Yu Y, Bae SC, Granick S. J. Am. Chem. Soc. 2006;128:9026–9027. doi: 10.1021/ja062620r. [DOI] [PubMed] [Google Scholar]
- 17.Yoshina-Ishii C, Boxer SG. J. Am. Chem. Soc. 2003;125:3696–3697. doi: 10.1021/ja029783+. [DOI] [PubMed] [Google Scholar]
- 18.Ballauff M. Prog. Polym. Sci. 2007;32:1135–1151. [Google Scholar]
- 19.Schrinner M, Ballauff M, Talmon Y, Kauffmann Y, Thun J, Möller M, Breu J. Science. 2009;323:617–620. doi: 10.1126/science.1166703. [DOI] [PubMed] [Google Scholar]
- 20.Mei Y, Wittemann A, Sharma G, Ballauff M, Koch Th., Gliemann H, Horbach J, Schimmel Th. Macromolecules. 2003;36:3452–3456. [Google Scholar]
- 21.Weisman S, Hirsch-Lerner D, Barenholz Y, Talmon Y. Biophys. J. 2004;87:609–614. doi: 10.1529/biophysj.103.033480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.