Abstract
Cisplatin, a chemotherapeutic agent of choice for the treatment of solid tumors, produces hearing loss in approximately half a million new cancer patients annually in the United States. The hearing loss is due, in part, to increased generation of reactive oxygen species (ROS) in the cochlea, leading to lipid peroxidation and damage or death of outer hair cells in the organ of Corti. The cochlea expresses the transient receptor potential vanilloid 1 (TRPV1), which are normally expressed on small diameter neurons in the peripheral nervous system and mediate thermal sensitivity, but whose role in the cochlea is unclear. In this study, we show that TRPV1 is coregulated along with the NADPH oxidase isoform, NOX3, by cisplatin. Induction of these proteins by cisplatin is dependent on ROS generation, since it is reversed by systemic lipoic acid administration. In organ of Corti hair cell cultures (UB/OC-1 cells), cisplatin activates and induces TRPV1 and NOX3, leading to apoptosis of these cells. Inhibition of TRPV1 by capsazepine or ruthenium red reduced the apoptosis, implicating TRPV1 in this process. Treatment of UB/OC-1 cultures with short interfering RNA (siRNA) against either TRPV1 or NOX3 reduced cisplatin-induced apoptosis, while round window application of TRPV1 siRNA to rats reduced TRPV1 expression, decreased damage to outer hair cells and reduced cisplatin-induced hearing loss. These data provide a link between NOX3 and TRPV1 in cisplatin-induced hearing loss and suggest that targeting these proteins for knockdown by siRNA could serve as a novel approach in treating cisplatin ototoxicity.
Keywords: transient receptor potential vanilloid 1 channel, cochlea, cisplatin, NADPH oxidase, hair cells, short interfering RNA
Introduction
Administration of platinum containing drugs, such as cisplatin, produces significant hearing loss, which is usually permanent and cumulative. Several reports have concluded that the generation of reactive oxygen species (ROS) is linked to cisplatin ototoxicity (Rybak and Ramkumar, 2007). The organ of Corti represents a major site for cisplatin induced hearing loss (Rybak and Kelly, 2003), where the drug produces permanent loss of outer hair cells (Kopke et al., 1997). Currently, there are no effective treatments against cisplatin, although antioxidant therapy has proven beneficial in animal models of cisplatin ototoxicity (Rybak and Kelly, 2003). However, concerns that antioxidants could interfere with the anticancer efficacy of cisplatin could limit their therapeutic usefulness in treating ototoxicity. Therefore, new or improved methods are needed for alleviating cisplatin ototoxicity.
Transient receptor potential vanilloid 1 (TRPV1) is a member of the transient receptor potential (TRP) channel family, expressed primarily by small diameter neurons (Aδ and C fibers) comprising the pain pathway. It is a nonselective cation channel which demonstrates responsivity to heat (Caterina et al., 1997). TRPV1 receptor expression has also been demonstrated in non-neuronal tissues including organ of Corti, keratinocytes and bladder urothelium (Southall et al., 2003; Zheng et al., 2003), suggesting roles in addition to the regulation of thermal pain sensation. Since TRPV1 has been shown to be induced by ROS (Puntambekar et al., 2005), we determined whether its expression is regulated by cisplatin and whether this channel contributes to cisplatin ototoxicity.
In the present study, we show that ROS produced by cisplatin promote activation and induction of TRPV1 and the NOX3 isoform of NADPH oxidase in the rat organ of Corti and spiral ganglion cells and in vitro organ of Corti (UB/OC-1) cell cultures. Furthermore, reduction in TRPV1 expression in these cultures and in the cochlea by short interfering RNA (siRNA) decreased cisplatin-induced damage to UB/OC-1 cultures and protected against hearing loss in the rat. These data provide evidence that inhibiting TRPV1 expression by siRNA could prove a useful strategy for protecting against cisplatin-induced hearing loss.
Materials and Methods
Reagents.
The various reagents: cisplatin, diphenyleneiodonium (DPI), 4-(2-aminoethyl) benzene sulfonylfluoride (AEBSF), capsezapine, capsaicin, ruthenium red, TRI reagent and 2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA) were purchased from Sigma-Aldrich. 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) dye was purchased from EMD Biosciences. TRPV1 antibody was from Neuromics and secondary goat anti rabbit antibody was purchased from Santa Cruz Biotechnology.
Animal procedures and sample collection.
Male Wistar rats were used for this study. Pretreatment auditory brainstem responses (ABRs) were performed 2–3 d before round window application of siRNA against TRPV1 or a scrambled siRNA sequence which served as a control. For administering siRNAs, the bulla was opened to expose the round window. The scrambled or TRPV1 siRNAs were then applied to the round window membrane in a volume of 3 μl for 15 min. After this period, animals were allowed to recover for 48 h before administering cisplatin. For cisplatin administration, rats were first anesthetized with a mixture of xylazine 5.5 mg/kg and ketamine 172.4 mg/kg treated with cisplatin (13 mg/kg) by intraperitoneal (i.p.) injections over a period of 30 min. Rats were then killed at intervals of 24, 48 or 72 h after cisplatin administration. The cochleae were dissected and used for the preparation of total RNA or total protein extracts, or perfused with 2.5% glutaraldehyde for morphological studies by SEM or with 4% paraformaldehyde for immunocytochemical analyses. For experiments involving lipoic acid, this agent was administered by i.p. injections at a dose of 50 mg/kg/i.p. just before the administration of