Abstract
Mechanical stimuli generated by head movements and changes in sound pressure are detected by hair cells with amazing speed and sensitivity. The mechanosensitive organelle, the hair bundle, is a highly elaborated structure of actin-based stereocilia arranged in precise rows of increasing height. Extracellular linkages contribute to its cohesion and convey forces to mechanically gated channels. Channel opening is nearly instantaneous and is followed by a process of sensory adaptation that keeps the channels poised in their most sensitive range. This process is served by motors, scaffolds, and homeostatic mechanisms. The molecular constituents of this process are rapidly being elucidated, especially by the discovery of deafness genes and antibody targets.
Keywords: adaptation, mechanoelectrical transduction, hair cell, inner ear, deafness
INTRODUCTION
Hair cells of the inner ear execute the fundamental process by which mechanical stimuli, originating from head movements or acoustic waves, are converted to neural signals. At the heart of the process is deflection of the hair bundle by mechanical force (see Figure 1), which causes the rapid opening of transduction channels. Depolarizing current flowing through transduction channels modulates neurotransmitter release onto afferent fibers of the eighth nerve, which encode and carry auditory and vestibular information to the brain.
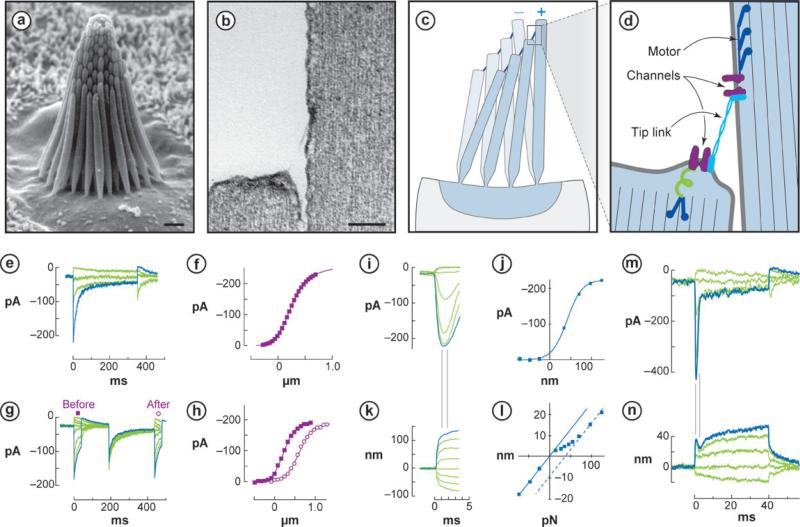
Figure 1.
Hair cell anatomy and physiology. (a) Hair bundle in a bullfrog saccule, comprising ~60 stereocilia and a single kinocilium (adjacent to tallest stereocilia) (scale bar = 1 μm). (b) Two stereocilia and the tip link extending between them (scale bar = 0.1 μm). (c) Deflection of hair bundle. Stereocilia tips remain in contact but shear with deflection. (d) Schematic model of transduction. Shearing with positive deflection increases tension in tip links, which pull open a transduction channel at each end. Myosin motors slip or climb to restore resting tension. An elastic gating spring likely exists between a channel and the actin cytoskeleton. (e) Mouse utricular hair cell transduction currents. Step deflections (not shown) from –0.3 to +0.7 μm elicit inward currents that adapt over 10–50 ms. (f) Activation or I(X) curve shows an operating range of ~0.7 μm, with ~12% of channels open at rest. Inward current is shown here as upward to reflect open probability. (g) Multiple short stimulus steps (not shown) map the I(X) curve before (square) and after (open circle) a 250-ms, 0.4-μm adapting step. (h) Adaptation occurs as a shift of the activation curve in the stimulus direction to an extent of 80%–90% of the stimulus amplitude. (i) Bullfrog saccular hair cell transduction currents, measured 1 ms after onset of a force stimulus delivered by an optical trap. (j) The I(X) curve is very narrow (~0.1 μm) when measured with fast stimuli. (k) Corresponding bundle movements elicited by the force stimuli. (l) Force-displacement relation measured 1 ms after onset of the stimulus. The bundle shows simple elastic movements for stimuli where channels are all closed or all open but is more compliant over the range of channel opening. (m) Fast and slow adaptation in a bullfrog hair cell. Positive force steps (not shown) elicit rapid channel opening followed by adaptation occurring in two phases, a fast phase of 1–3 ms and a slow phase of 10–50 ms. (n) Corresponding bundle movements reveal correlates of adaptation: a quick negative movement associated with fast adaptation and a slower positive movement with slow adaptation.
For more than 30 years, researchers have worked to elucidate the mechanism of transduction at the single-cell level. The result has been a detailed understanding of the ultrastructure and biophysics of transduction. Poorly understood, however, are the specific proteins that create these structures and mediate the biophysics. The cloning of deafness genes and the identification of hair-bundle antigens in recent years have provided some candidate molecules (see Table 1). Here, we summarize what we know of the physiology and ask which roles these candidate proteins might play in hair cell function.
Table 1.
Mutations of putative mechanosensation genes
| Putative function |
Gene (name) |
Mutant mice |
Human deafness genes |
|---|---|---|---|
| Adaptation | Myo7a (myosin 7a) | shaker-1, Myo7a nmf371, Myo7a6J, Myo7a4626SB | DFNB2, DFNA11, USH1B |
| |
Myo1c (myosin 1c) |
Y61G knockin, Y61G transgenic |
|
| Homeostasis | Atp2B2 (PMCA2) | deafwaddler, wriggle, dfw2J | |
| Ush1g (Sans) | Jackson shaker | USH1G | |
| |
Ush1c (Harmonin) |
Deafcircler, deaf circler 2 Jackson |
DFNB18, USH1C |
| Stereociliary Links | Gpr98 (Vlgr1) | Vlgr1 knockout | DFNB6, USH2C |
| Pcdh15 (Protocadherin 15) | Ames Waltzer | DFNB23, USH1F | |
| Cdh23 (cadherin 23) | Age-related hearing loss 1, bobby, bustling, modifier of deaf waddler, otocadherin, waltzer, Cdh23 nmf112, Cdh23 nmf181, Cdh23 nmf252 | DFNB12, USH1D | |
| Ush2a (Usherin) | USH2A | ||
| |
Ptprq
|
Ptprq knockout |
|
| Transduction Channels | TrpN1 | nompC (D. melanogaster) | |
| TrpV4 | TrpV4 knockout | ||
| TrpA1 | TrpA1 knockout | ||
| Mcoln3 (TRPML3) | veratint-waddler, varitint-waddler-J | ||
| Tmhs | hurry scurry | DFNB67 | |
| P2rx2 (P2X2) | P2X2 knockout |
STRUCTURE AND BIOPHYSICS
Bundle Structure and Development
Hair cells in vertebrate inner ear or lateral line organs bear a single bundle composed of tens to hundreds of actin-based stereocilia and (except in mature mammalian cochlear hair cells) a single microtubule-based kinocilium (see Figure 1). The tapered bases of stereocilia insert into an actin-rich cuticular plate; the kinocilium is anchored by a basal body adjacent to the cuticular plate. These structures anchor the bundle, and the taper creates a flexion point that allows the rigid stereocilia to pivot at their bases (Crawford & Fettiplace 1985). The eccentric placement of the kinocilium and the monotonic increase in stereocilia heights, with the tallest nearest the kinocilium, define the bundle's morphological axis. Deflections of the hair bundle toward the tallest stereocilia (positive deflections) open transduction channels, defining a corresponding physiological axis. Even within a species, hair bundles in different regions of an inner ear organ can have diverse morphology, with stereocilia varying in number, width, height, and arrangement. Hair bundle morphology likely contributes to variation in mechanical properties and frequency tuning (Cotton & Grant 2004).
A variety of extracellular linkages connect adjacent stereocilia (discussed in detail below). Of special note are the tip links that extend from the tip of each stereocilium to the side of the tallest adjacent stereocilium (see Figure 1b). Tip links are thus arrayed along the bundle's excitatory axis and not from side to side.
Proper development of the hair bundle is essential for hair-cell mechanotransduction, and, not surprisingly, defects in the participating proteins are causes of inherited deafness. The development of the hair bundle has been comprehensively described for the mouse and chick (reviewed in Frolenkov et al. 2004, Tilney et al. 1992). At early stages, the apical surfaces of hair cells and supporting cells are covered with short actin-based microvilli and a single kinocilium. Kinocilium migration to one edge of the cell then determines hair bundle polarity. Microvilli nearest the kinocilium elongate first by adding actin monomers at their tips. Successive rows grow out, and tip links soon connect adjacent rows of stereocilia. Stereocilia then expand in width, concomitant with tapering at their bases. Over the next several days, β- and γ-actin are crosslinked by espin and fimbrin forming the core of the stereocilia. Finally, stereocilia grow to reach their mature length (Tilney et al. 1988).
Mechanotransduction
Step deflections of hair bundles in the mouse utricle reveal the characteristic features of transduction (see Figure 1e, f). Positive deflections of <1 μm elicit inward currents of up to ~1 nA; negative deflections turn off channels that are open at rest. The activation curve, characterized by the current as a function of deflection [I(X)], has a typical range of 0.5 μm or less. With the hair bundle in its resting position, the channel is poised in the steepest portion of its operating range, with 10%–20% of channels open.
Channel opening is extremely fast, with time constants ranging from ~1000 μs in turtle at 3°C to <10 μs in mammals at 37°C, ruling out second-messenger-mediated activation of channels (Corey & Hudspeth 1979b, Crawford et al. 1989, Ricci et al. 2005). Instead, channels are thought to be directly opened by mechanical force conveyed to the channel by stretching an elastic “gating spring” element (Corey & Hudspeth 1983b). Sensitive measurements of bundle mechanics confirmed this model by detecting the mechanical correlate of channel opening (Howard & Hudspeth 1988). Specifically, the stiffness of the bundle is constant when channels are all closed or all open but is reduced when channels can open or close (see Figure 1i–l). From such measurements, recently confirmed and extended (Cheung & Corey 2006), one sees that the channel moves by ~2.5 nm on opening and that the gating spring has a stiffness ~1000 μN/m.
Transduction channels are permeable to small cations, up to ~1.2 nm in diameter (Corey & Hudspeth 1979a, Farris et al. 2004) and pass Ca2+ particularly well (Lumpkin et al. 1997). Thus the bulk of the transduction current in vivo, where bundles are bathed in a high-K+ low-Ca2+ endolymph, is carried by K+. Both Ca2+ and K+ must then be transported out of the hair cell; whereas the electrochemical gradient for K+ allows passive efflux through channels in the cell's basolateral surface, active pumps are required to extrude Ca2+ from stereocilia.
The mechanosensitive transduction channels are located in the distal tips of the stereocilia (Hudspeth 1982, Denk et al. 1995). The discovery of tip links in the same area suggested a structural model for transduction (see Figure 1c,d), in which positive deflection of stereocilia increases tension in tip links, which pulls the channels open (Pickles et al. 1984). Although the tip link was initially suggested to be elastic like the physiologically defined gating spring, better images suggest that the tip link does not stretch (more like a cable than a spring) and imply that the gating spring is intracellular instead (Kachar et al. 2000).
Stereocilium: rigid, elongated microvillus filled with a paracrystalline array of actin filaments that forms the building block of the hair bundle
Mechanotransduction: conversion of mechanical stimuli into electrical signals
Adaptation
It is remarkable that hair cells can detect hair bundle deflections smaller than 1 nm, as they do at human auditory threshold, but even more so that this high sensitivity can be maintained in the presence of large static stimuli. Several Ca2+-dependent processes work in parallel to maintain the proper bias on transduction channels to keep them at their most sensitive range. In doing so, they produce a rapid adaptation of the response to deflection, which limits the sensitivity to slow or tonic stimuli and may also amplify the response to rapid sinusoidal stimuli.
Adaptation : process by which sensory cells adjust to static stimuli allowing for detection of stimulus changes
Adaptation can be seen in the response to step deflections, as a relaxation in channel open probability occurring over ~50 ms. During positive deflections, channels tend to reclose; during negative deflections they reopen (see Figure 1e). Mapping the activation curve with short steps before and at the end of a maintained adapting step (see Figure 1g) reveals that adaptation is manifest as a shift of the activation curve in the direction of the stimulus, without significant change in the number or conductance of available channels (see Figure 1h) (Corey & Hudspeth 1983a, Eatock et al. 1987).
On an expanded timescale (see Figure 1m), adaptation clearly occurs on two distinct timescales, termed fast (0.3–3 ms) and slow (5–50 ms) adaptation. An attractive and well-supported model for slow adaptation is that a motor complex at the upper end of each tip link, comprising ~100 myosin molecules and associated regulatory and scaffolding proteins, continuously exerts a bias force on the channels, keeping them open 10%–20% of the time. Increased tension during positive deflection drags the motor complex down, relaxing tension on the channels and allowing them to close (Howard & Hudspeth 1987). Negative deflections allow the complex to climb and restore tension. Slow adaptation resulting from positive deflection is correlated with a slow positive movement of the bundle (see Figure 1n), consistent with slipping of a motor. Slow adaptation is an active process that requires the hydrolysis of ATP and involves a myosin motor (Gillespie & Hudspeth 1993). Ca2+ that enters the tips of stereocilia through open transduction channels accelerates the process in both the climbing and slipping directions, also consistent with regulation of a myosin motor.
In general, adaptation is never complete, in that the activation curve moves by only 80%–90% of the stimulus amplitude. To account for the incompleteness, the mechanical model for slow adaptation was expanded to incorporate an additional “extent spring” that acts to limit the degree of motor slipping or climbing (Shepherd & Corey 1994, Vollrath & Eatock 2003). The structural and molecular correlates of the extent spring are not known, and it may be that the extent is limited by something more complicated than an elastic element.
Fast adaptation is both less well understood and perhaps more important for auditory perception. It manifests as a rapid closure of channels during positive deflections, sometimes in less than 0.1 ms (see Figure 1m) (Howard & Hudspeth 1987, Kennedy et al. 2003, Ricci et al. 2000). Fast adaptation is mediated by Ca2+ that enters through open transduction channels. Its rapid time course along with estimates of stereociliary calcium buffering and diffusion suggest that calcium acts within 20–40 nm of the inner mouth of the channel (Lumpkin & Hudspeth 1995, Ricci et al. 1998). Like slow adaptation, fast adaptation has a mechanical correlate, but in the opposite direction: As channels close following Ca2+ entry, the bundle displays a small negatively directed “twitch,” indicating increased tension in the tip links (see Figure 1n). The twitch is caused by closure of transduction channels because it does not occur for extreme bundle positions where the channels remain closed or open (Benser et al. 1996, Cheung & Corey 2006).
Researchers have proposed three distinct Ca2+-dependent mechanisms for fast adaptation, which could produce both the decline in current and the corresponding bundle twitch. First, Ca2+ could bind directly to the transduction channel to promote closure, and the closing channels would pull on tip links to move the bundle negatively (Cheung & Corey 2006, Howard & Hudspeth 1988, Wu et al. 1999). Second, Ca2+ could bind to an elastic “reclosure element” in series with the channel, decreasing its stiffness to reduce tension. The reduced tension would allow channels to close, and as they snap shut they would paradoxically increase tip link tension to move the bundle (Bozovic & Hudspeth 2003). Finally, Ca2+ could bind to a “release element” in series with the channel, causing it to lengthen. Again, the reduced tension would close channels, and the closure would increase tension to move the bundle (Stauffer et al. 2005). A quantitative analysis of the movement's dependence on bundle position supported only the first model and suggested that a Ca2+-bound channel requires ~3 pN more force to open (Cheung & Corey 2006). However, chemical inhibition of myosin-1c, the myosin of the slow adaptation motor, also slowed fast adaptation, suggesting a more complex interaction of myosin-1c with the fast adaptation process (Stauffer et al. 2005).
Both slow and fast adaptation have mechanical correlates, but slow adaptation causes a relaxation in the direction of the stimulus, whereas fast adaptation causes force production in the opposite direction. Timed properly, fast adaptation can contribute energy to bundle movement. For sinusoidal stimuli (such as acoustic vibrations in the cochlea), the positive phase of a stimulus would cause, with a delay, a negative twitch that could amplify the next negative phase. Like pushing a child on a swing, small forces could build up the vibration of the bundle. In principle, forces generated by stereocilia could amplify the overall vibration of the cochlea's basilar membrane, both increasing the sensitivity and sharpening the tuning (Howard & Hudspeth 1987, Hudspeth 2005).
Which specific proteins, then, form the mechanotransduction machinery and execute this complex physiology?
MOLECULES CONTRIBUTING TO EXTRACELLULAR LINKS
Individual stereocilia are connected by a variety of extracellular links, named for their location along the stereocilia. Link development has been best described in chick and mouse (Goodyear et al. 2005, Tsuprun et al. 2004), but links vary among species and inner ear organs and may be molecularly heterogeneous. Clues to their molecular identity come from diverse sources: mouse deafness models with disorganized bundles and missing links; high-resolution electron microscopy; identification of the epitopes for stereocilia-binding antibodies; comparison of the developmental timecourse of links and candidate proteins; and comparison of link sensitivity to enzymes and calcium chelators.
Ankle Links
Ankle links connect the stereocilia at the taper region near the apex of the cell. They are composed of small globular structures regularly extending ~150 nm from the ankle of one stereocilia and forming a zipper-like structure with the ectodomain of the adjacent ankle link (Tsuprun et al. 2004). These links are sensitive to both subtilisin and BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid). They persist in mature vestibular hair cells in a variety of species (chick: Goodyear & Richardson 1992, Goodyear & Richardson 1999; mouse: Goodyear et al. 1995; human: Jeffries et al. 1986) but are lost in mature mouse cochlear hair cells (Goodyear et al. 1995).
Vlgr1
One component of the ankle links in chick hair cells, recognized by a monoclonal antibody and called the ankle link antigen (ALA) (Goodyear & Richardson 1999), was recently identified as the very large G protein–coupled receptor 1 (Vlgr1) (McGee et al. 2006). VLGR1, a huge protein of ~6300 amino acids, has an extended N-terminus composed primarily of calx beta domains, a domain found in Na+-Ca2+ exchangers and the integrin subunit beta4 that is usually cytoplasmic and that binds Ca2+ (see Figure 2). The C-terminus has a proteolytic site and seven transmembrane domains typical of G protein–coupled receptors, suggesting a signaling function as well as a structural function.
Usher syndrome: group of genetic disorders characterized by varying degrees of congenital or progressive auditory, vestibular, and visual dysfunction
Figure 2.
Proteins associated with links between stereocilia. (a) Position of different links between stereocilia. (b) Links at tips of stereocilia. (c) Protein domain structures. CA, cadherin domain; tm, transmembrane domain; FN3, fibronectin type 3 domain; PTPc, protein tyrosine phosphatase catalytic domain; LamNT, laminin N-terminal domain; EGF lam, laminin-type epidermal growth factor–like domain; LamG, laminin G domain; calx beta, Ca2+ exchanger/integrin-beta4 domain; EPTP, epitempin-like domain; GPS, G protein–coupled receptor proteolytic site domain; GPCR7tm, G protein–coupled receptor seven-transmembrane domain.
Mutations in the human ortholog of Vlgr1 cause Usher syndrome 2C. Vlgr1-mutant mice lack ankle links. They are severely deaf by three weeks of age, although vestibular hair cells retain normal transduction currents (McGee et al. 2006). Vlgr1-mutant mice are predisposed to audiogenic seizures (McMillan & White 2004).
Usherin
Usherin, a protein defective in Usher syndrome 2A, is another candidate for the ankle links. Although originally described as a basement membrane protein (Bhattacharya et al. 2002), a long isoform was recently described which is restricted to the ankle-link region (Adato et al. 2005a). Usherin, a large protein of almost 5200 amino acids, has an extended N-terminus with a variety of protein interaction domains preceding a single transmembrane domain (see Figure 2). They include domains (LamNT, EGF lam, and LamG) found in the basement membrane adhesion protein laminin and 37 fibronectin-type-3 domains. The ectodomain is predicted to be 125–170 nm in length in different isoforms. It is possible that Usherin and Vlgr1 multimerize to form the ankle link, but this remains to be shown experimentally.
Although loss of these candidate ankle link proteins contributes to deafness in Usher syndrome and improper hair cell development, hair cell transduction is unlikely to depend on the ankle links because transduction persists after removal of ankle links with subtilisin.
Shaft Connectors
Ptprq
Shaft connectors span the region between neighboring stereocilia above the ankle links (see Figure 2). A component of the shaft connectors, recognized by an antibody and called the hair cell antigen (HCA) (Goodyear & Richardson 1992), has been identified as Ptprq, a member of the receptor-like protein tyrosine phosphatase family (Goodyear et al. 2003). Ptprq has an extended N-terminus composed of 17 fibronectin-type-3 domains, which mediate binding to extracellular proteins. There is a single transmembrane domain (see Figure 2). A C-terminal domain, similar to the protein tyrosine phosphatase type C domain, apparently functions as a phosphatidylinositol lipid phosphatase (Oganesian et al. 2003).
Ptprq-deficient mice lack shaft connectors, but hair bundles do not significantly splay or separate. Transduction currents, although smaller than normal, adapt normally (Goodyear et al. 2003). Instead, cochlear stereocilia become fused and degenerate. Ptprq may be a structural element that properly spaces stereocilia; alternatively, phosphatase activity may maintain the appropriate lipid content necessary for stereocilia integrity.
Horizontal Top Connectors
In the mouse cochlea, horizontal top connectors—also referred to as lateral links—are a zipper-like array of links, 12–14 nm long, 4–8 nm wide, and spaced ~20 nm apart, that connect the upper shafts of the stereocilia (Goodyear et al. 2005). In outer hair cells they consist of 1–3 strands composed of small globular domains spaced 6–8 nm apart. Pairs of links overlap and end in a prominent varicosity (Tsuprun et al. 2003). These links persist in mature hair cells and are insensitive to subtilisin and BAPTA. At present their molecular identity is unknown, and the lack of agents that disrupt them makes it difficult to test their function. However, the continued cohesion of the bundle after all other links are severed suggests that horizontal top connectors mediate a sliding adhesion of stereocilia extending to high frequencies (Karavitaki & Corey 2006).
Tip Links
Although all links contribute to hair bundle development and mechanics, only the tip link has a clear role in mechanosensation. Tip links are disrupted by BAPTA, EGTA (ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid), elastase, or La3+, but not by subtilisin; these abolish mechanosensitivity and move the bundle by a distance consistent with releasing resting tension on transduction channels (Assad et al. 1991, Crawford et al. 1991). Furthermore, tip links and mechanotransduction return on the same time scale of 5–10 h (Zhao et al. 1996). The protein synthesis inhibitor cyclohexamide does not prevent regeneration (Zhao et al. 1996), suggesting a reserve pool of tip links or transduction complexes.
Electron micrographs reveal the tip link as 8–11 nm in diameter and ~150 nm in length and as a pair of coiled filaments composed of uniform globular units (Tsuprun et al. 2004, Kachar et al. 2000). At its upper and lower attachment points the link bifurcates into two or three strands, respectively.
Cadherin 23
Cadherin 23 has an extended extracellular N-terminal domain comprising 27 cadherin repeats and a single transmembrane domain (see Figure 2). The cadherin domains mediate Ca2+-dependent antiparallel homotypic binding between adjacent membranes. Steered molecular dynamics simulations of individual cadherin repeats suggest a very stiff structure, consistent with the indications from electron microscopy that the tip link is inextensible (Sotomayor et al. 2005).
Mutations in the human CDH23 gene cause Usher syndrome 1D. Mice with mutations in Cdh23 display aberrant stereocilia organization early in hair-cell development and show progressive hearing loss (Di Palma et al. 2001). In developing hair bundles, cadherin 23 is located along the length of the stereocilia, where it likely contributes to transient lateral links and kinociliary links (Michel et al. 2005, Rzadzinska et al. 2005, Siemens et al. 2004). Conflicting results have been reported for mature hair cells. An antibody to the cytoplasmic region of Cadherin 23 showed intense labeling along the kinocilia and in the tallest stereocilia, with more specific labeling at the tips. Immunogold labeling appears near the tip link insertions (Siemens et al. 2004, Sollner et al. 2004). In contrast, labeling with other N- and C-terminal antibodies disappears in mature hair bundles, inconsistent with the continuing presence of tip links (Boeda et al. 2002, Lagziel et al. 2005, Michel et al. 2005). Cadherin 23 can be immunoprecipitated with myosin-1c, a part of the transduction complex, although in vitro association may not reflect binding in hair cells.
The chemical sensitivity of these epitopes is also confusing. Treatment with EGTA, which breaks tip links, apparently eliminates cadherin 23 immunoreactivity from the tips of the hair bundle and increases immunoreactivity at the apical surface. Like tip links, recombinant cadherin 23 is cleaved by elastase but not by subtilisin (Siemens et al. 2004). A different study found that cadherin 23 staining in the hair bundle was insensitive to BAPTA or La+3 but was abolished with subtilisin (Michel et al. 2005). These differences may be attributable to scarcity of tip link protein and the presence of different splice variants.
Protocadherin 15
Like cadherin 23, protocadherin 15 (Pcdh15) has a large extracellular domain with 11 cadherin repeats and a single transmembrane domain (see Figure 2). A component of the tip link and kinocilial links in chick hair cells called the tip link antigen (TLA) was recently identified as Pcdh15 (Ahmed et al. 2006). Its immunoreactivity follows a developmental pattern similar to cadherin 23, being widely expressed early and later restricted to the tips of stereocilia and kinociliary links (Goodyear & Richardson 2003). Furthermore, TLA immunoreactivity is lost with BAPTA treatment and recovers on the same timescale as tip links.
At least four isoforms of protocadherin 15 are expressed in the inner ear. Three contain unique cytoplasmic domains; a fourth is likely secreted. Staining with isoform-specific antibodies shows that each has a distinct spatial and temporal expression pattern as well as differing chemical sensitivities (Ahmed et al. 2006). Because the labeling pattern of no single isoform completely overlaps the TLA, protocadherin 15 may contribute to a variety of links at different times in development.
Mutations in the human PCDH15 gene are responsible for Usher syndrome 1F and for nonsyndromic deafness, DFNB23 (Alagramam et al. 2001b, Ahmed et al. 2001). Pcdh15 is mutated in Ames waltzer mice, which are deaf and exhibit vestibular dysfunction (Alagramam et al. 2001a, Pawlowski et al. 2006). In these mice the cochlear and saccular epithelia degenerate, but the crista and utricle are unaffected (Alagramam et al. 1999, 2000). Furthermore, the horizontal top connectors and ankle links are intact, suggesting protocadherin 15 is not a major component of either linkage. More severe mutant Pcdh15 alleles disrupt the stereociliary rootlets and actin meshwork of the cuticular plate (Pawlowski et al. 2006), and they have splayed and poorly formed bundles, suggesting an important role in bundle development. Cochlear and vestibular hair cells lack transduction currents; however, this may be secondary to bundle malformations.
MOLECULES CONTRIBUTING TO TRANSDUCTION AND ADAPTATION
Motor Proteins for Adaptation
Slow adaptation represents a change in the force stimulus reaching the transduction channels (Corey & Hudspeth 1983a,b). When it was recognized that tip links could convey the force (Pickles et al. 1984), that they were near the actin cores of stereocilia, and that slow adaptation corresponded to a mechanical relaxation of the elements within the bundle, investigators suggested that a myosin-based motor regulates the force on channels (Howard & Hudspeth 1987). A search for the myosin then followed (for review see Gillespie & Cyr 2004).
Myosin-1c
Phosphate analogs such as vanadate bind to the ATP-binding site of myosins and can block force production. Such treatment of hair cells slowed the adaptation motor and reduced its apparent resting tension (Yamoah & Gillespie 1996), supporting the idea that the adaptation motor is indeed a myosin. Labeling of purified frog stereocilia proteins in the presence of vanadate revealed three potential myosin candidates. One, of ~120 kDa, was labeled by antibodies to bovine myosin-1c; these antibodies also labeled the distal hair bundles of frog hair cells (Gillespie et al. 1993). In addition, antibodies made to a myosin-1c cloned from frog saccule labeled the distal stereocilia of hair cells, especially their tips (Hasson et al. 1997, Solc et al. 1994). Quantitative immunogold localization showed myosin-1c was at its highest concentration at either end of the tip link, within ~100 nm of its insertions (Garcia et al. 1998).
Myosin-1c is a type-I unconventional myosin, smaller (at 1028 amino acids) than conventional muscle myosins. It has a traditional myosin head that binds actin and hydrolyzes ATP, a linear neck region of three or four light-chain-binding IQ domains, and a globular C-terminus, which apparently mediates binding to membranes and resembles a binding domain found in transcriptional regulators (see Figure 3). The tail portion of myosin-1c is unlike the coiled-coil domain of conventional myosins, which dimerize to form a two-headed motor protein that binds and translocates along actin filaments. Instead, the C-terminus of type-I myosins contains a putative anionic phospholipid-binding domain, which can adhere to phosphatidylserine and phosphatidylinositol 4,5 bisphosphate (PIP2). Type-I myosins tend to be unbound from actin 90%–95% of the time. Thus to move and not diffuse away, myosin-1c must be clustered by a scaffolding protein or through binding to an immobile element such as a lipid raft. Protein purification and antibody labeling suggested that there are 100–500 myosin-1c molecules per stereocilium (Garcia et al. 1998, Gillespie et al. 1993), fitting well with estimates of 100–200 myosins in the adaptation motor, of which 5–10 are in series with the transduction channel at any one time (Gillespie & Cyr 2004). Myosin-1c binds 2–3 molecules of calmodulin under low-Ca2+ conditions (Cyr et al. 2002), but binding is disrupted by increased Ca2+ concentrations, consistent with the adaptation rate's Ca2+ dependence.
Figure 3.
Proteins associated with adaptation. Myosin-1c is located in the hair bundle, especially in the distal third, and is most concentrated at either end of the tip link. Myosin-7a is found throughout the bundle. Both are found in the vesicle-rich pericuticular zone (pz) between the cuticular plate (cp) and the membrane. IQ, regulatory light-chain-binding domain; HDACI, histone deacetylase interacting domain; EFH, EF hand (Ca2+-binding) domain; cc, coiled-coil domain; MyTH4, myosin tail homology domain 4; FERM, 4.1/ezrin/radixin/moesin-like domain; SH3, Src homology 3 domain; PDZ, PSD-95/Dlg/ZO-1-like domain.
To test myosin-1c involvement in the adaptation motor, Gillespie and colleagues (1999) introduced a mutation that expanded the ATP-binding pocket of myosin-1c (tyrosine 61 to glycine, Y61G) so that a larger ADP analog, N6 2-methyl butyl ADP (NMB-ADP), could bind and inhibit motility. Mutant myosin-1c in vitro, inhibited with NMB-ADP, blocked motility of wild-type myosin-1c that was mechanically coupled to it. Thus the mutant acted in a dominant-negative manner. Adaptation was then tested in transgenic mice carrying an additional gene for the Y61G myosin-1c. Hair cells from this mouse had normal transduction and adaptation in the absence of NMB-ADP. When NMB-ADP diffused into hair cells from the recording pipette, however, adaptation was blocked in minutes (Holt et al. 2002). Similar results were seen in a knock-in mouse where all myosin-1c carried the Y61G mutation (Stauffer et al. 2005). Myosin-1c involvement in the slow adaptation motor seems incontrovertible at this point.
Surpisingly, inhibition of myosin-1c in the Y61C knock-in mouse (but not in the transgenic) reduced fast adaptation as well (Stauffer et al. 2005). Myosin-1c may also be an important mechanical component mediating fast channel closure, or slow adaptation may poise the channel in an appropriate range for more direct Ca2+-dependent closure.
Calmodulin
Calmodulin is a classic, small, Ca2+-binding regulatory protein with four EF-hand domains (see Figure 3). Calmodulin is detected at the distal tips of stereocilia (Shepherd et al. 1989), near the insertion points at both ends of the tip link (Furness et al. 2002). A higher density of molecules was observed in the side plaques of the taller stereocilia, consistent with calmodulin binding myosin-1c and with its putative role in mediating the Ca2+ dependence of adaptation.
Myosin-7a
Myosin-7a, first identified in PCR screens in cell lines and inner ear (Bement et al. 1994, Solc et al. 1994), became of special interest when it was recognized as the gene product mutated in the human deafness Usher syndrome 1B (Weil et al. 1995) and in the deaf, shaker-1 mouse (Gibson et al. 1995).
Myosin-7a is a larger unconventional myosin with a traditional head domain and five IQ domains. A short coiled-coil domain may mediate dimerization. An extended tail has a tandem arrangement of likely cargo-binding domains (MyTH4 and FERM domains) found in myosins and in a kinesin as well as an SH3 domain also associated with protein interaction (see Figure 3). Myosin-7a is widely expressed in epithelial cells that have cilia or microvilli (Hasson et al. 1997) and may anchor membrane proteins to the cytoskeleton. Myosin-7a has also been associated with transport of intracellular organelles in a variety of cells. It is located throughout the hair bundle of mouse hair cells (although it is restricted to the ankle-link region in frog) and found in the cuticular plate and pericuticular zone (Boeda et al. 2002, el-Amraoui et al. 1996, Hasson et al. 1997).
In the shaker-1 mouse mutants, the severity of phenotype depends on the specific allele. But in general, hair bundles are disorganized with stereocilia sometimes separated into clusters. Within clusters, however, stereocilia retain their staircase pattern and are interconnected with horizontal top connectors and tip links. Myosin-7a may thus play a role in linking adjacent stereocilia, especially during development (Self et al. 1998).
In shaker-1 mice, transduction channels are closed in a resting hair bundle and require large positive deflections to be opened, as if resting tension on the transduction channels is greatly reduced. Furthermore, adaptation is much faster in shaker-1 hair cells, as if the adaptation motor cannot exert much force (Kros et al. 2002). This suggests that myosin-7a may also be involved in the adaptation motor complex. However, the evidence for myosin-1c as the adaptation motor is very strong, and myosin-7a is not concentrated near stereocilia tips. Instead, defects in myosin-7a may prevent the proper transport or assembly of adaptation motor components.
A large number of putative myosin-7a-binding proteins have been discovered and localized within hair cells (Boeda at al. 2002); some of these are the products of genes defective in other forms of Usher syndrome. Despite clear phenotypes, no clear function for myosin-7a in hair cells has been found.
Scaffolding Proteins
Two scaffolding proteins, harmonin b and SANS, are essential for proper hair bundle development and may interact with extracellular links and components of the transduction complex. Numerous interactions have been found using in vitro approaches (for review, see Adato et al. 2005b, Reiners et al. 2006), most which remain to be verified in hair cells.
Harmonin b
The gene defective in Usher 1C encodes harmonin, a protein that occurs in a variety of splice forms. All are intracellular scaffolding proteins with 2–3 PDZ domains and a coiled-coil region (see Figure 3). Harmonin b is localized specifically to stereocilia and binds both cadherin 23 and myosin-7a (Boeda et al. 2002), thus creating a link by which myosin-7a could be involved in connections between stereocilia. Further evidence for harmonin interaction with myosin-7a is its failure to localize to stereocilia in the shaker-1 mouse. Harmonin b also reportedly binds protocadherin 15, VLGR1, and usherin.
MODULATORS OF ADAPTATION AND HOMEOSTATIC MECHANISMS
Anionic Phospholipids
PIP2
The anionic lipid phosphatidylinositol-4,5-bisphosphate (PIP2) is found in the distal portions of frog saccular stereocilia. Ptprq, a lipid phosphatase also thought to contribute to shaft connectors, is found in the PIP2-free regions and may keep PIP2 concentrations low by removing phosphate groups to form PI(4)P or PI(5)P. Ptprq-deficient mice have cochlear hair bundles that become disorganized after birth; the hair cells eventually degenerate (Goodyear et al. 2003).
PIP2 is required for proper mechanotransduction and adaptation. It binds to myosin-1c both on the C-terminus (Tang et al. 2002) and the IQ domains (Hirono et al. 2004). Pharmacological agents that deplete PIP2 reduce the rates of slow and fast adaptation and increase the resting open probability of the transduction channel (and ultimately abolish hair cell transduction; Hirono et al. 2004). One interpretation is that PIP2 binding tends to separate myosin-1c from the actin core, reducing the overall force of the motor and accelerating adaptation. Indeed, elevated Ca2+ increases binding of myosin-1c to PIP2, perhaps by releasing calmodulin and exposing IQ domains, and it also accelerates adaptation. Without PIP2, myosin-1c is less tightly associated with the membrane and could bind more tightly to actin to reduce the rate of slow adaptation.
Buffers and Pumps
Ca2+ concentration in the stereocilia affects the resting open probability of the transduction channel as well as the rate and extent of adaptation (Assad et al. 1989; Ricci et al. 1998, 2003; Ricci & Fettiplace 1997). Hair cells regulate calcium concentration with fixed and mobile calcium buffers and through active extrusion.
Calcium Buffers
Hair cells express many different calcium-binding proteins including calbindin D28k, calretinin, parvalbumin-α, and parvalbumin-β (oncomodulin). Calmodulin may also contribute to calcium buffering. Quantitative immunogold electron microscopy has shown that the concentration of calcium buffers varies along the tonotopic axis of the cochlea and with hair cell type (Hackney et al. 2003, 2005). In stereocilia the concentrations of calbindin-D28k and oncomodulin are estimated at 20 and 969 μM, respectively (Hackney et al. 2005). Calbindin-D28k is a small (261 a.a.) protein with six Ca2+-binding EF hands (see Figure 4). Oncomodulin has just two EF hand domains (see Figure 4). Electrophysiological measurements from turtle cochlear hair cells show a tonotopic gradient of calcium buffering capacity, equivalent to between 0.1 and 0.4 mM BAPTA for low- and high-frequency cells, respectively (Ricci & Fettiplace 1997).
Figure 4.
Proteins associated with ion homeostasis. All are located in stereocilia; the Ca2+-binding proteins calbindin and oncomodulin are in the soma as well. ATPase, cation transporter ATPase domain; hydrolase, haloacid dehalogenase-like hydrolase domain.
PMCA2
Even without stimulation, transduction channels open at rest may elevate stereociliary calcium by ~1 mM/s (Ricci & Fettiplace 1998). To extrude Ca2+, stereocilia contain ~2000 molecules/μm2 of plasma membrane Ca2+ ATPase (PMCA). PMCA is an electrogenic Ca2+/H+ exchanger, accepting one proton for every Ca2+ extruded (Hao et al. 1994). Its structure—10 transmembrane domains with interspersed intracellular ATP-binding, phosphorylation, and ATP-hydrolysis domains (see Figure 4)—is typical of ATP-dependent Ca2+ and Na+ pumps. PMCA2 was identified in hair cells on the basis of its pharmacological and electrophysiological properties (Yamoah et al. 1998). The outward current produced by the pump is dependent on Ca2+ entering through transduction channels and is blocked by vanadate, a phosphate analog that prevents ATP hydrolysis and blocks PMCA's enzymatic activity.
PMCA exchangers are the main pathway in the hair bundle for calcium extrusion (Dumont et al. 2001, Lumpkin & Hudspeth 1998, Yamoah et al. 1998). Deafwaddler mice with mutated PMCA2 are deaf and have vestibular defects (Street et al. 1998). PMCA2 apparently maintains the endolymphatic calcium level; in deafwaddler mice it is reduced from the normal ~30 μM to ~7 μM (Wood et al. 2004).
Proton Pumps
PMCA exchanges Ca2+ for H+, which could lead to acidification of the stereocilia cytoplasm (Yamoah et al. 1998). To maintain physiological pH, stereocilia must extrude protons. Gillespie and colleagues (Hill et al. 2006) have shown that hair cells extrude protons at ~1 mM/s. Several proton pumps are expressed in hair cells. ATP6V1E, a H+ ATPase, is prominent in the cell body of both cochlear and vestibular hair cells but not in stereocilia (Stankovic et al. 1997). Similarly, members of the family of electrogenic Na+/H+ exchangers, NHE1 and 3, have been detected in the hair cell body (Bond et al. 1998). However, the apical hair cell membrane is bathed in endolymph, which lacks the Na+ necessary to drive these pumps. Recently, two other members of the NHE family, NHE6 and -9, each with 12 transmembrane domains (see Figure 4), have been described in hair cell stereocilia (Hill et al. 2006). NHE6 and -9 operate as K+/H+ exchangers and are generally located in intracellular organelles where they control pH. In stereocilia, they would be powered by the electrochemical gradient for K+.
MECHANICALLY GATED ION CHANNELS
Clues to the molecular identity of the hair cell transduction channel can be gleaned from its physiological properties. It is a nonselective cation channel with high Ca2+ permeability (Corey & Hudspeth 1979a, Lumpkin et al. 1997, Ohmori 1985). In endolymph-like solutions with high K+, up to 20% of the current is carried by Ca2+ (Ricci & Fettiplace 1998), but millimolar Ca2+ blocks the channel. The large (100–300 pS) single-channel conductance (Crawford et al. 1991, Geleoc et al. 1997, Ricci et al. 2003) and permeability to small organic cations like the styryl dye FM1-43 (Corey & Hudspeth 1979a, Farris et al. 2004, Gale et al. 2001, Meyers et al. 2003) indicate a pore of at least 1.3 nm. Although there are no high-affinity blockers, amiloride and its analogs block at low micromolar concentrations in a voltage-dependent manner (Jorgensen & Ohmori 1988, Kroese et al. 1989, Marcotti et al. 2005, Rusch et al. 1994).
Candidate channel proteins should be localized at the tips of stereocilia and should be present from the onset of mechanosensitivity through adulthood. Because the single-channel conductance and kinetics vary in cochlear hair cells tuned to different frequencies (Ricci et al. 2003), multiple subunits or splice variants may form the channel.
Transient Receptor Potential Channels
A growing number of transient receptor potential (TRP) channels have been implicated in sensory transduction and mechanosensation in both vertebrates and invertebrates (Clapham 2003, Lin & Corey 2005, Sukharev & Corey 2004). In addition, TRP channels generally have high single-channel conductances and nonselective pores with high Ca2+ permeability (Owsianik et al. 2006). TRP channels operate as tetramers, but their combinatorial coassembly is still largely unknown.
NOMPC/TRPN1
Great excitement greeted a novel ion channel discovered in a genetic screen for mechanosensitive genes in D. melanogaster (Kernan et al. 1994, Walker et al. 2000). The mutated gene nompC (no mechanoreceptor potential in the bristle organs) is a homolog of mammalian TRP channels. Although the precise location of nompC within the bristle complex is unknown, bristle mechanoreceptors share many hallmarks of hair cell transduction, including fast-latency responses and nonselective currents that adapt with sustained deflection. Mutant alleles of nompC abolish or alter the large, transient portion of the receptor potential, implying that nompC is intimately linked to mechanosensation in the fly bristle (Walker et al. 2000).
Vertebrate orthologs of nompC, TRPN1, have been found in zebrafish (Sidi et al. 2003) and two frog species, Xenopus laevis (Shin et al. 2005) and Rana catesbeiana (N.D. Stern, K.Y. Kwan, and D.P. Corey, unpublished observations). TRPN1 is a large channel with 29 ankyrin repeats in an extended, probably intracellular, N-terminus. Ankyrin repeats are commonly protein-binding domains, but they also fold into a curved elastic structure. Six transmembrane domains with a pore domain between the fifth and sixth are typical of TRP channels; two others are predicted by hydropathy but not confirmed (see Figure 5). Transcripts for TRPN1 are detectable 48 h postfertilization in zebrafish inner ear hair cells during the developmental window when hair cells become mechanosensitive. Inhibiting TRPN1 expression with morphlino oligonucleotides leads to auditory and vestibular defects assessed by a loss of acoustic-startle response and uncoordinated swimming (Sidi et al. 2003). In fish without startle responses, mechanotransduction is impaired: Lateral line hair cells failed to accumulate the fluorescent dye FM1-43, a marker of functional transduction channels. Hair bundle deflections could not elicit microphonic potentials, an extracellular measure of current flow into hair cells.
Figure 5.
Protein candidates for the mechanosensory transduction channel. The channel is located most likely at either end of the tip link. A, ankyrin domain. Lightly-shaded transmembrane domains are predicted by hydropathy but not common to other members of the TRP channel family.
In Xenopus, a TRPN1 antibody labels the embryonic lateral line and adult saccular hair cells. However, saccular hair cell labeling can be observed only in the kinocilia, and not in the stereocilia where the transduction channels are located (Shin et al. 2005). TRPN1 may play a yet-undefined role in the kinocilia, supported by its presence in another class of ciliated epidermal cells located in the tadpole tail.
Despite some enticing evidence in insects and lower vertebrates, TRPN1 clearly cannot mediate transduction in all vertebrates because the gene is absent in mammals and birds as well as in two pufferfish species.
TRPA1
TRPA1 is unique among mammalian TRP channels owing to its large number of N-terminal ankyrin repeats. TRPA1 has 17 ankyrin repeats preceding the six transmembrane domains typical of TRP channels. A highly conserved coiled-coil domain in the C-terminus may mediate multimerization (see Figure 5). Steered molecular dynamics simulations suggest that the ankyrin repeat domain has elasticity like that of the hair cell gating spring (Lee et al. 2006, Sotomayor et al. 2005).
TRPA1 mRNA is detected in mouse hair cells as early as embryonic day 17, coincident with the onset of mechanosensation (Geleoc & Holt 2003). TRPA1 antibodies label the tips of bullfrog hair cell stereocilia; this labeling is eliminated by treatment with BAPTA or La3+ (Corey et al. 2004), which suggests that the channel may be recycled along with broken tip link components. Transient knockdown of TRPA1 with morpholino oligonucleotides in larval zebrafish or with adenovirus-mediated siRNAs in cultured embryonic mouse utricles reduced transducer channel function (Corey et al. 2004). These data, along with its structural similarity to TRPN1, strongly implicated TRPA1 as a candidate for both the mammalian mechanically sensitive channel and the gating spring.
However, some properties of TRPA1 expressed in heterologous cell lines do not match the native transduction channel. Single-channel conductances are large (100–300 pS in hair cells, but only 50–100 pS in cell lines; Nagata et al. 2005), but a known activator of TRPA1, allyl isothiocyanate, does not affect hair cell transduction (M.A. Vollrath & D.P. Corey, unpublished observations; Jordt et al. 2004). Furthermore, blocker sensitivities vary dramatically. The transduction channel is 10–20 times more sensitive to amiloride than is TRPA1 (Nagata et al. 2005), and it is 100 times less sensitive to Gd3+. Whereas TRPA1 is thought to be activated by intracellular Ca2+ (Jordt et al. 2004, Zuborg et al. 2007), Ca2+ inhibits the transduction channel (Cheung & Corey 2006, Howard & Hudspeth 1987).
More damning are findings that TRPA1-knockout mice have normal auditory and vestibular behavior (Bautista et al. 2006, Kwan et al. 2006), and transduction and adaptation are normal in single mouse utricular hair cells (Kwan et al. 2006). TRPA1 cannot be a critical component of the transduction complex unless another channel compensates for its function when it is deleted.
TRPML3
TRPML3 (mucolipin-3) is a small TRP channel with a large loop between the first and second transmembrane domains (see Figure 5). The varitint-waddler mouse has point mutations in Trpml3 and shows pigmentation defects, deafness, and circling behavior. The inner-ear phenotype is attributable to early-onset hair bundle disorganization and progressive hair cell degeneration (Cable & Steel 1998, Di Palma et al. 2002). Antibodies to TRPML3 label hair cell cytoplasm and vesicles in the pericuticular zone, but stereocilia are also labeled (Di Palma et al. 2002). TRPML3 also functions in the melanocytes of the stria vascularis, which maintain the endocochlear potential of the endolymphatic compartment. Although a role in transduction cannot be ruled out, the phenotype may be attributable to defects in vesicle trafficking and ion homeostasis because other TRP channels in its subfamily, TRPML1 and TRPML2, coassemble with TRPML3 and associate with lysosomal compartments (Venkatachalam et al. 2006).
TRPV4
TRPV4 is a typical TRP channel, with five ankyrin domains preceding its six transmembrane domains (see Figure 5). It may be activated by osmotic (Liedtke et al. 2000), mechanical (Liedtke et al. 2003), or thermal (Guler et al. 2002) stimuli. TRPV4 transcripts are present in hair cells, stria vascularis, and the spiral ganglion of the mouse cochlea. Although TRPV4 knockout mice exhibit hearing loss, hearing is normal during the first 8 postnatal weeks and later diminishes in sensitivity by just 20 dB (Tabuchi et al. 2005). Shen et al. (2006) recently demonstrated that osmotic and TRPV4 activators evoke responses in hair cells from wild-type but not TRPV4-deficient mice. Thus, TRPV4's function is still unresolved.
Other Channels that Could Play a Role in Mechanosensation
TMHS
Several members of the large tetraspan superfamily of membrane proteins have been implicated in human deafness; these include claudins, clarins, and connexins. A new member called TMHS, for transmembrane protein of hair cell stereocilia, has been implicated in human deafness (DFNB67) (Kalay et al. 2006, Shabbir et al. 2006). TMHS is a small (219 a.a.) protein with four putative transmembrane helices and two short cysteine-containing extracellular loops (see Figure 5).
Longo-Guess et al. (2005) also found a spontaneous mutation in TMHS in Hurry-scurry mice, which have auditory and vestibular defects. Cochlear hair bundles become disorganized and splayed by P8, and the cochlea degenerates by 4 months of age. A TMHS antibody indicates transient expression in cochlear hair cell stereocilia between E16.5 and P3 and in immature vestibular hair cells (Longo-Guess et al. 2005, Shabbir et al. 2006). TMHS's phenotype, location, and early time course of peak expression all suggest it plays a role in hair bundle morphogenesis.
P2X2
P2X2, a member of the P2X family of ATP-gated channels, has just two transmembrane domains with a large extracellular loop (see Figure 5). P2X receptors share some properties with the hair cell transduction channel. Both are expressed in the apical part of the hair cell and are blocked by amiloride. However, the ATP-activated and mechanically activated currents in hair cells are distinct. Futhermore, D-tubocurarine blocks both channels but with different KDs (Glowatzki et al. 1997). Finally, P2X1, 2, and 3 individual knockout animals and their combinations lack overt auditory or vestibular defects.
The criteria for the hair cell transduction channel have not been met by any of the current candidate channels known to be expressed in hair cells. Other candidates with high conductance and/or nonselective pores include other TRPs, acetylcholine receptors, glutamate receptors, cyclic-nucleotide-gated channels, and connexin hemichannels.
SUMMARY POINTS.
Although the anatomy and physiology of hair-cell transduction are well understood, the corresponding proteins have not, for the most part, been identified.
Cloning of deafness genes and hair-cell antigens has provided a number of candidates. Usher syndrome genes, in particular, have been illuminating.
Links between stereocilia are tentatively identified, with usherin and VLGR1 likely in the ankle links, PTPRQ in the shaft connectors, and protocadherin 15 and cadherin 23 possibly in the tip links. The horizontal top connector's identity is yet unknown.
PMCA2 pumps Ca2+ from the stereocilia but acidifies the cytoplasm; NHE6 and 9 then use the electrochemical gradient for K+ to pump protons out of stereocilia.
Myosin-1c is almost certainly the adaptation motor that regulates bias tension on transduction channels. Myosin-7a, after ten years and many putative binding partners, still does not have a clear function.
The transduction channel itself is conspicuously mysterious, despite the discovery of several promising candidates.
FUTURE ISSUES.
The key proteins of the transduction apparatus are the transduction channel, the tip link, and the adaptation motor. Only one has been firmly identified (myosin-1c is the motor). Finding the other two, and additional proteins that bind to them, will start to reveal the micromachinery of transduction. Only then can we start to correlate biophysical events with specific protein domains and to modify those domains to test biophysical theories. Only then can we test theories that might explain the exquisite sensitivity and frequency discrimination of vertebrate hearing.
The remarkable geometry and complexity of the transduction apparatus begs the question of how the components of the transduction complex are assembled during development and maintained and regenerated over the lifetime of the animal.
For many of the candidate molecules, knockouts and mutant mice have severe defects in hair cell development. Differentiating their roles in bundle development from those in the transduction complex may require chemical-genetic strategies like those used for myosin-1c, where function can be transiently disabled.
ACKNOWLEDGMENTS
Research in our laboratory is supported by the National Institutes of Health, the Mathers Foundation, the National Organization for Hearing Research, and the Howard Hughes Medical Institute. M.A.V. and K.Y.K. are Associates, and D.P.C. is an Investigator of the Howard Hughes Medical Institute.
LITERATURE CITED
- Adato A, Lefevre G, Delprat B, Michel V, Michalski N, et al. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum. Mol. Genet. 2005a;14:3921–32. doi: 10.1093/hmg/ddi416. [DOI] [PubMed] [Google Scholar]
- Adato A, Michel V, Kikkawa Y, Reiners J, Alagramam KN, et al. Interactions in the network of Usher syndrome type 1 proteins. Hum. Mol. Genet. 2005b;14:347–56. doi: 10.1093/hmg/ddi031. [DOI] [PubMed] [Google Scholar]
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, et al. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J. Neurosci. 2006;26:7022–34. doi: 10.1523/JNEUROSCI.1163-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ZM, Riazuddin S, Bernstein SL, Ahmed Z, Khan S, et al. Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am. J. Hum. Genet. 2001;69:25–34. doi: 10.1086/321277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagramam KN, Kwon HY, Cacheiro NL, Stubbs L, Wright CG, et al. A new mouse insertional mutation that causes sensorineural deafness and vestibular defects. Genetics. 1999;152:1691–99. doi: 10.1093/genetics/152.4.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagramam KN, Murcia CL, Kwon HY, Pawlowski KS, Wright CG, Woychik RP. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat. Genet. 2001a;27:99–102. doi: 10.1038/83837. [DOI] [PubMed] [Google Scholar]
- Alagramam KN, Yuan H, Kuehn MH, Murcia CL, Wayne S, et al. Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum. Mol. Genet. 2001b;10:1709–18. doi: 10.1093/hmg/10.16.1709. [DOI] [PubMed] [Google Scholar]
- Alagramam KN, Zahorsky-Reeves J, Wright CG, Pawlowski KS, Erway LC, et al. Neuroepithelial defects of the inner ear in a new allele of the mouse mutation Ames waltzer. Hear. Res. 2000;148:181–91. doi: 10.1016/s0378-5955(00)00152-0. [DOI] [PubMed] [Google Scholar]
- Assad JA, Hacohen N, Corey DP. Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc. Natl. Acad. Sci. USA. 1989;86:2918–22. doi: 10.1073/pnas.86.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad JA, Shepherd GM, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7:985–94. doi: 10.1016/0896-6273(91)90343-x. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–82. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bement WM, Hasson T, Wirth JA, Cheney RE, Mooseker MS. Identification and overlapping expression of multiple unconventional myosin genes in veterbrate cell types. Proc. Natl. Acad. Sci. USA. 1994;91:6549–53. doi: 10.1073/pnas.91.14.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benser ME, Marquis RE, Hudspeth AJ. Rapid, active hair bundle movements in hair cells from the bullfrog's sacculus. J. Neurosci. 1996;16:5629–43. doi: 10.1523/JNEUROSCI.16-18-05629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya G, Miller C, Kimberling WJ, Jablonski MM, Cosgrove D. Localization and expression of usherin: a novel basement membrane protein defective in people with Usher's syndrome type IIa. Hear. Res. 2002;163:1–11. doi: 10.1016/s0378-5955(01)00344-6. [DOI] [PubMed] [Google Scholar]
- Boeda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, et al. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J. 2002;21:6689–99. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond BR, Ng LL, Schulte BA. Identification of mRNA transcripts and immunohisto-chemical localization of Na/H exchanger isoforms in gerbil inner ear. Hear. Res. 1998;123:1–9. doi: 10.1016/s0378-5955(98)00089-6. [DOI] [PubMed] [Google Scholar]
- Bozovic D, Hudspeth AJ. Hair-bundle movements elicited by transepithelial electrical stimulation of hair cells in the sacculus of the bullfrog. Proc. Natl. Acad. Sci. USA. 2003;100:958–63. doi: 10.1073/pnas.0337433100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cable J, Steel KP. Combined cochleo-saccular and neuroepithelial abnormalities in the Varitint-waddler-J (VaJ) mouse. Hear. Res. 1998;123:125–36. doi: 10.1016/s0378-5955(98)00107-5. [DOI] [PubMed] [Google Scholar]
- Cheung EL, Corey DP. Ca2+ changes the force sensitivity of the hair-cell transduction channel. Biophys. J. 2006;90:124–39. doi: 10.1529/biophysj.105.061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–24. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–30. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- Corey DP, Hudspeth AJ. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979a;281:675–77. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- Corey DP, Hudspeth AJ. Response latency of vertebrate hair cells. Biophys. J. 1979b;26:499–506. doi: 10.1016/S0006-3495(79)85267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, Hudspeth AJ. Analysis of the microphonic potential of the bullfrog's sacculus. J. Neurosci. 1983a;3:942–61. doi: 10.1523/JNEUROSCI.03-05-00942.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, Hudspeth AJ. Kinetics of the receptor current in bullfrog saccular hair cells. J. Neurosci. 1983b;3:962–76. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton J, Grant W. Computational models of hair cell bundle mechanics: II. Simplified bundle models. Hear. Res. 2004;197:105–11. doi: 10.1016/j.heares.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Crawford AC, Evans MG, Fettiplace R. Activation and adaptation of transducer currents in turtle hair cells. J. Physiol. (London) 1989;419:405–34. doi: 10.1113/jphysiol.1989.sp017878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Evans MG, Fettiplace R. The actions of calcium on the mechano-electrical transducer current of turtle hair cells. J. Physiol. (London) 1991;434:369–98. doi: 10.1113/jphysiol.1991.sp018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J. Physiol. (London) 1985;364:359–79. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr JL, Dumont RA, Gillespie PG. Myosin-1c interacts with hair-cell receptors through its calmodulin-binding IQ domains. J. Neurosci. 2002;22:2487–95. doi: 10.1523/JNEUROSCI.22-07-02487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Holt JR, Shepherd GMG, Corey DP. Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron. 1995;15:1311–21. doi: 10.1016/0896-6273(95)90010-1. [DOI] [PubMed] [Google Scholar]
- Di Palma F, Belyantseva IA, Kim HJ, Vogt TF, Kachar B, Noben-Trauth K. Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc. Natl. Acad. Sci. USA. 2002;99:14994–99. doi: 10.1073/pnas.222425399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Palma F, Holme RH, Bryda EC, Belyantseva IA, Pellegrino R, et al. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat. Genet. 2001;27:103–7. doi: 10.1038/83660. [DOI] [PubMed] [Google Scholar]
- Dumont RA, Lins U, Filoteo AG, Penniston JT, Kachar B, Gillespie PG. Plasma membrane Ca2+-ATPase isoform 2a is the PMCA of hair bundles. J. Neurosci. 2001;21:5066–78. doi: 10.1523/JNEUROSCI.21-14-05066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA, Corey DP, Hudspeth AJ. Adaptation of mechanoelectrical transduction in hair cells of the bullfrog's sacculus. J. Neurosci. 1987;7:2821–36. doi: 10.1523/JNEUROSCI.07-09-02821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Amraoui A, Sahly I, Picaud S, Sahel J, Abitbol M, Petit C. Human Usher 1B/mouse shaker-1: the retinal phenotype discrepancy explained by the presence/absence of myosin VIIA in the photoreceptor cells. Hum. Mol. Genet. 1996;5:1171–78. doi: 10.1093/hmg/5.8.1171. [DOI] [PubMed] [Google Scholar]
- Farris HE, LeBlanc CL, Goswami J, Ricci AJ. Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J. Physiol. 2004;558:769–92. doi: 10.1113/jphysiol.2004.061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolenkov GI, Belyantseva IA, Friedman TB, Griffith AJ. Genetic insights into the morphogenesis of inner ear hair cells. Nat. Rev. Genet. 2004;5:489–98. doi: 10.1038/nrg1377. [DOI] [PubMed] [Google Scholar]
- Furness DN, Karkanevatos A, West B, Hackney CM. An immunogold investigation of the distribution of calmodulin in the apex of cochlear hair cells. Hear. Res. 2002;173:10–20. doi: 10.1016/s0378-5955(02)00584-1. [DOI] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J. Neurosci. 2001;21:7013–25. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JA, Yee AG, Gillespie PG, Corey DP. Localization of myosin-Ibeta near both ends of tip links in frog saccular hair cells. J. Neurosci. 1998;18:8637–47. doi: 10.1523/JNEUROSCI.18-21-08637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleoc GS, Holt JR. Developmental acquisition of sensory transduction in hair cells of the mouse inner ear. Nat. Neurosci. 2003;6:1019–20. doi: 10.1038/nn1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geleoc GS, Lennan GW, Richardson GP, Kros CJ. A quantitative comparison of mechanoelectrical transduction in vestibular and auditory hair cells of neonatal mice. Proc. R. Soc. London B. Biol. Sci. 1997;264:611–21. doi: 10.1098/rspb.1997.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson F, Walsh J, Mburu P, Varela A, Brown KA, et al. A type VII myosin encoded by the mouse deafness gene Shaker-1. Nature. 1995;374:62–64. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- Gillespie PG, Cyr JL. Myosin-1c, the hair cell's adaptation motor. Annu. Rev. Physiol. 2004;66:521–45. doi: 10.1146/annurev.physiol.66.032102.112842. [DOI] [PubMed] [Google Scholar]
- Gillespie PG, Gillespie SK, Mercer JA, Shah K, Shokat KM. Engineering of the myosin-I beta nucleotide-binding pocket to create selective sensitivity to N(6)-modified ADP analogs. J. Biol. Chem. 1999;274:31373–81. doi: 10.1074/jbc.274.44.31373. [DOI] [PubMed] [Google Scholar]
- Gillespie PG, Hudspeth AJ. Adenine nucleoside diphosphates block adaptation of mechanoelectrical transduction in hair cells. Proc. Natl. Acad. Sci. USA. 1993;90:2710–14. doi: 10.1073/pnas.90.7.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, Wagner MC, Hudspeth AJ. Identification of a 120 kd hair-bundle myosin located near stereociliary tips. Neuron. 1993;11:581–94. doi: 10.1016/0896-6273(93)90071-x. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Ruppersberg JP, Zenner HP, Rusch A. Mechanically and ATP-induced currents of mouse outer hair cells are independent and differentially blocked by d-tubocurarine. Neuropharmacology. 1997;36:1269–75. doi: 10.1016/s0028-3908(97)00108-1. [DOI] [PubMed] [Google Scholar]
- Goodyear R, Holley M, Richardson G. Hair and supporting-cell differentiation during the development of the avian inner ear. J. Comp. Neurol. 1995;351:81–93. doi: 10.1002/cne.903510108. [DOI] [PubMed] [Google Scholar]
- Goodyear R, Richardson G. Distribution of the 275 kD hair cell antigen and cell surface specialisations on auditory and vestibular hair bundles in the chicken inner ear. J. Comp. Neurol. 1992;325:243–56. doi: 10.1002/cne.903250208. [DOI] [PubMed] [Google Scholar]
- Goodyear R, Richardson G. The ankle-link antigen: an epitope sensitive to calcium chelation associated with the hair-cell surface and the calycal processes of photoreceptors. J. Neurosci. 1999;19:3761–72. doi: 10.1523/JNEUROSCI.19-10-03761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear RJ, Legan PK, Wright MB, Marcotti W, Oganesian A, et al. A receptor-like inositol lipid phosphatase is required for the maturation of developing cochlear hair bundles. J. Neurosci. 2003;23:9208–19. doi: 10.1523/JNEUROSCI.23-27-09208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear RJ, Marcotti W, Kros CJ, Richardson GP. Development and properties of stereociliary link types in hair cells of the mouse cochlea. J. Comp. Neurol. 2005;485:75–85. doi: 10.1002/cne.20513. [DOI] [PubMed] [Google Scholar]
- Goodyear RJ, Richardson GP. A novel antigen sensitive to calcium chelation that is associated with the tip links and kinocilial links of sensory hair bundles. J. Neurosci. 2003;23:4878–87. doi: 10.1523/JNEUROSCI.23-12-04878.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina MJ. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002;22:6408–14. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney CM, Mahendrasingam S, Jones EM, Fettiplace R. The distribution of calcium buffering proteins in the turtle cochlea. J. Neurosci. 2003;23:4577–89. doi: 10.1523/JNEUROSCI.23-11-04577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney CM, Mahendrasingam S, Penn A, Fettiplace R. The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J. Neurosci. 2005;25:7867–75. doi: 10.1523/JNEUROSCI.1196-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Rigaud JL, Inesi G. Ca2+/H+ countertransport and electrogenicity in proteoliposomes containing erythrocyte plasma membrane Ca-ATPase and exogenous lipids. J. Biol. Chem. 1994;269:14268–75. [PubMed] [Google Scholar]
- Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y, et al. Unconventional myosins in inner-ear sensory epithelia. J. Cell Biol. 1997;137:1287–307. doi: 10.1083/jcb.137.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JK, Brett CL, Chyou A, Kallay LM, Sakaguchi M, et al. Vestibular hair bundles control pH with (Na+, K+)/H+ exchangers NHE6 and NHE9. J. Neurosci. 2006;26:9944–55. doi: 10.1523/JNEUROSCI.2990-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono M, Denis CS, Richardson GP, Gillespie PG. Hair cells require phosphatidylinositol 4,5-bisphosphate for mechanical transduction and adaptation. Neuron. 2004;44:309–20. doi: 10.1016/j.neuron.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Holt JR, Gillespie SK, Provance DW, Shah K, Shokat KM, et al. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell. 2002;108:371–81. doi: 10.1016/s0092-8674(02)00629-3. [DOI] [PubMed] [Google Scholar]
- Howard J, Hudspeth AJ. Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog's saccular hair cell. Proc. Natl. Acad. Sci. USA. 1987;84:3064–68. doi: 10.1073/pnas.84.9.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J, Hudspeth AJ. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog's saccular hair cell. Neuron. 1988;1:189–99. doi: 10.1016/0896-6273(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. Extracellular current flow and the site of transduction by vertebrate hair cells. J. Neurosci. 1982;2:1–10. doi: 10.1523/JNEUROSCI.02-01-00001.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ. How the ear's works work: mechanoelectrical transduction and amplification by hair cells. C. R. Biol. 2005;328:155–62. doi: 10.1016/j.crvi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Jeffries DJ, Pickles JO, Osborne MP, Rhys-Evans PH, Comis SD. Crosslinks between stereocilia in hair cells of the human and guinea pig vestibular labyrinth. J. Laryngol. Otol. 1986;100:1367–74. doi: 10.1017/s002221510010115x. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–65. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Jorgensen F, Ohmori H. Amiloride blocks the mechano-electrical transduction channel of hair cells of the chick. J. Physiol. 1988;403:577–88. doi: 10.1113/jphysiol.1988.sp017265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B, Parakkal M, Kurc M, Zhao Y, Gillespie PG. High-resolution structure of hair-cell tip links. Proc. Natl. Acad. Sci. USA. 2000;97:13336–41. doi: 10.1073/pnas.97.24.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalay E, Li Y, Uzumcu A, Uyguner O, Collin RW, et al. Mutations in the lipoma HMGIC fusion partner-like 5 (LHFPL5) gene cause autosomal recessive nonsyndromic hearing loss. Hum. Mutat. 2006;27:633–39. doi: 10.1002/humu.20368. [DOI] [PubMed] [Google Scholar]
- Karavitaki KD, Corey DP. Hair bundle mechanics at high frequencies: a test of series or parallel transduction. In: Nuttall AL, editor. Auditory Mechanisms: Processes and Models. World Sci.; Singapore: 2006. pp. 286–92. [Google Scholar]
- Kennedy HJ, Evans MG, Crawford AC, Fettiplace R. Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat. Neurosci. 2003;6:832–36. doi: 10.1038/nn1089. [DOI] [PubMed] [Google Scholar]
- Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- Kroese AB, Das A, Hudspeth AJ. Blockage of the transduction channels of hair cells in the bullfrog's sacculus by aminoglycoside antibiotics. Hear. Res. 1989;37:203–17. doi: 10.1016/0378-5955(89)90023-3. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, et al. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat. Neurosci. 2002;5:41–47. doi: 10.1038/nn784. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–89. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Lagziel A, Ahmed ZM, Schultz JM, Morell RJ, Belyantseva IA, Friedman TB. Spatiotemporal pattern and isoforms of cadherin 23 in wild type and waltzer mice during inner ear hair cell development. Dev. Biol. 2005;280:295–306. doi: 10.1016/j.ydbio.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Lee G, Abdi K, Jiang Y, Michaely P, Bennett V, Marszalek PE. Nanospring behavior of ankyrin repeats. Nature. 2006;440:246–49. doi: 10.1038/nature04437. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–35. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2003;100(Suppl. 2):14531–36. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Corey DP. TRP channels in mechanosensation. Curr. Opin. Neurobiol. 2005;15:350–57. doi: 10.1016/j.conb.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Longo-Guess CM, Gagnon LH, Cook SA, Wu J, Zheng QY, Johnson KR. A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. Proc. Natl. Acad. Sci. USA. 2005;102:7894–99. doi: 10.1073/pnas.0500760102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Hudspeth AJ. Detection of Ca2+ entry through mechanosensitive channels localizes the site of mechanoelectrical transduction in hair cells. Proc. Natl. Acad. Sci. USA. 1995;92:10297–301. doi: 10.1073/pnas.92.22.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Hudspeth AJ. Regulation of free Ca2+ concentration in hair-cell stereocilia. J. Neurosci. 1998;18:6300–18. doi: 10.1523/JNEUROSCI.18-16-06300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Marquis RE, Hudspeth AJ. The selectivity of the hair cell's mechanoelectrical-transduction channel promotes Ca2+ flux at low Ca2+ concentrations. Proc. Natl. Acad. Sci. USA. 1997;94:10997–1002. doi: 10.1073/pnas.94.20.10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J. Physiol. 2005;567:505–21. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee J, Goodyear RJ, McMillan DR, Stauffer EA, Holt JR, et al. The very large G-protein-coupled receptor VLGR1: a component of the ankle link complex required for the normal development of auditory hair bundles. J. Neurosci. 2006;26:6543–53. doi: 10.1523/JNEUROSCI.0693-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DR, White PC. Loss of the transmembrane and cytoplasmic domains of the very large G-protein-coupled receptor-1 (VLGR1 or Mass1) causes audiogenic seizures in mice. Mol. Cell Neurosci. 2004;26:322–29. doi: 10.1016/j.mcn.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, et al. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J. Neurosci. 2003;23:4054–65. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel V, Goodyear RJ, Weil D, Marcotti W, Perfettini I, et al. Cadherin 23 is a component of the transient lateral links in the developing hair bundles of cochlear sensory cells. Dev. Biol. 2005;280:281–94. doi: 10.1016/j.ydbio.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 2005;25:4052–61. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Mechanoelectrical transduction currents in isolated vestibular hair cells of the chick. J. Physiol. 1985;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oganesian A, Poot M, Daum G, Coats SA, Wright MB, et al. Protein tyrosine phosphatase RQ is a phosphatidylinositol phosphatase that can regulate cell survival and proliferation. Proc. Natl. Acad. Sci. USA. 2003;100:7563–68. doi: 10.1073/pnas.1336511100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu. Rev. Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- Pawlowski KS, Kikkawa YS, Wright CG, Alagramam KN. Progression of inner ear pathology in Ames waltzer mice and the role of protocadherin 15 in hair cell development. J. Assoc. Res. Otolaryngol. 2006;7:83–94. doi: 10.1007/s10162-005-0024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles JO, Comis SD, Osborne MP. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear. Res. 1984;15:103–12. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Reiners J, Nagel-Wolfrum K, Jurgens K, Marker T, Wolfrum U. Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease. Exp. Eye Res. 2006;83:97–119. doi: 10.1016/j.exer.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Ricci AJ, Crawford AC, Fettiplace R. Active hair bundle motion linked to fast transducer adaptation in auditory hair cells. J. Neurosci. 2000;20:7131–42. doi: 10.1523/JNEUROSCI.20-19-07131.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Crawford AC, Fettiplace R. Tonotopic variation in the conductance of the hair cell mechanotransducer channel. Neuron. 2003;40:983–90. doi: 10.1016/s0896-6273(03)00721-9. [DOI] [PubMed] [Google Scholar]
- Ricci AJ, Fettiplace R. The effects of calcium buffering and cyclic AMP on mechanoelectrical transduction in turtle auditory hair cells. J. Physiol. 1997;501(Pt. 1):111–24. doi: 10.1111/j.1469-7793.1997.111bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Fettiplace R. Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J. Physiol. (London) 1998;506:159–73. doi: 10.1111/j.1469-7793.1998.159bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Kennedy HJ, Crawford AC, Fettiplace R. The transduction channel filter in auditory hair cells. J. Neurosci. 2005;25:7831–39. doi: 10.1523/JNEUROSCI.1127-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Wu YC, Fettiplace R. The endogenous calcium buffer and the time course of transducer adaptation in auditory hair cells. J. Neurosci. 1998;18:8261–77. doi: 10.1523/JNEUROSCI.18-20-08261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch A, Kros CJ, Richardson GP. Block by amiloride and its derivatives of mechanoelectrical transduction in outer hair cells of mouse cochlear cultures. J. Physiol. (London) 1994;474:75–86. doi: 10.1113/jphysiol.1994.sp020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzadzinska AK, Derr A, Kachar B, Noben-Trauth K. Sustained cadherin 23 expression in young and adult cochlea of normal and hearing-impaired mice. Hear. Res. 2005;208:114–21. doi: 10.1016/j.heares.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Self T, Mahony M, Fleming J, Walsh J, Brown SD, Steel KP. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development. 1998;125:557–66. doi: 10.1242/dev.125.4.557. [DOI] [PubMed] [Google Scholar]
- Shabbir MI, Ahmed ZM, Khan SY, Riazuddin S, Waryah AM, et al. Mutations of human TMHS cause recessively inherited nonsyndromic hearing loss. J. Med. Genet. 2006;43:634–40. doi: 10.1136/jmg.2005.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Harada N, Kubo N, Liu B, Mizuno A, et al. Functional expression of transient receptor potential vanilloid 4 in the mouse cochlea. Neuroreport. 2006;17:135–39. doi: 10.1097/01.wnr.0000199459.16789.75. [DOI] [PubMed] [Google Scholar]
- Shepherd GMG, Barres BA, Corey DP. “Bundle Blot” purification and initial protein characterization of hair-cell stereocilia. Proc. Natl. Acad. Sci. USA. 1989;86:4973–77. doi: 10.1073/pnas.86.13.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GMG, Corey DP. The extent of adaptation in bullfrog saccular hair cells. J. Neurosci. 1994;14:6217–29. doi: 10.1523/JNEUROSCI.14-10-06217.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JB, Adams D, Paukert M, Siba M, Sidi S, et al. Xenopus TRPN1 (NOMPC) localizes to microtubule-based cilia in epithelial cells, including inner-ear hair cells. Proc. Natl. Acad. Sci. USA. 2005;102:12572–77. doi: 10.1073/pnas.0502403102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidi S, Friedrich RW, Nicolson T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science. 2003;301:96–99. doi: 10.1126/science.1084370. [DOI] [PubMed] [Google Scholar]
- Siemens J, Lillo C, Dumont RA, Reynolds A, Williams DS, et al. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature. 2004;428:950–55. doi: 10.1038/nature02483. [DOI] [PubMed] [Google Scholar]
- Solc CK, Derfler BH, Duyk GM, Corey DP. Molecular cloning of myosins from the bullfrog saccular macula: a candidate for the adaptation motor. Auditory Neurosci. 1994;1:63–75. [Google Scholar]
- Sollner C, Rauch GJ, Siemens J, Geisler R, Schuster SC, et al. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature. 2004;428:955–59. doi: 10.1038/nature02484. [DOI] [PubMed] [Google Scholar]
- Sotomayor M, Corey DP, Schulten K. In search of the hair-cell gating spring: elastic properties of ankyrin and cadherin repeats. Structure (Camb.) 2005;13:669–82. doi: 10.1016/j.str.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Stankovic KM, Brown D, Alper SL, Adams JC. Localization of pH regulating proteins H+ATPase and Cl-/HCO3- exchanger in the guinea pig inner ear. Hear. Res. 1997;114:21–34. doi: 10.1016/s0378-5955(97)00072-5. [DOI] [PubMed] [Google Scholar]
- Stauffer EA, Scarborough JD, Hirono M, Miller ED, Shah K, et al. Fast adaptation in vestibular hair cells requires myosin-1c activity. Neuron. 2005;47:541–53. doi: 10.1016/j.neuron.2005.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K. Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nat. Genet. 1998;19:390–94. doi: 10.1038/1284. [DOI] [PubMed] [Google Scholar]
- Sukharev S, Corey DP. Mechanosensitive channels: multiplicity of families and gating paradigms. Sci. STKE. 20042004:re4. doi: 10.1126/stke.2192004re4. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Suzuki M, Mizuno A, Hara A. Hearing impairment in TRPV4 knockout mice. Neurosci. Lett. 2005;382:304–8. doi: 10.1016/j.neulet.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Tang N, Lin T, Ostap EM. Dynamics of myo1c (myosin-ibeta) lipid binding and dissociation. J. Biol. Chem. 2002;277:42763–68. doi: 10.1074/jbc.M206388200. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, Cotanche DA. Actin filaments, stereocilia, and hair cells of the bird cochlea. V. How the staircase pattern of stereociliary lengths is generated. J. Cell Biol. 1988;106:355–65. doi: 10.1083/jcb.106.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, DeRosier DJ. Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu. Rev. Cell Biol. 1992;8:257–74. doi: 10.1146/annurev.cb.08.110192.001353. [DOI] [PubMed] [Google Scholar]
- Tsuprun V, Goodyear RJ, Richardson GP. The structure of tip links and kinocilial links in avian sensory hair bundles. Biophys. J. 2004;87:4106–12. doi: 10.1529/biophysj.104.049031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuprun V, Schachern PA, Cureoglu S, Paparella M. Structure of the stereocilia side links and morphology of auditory hair bundle in relation to noise exposure in the chinchilla. J. Neurocytol. 2003;32:1117–28. doi: 10.1023/B:NEUR.0000021906.08847.d2. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Hofmann T, Montell C. Lysosomal localization of TRPML3 depends on TRPML2 and the mucolipidosis-associated protein TRPML1. J. Biol. Chem. 2006;281:17517–27. doi: 10.1074/jbc.M600807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath MA, Eatock RA. Time course and extent of mechanotransducer adaptation in mouse utricular hair cells: comparison with frog saccular hair cells. J. Neurophysiol. 2003;90:2676–89. doi: 10.1152/jn.00893.2002. [DOI] [PubMed] [Google Scholar]
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–34. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, et al. Defective myosin VIIA gene responsible for Usher syndrome type IB. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- Wood JD, Muchinsky SJ, Filoteo AG, Penniston JT, Tempel BL. Low endolymph calcium concentrations in deafwaddler2J mice suggest that PMCA2 contributes to endolymph calcium maintenance. J. Assoc. Res. Otolaryngol. 2004;5:99–110. doi: 10.1007/s10162-003-4022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Ricci AJ, Fettiplace R. Two components of transducer adaptation in auditory hair cells. J. Neurophysiol. 1999;82:2171–81. doi: 10.1152/jn.1999.82.5.2171. [DOI] [PubMed] [Google Scholar]
- Yamoah EN, Gillespie PG. Phosphate analogs block adaptation in hair cells by inhibiting adaptation-motor force production. Neuron. 1996;17:523–33. doi: 10.1016/s0896-6273(00)80184-1. [DOI] [PubMed] [Google Scholar]
- Yamoah EN, Lumpkin EA, Dumont RA, Smith PJ, Hudspeth AJ, Gillespie PG. Plasma membrane Ca2+-ATPase extrudes Ca2+ from hair cell stereocilia. J. Neurosci. 1998;18:610–24. doi: 10.1523/JNEUROSCI.18-02-00610.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yamoah EN, Gillespie PG. Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc. Natl. Acad. Sci. USA. 1996;93:15469–74. doi: 10.1073/pnas.93.26.15469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca(2+). Nat. Neurosci. 2007 doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- Eatock RA. Adaptation in hair cells. Annu. Rev. Neurosci. 2000;23:285–314. doi: 10.1146/annurev.neuro.23.1.285. [DOI] [PubMed] [Google Scholar]
- Gillespie PG, Dumont RA, Kachar B. Have we found the tip link, transduction channel, and gating spring of the hair cell? Curr. Opin. Neurobiol. 2005;15:389–96. doi: 10.1016/j.conb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Ricci AJ, Kachar B, Gale J, Van Netten SM. Mechano-electrical transduction: new insights into old ideas. J. Membr. Biol. 2006;209:71–88. doi: 10.1007/s00232-005-0834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]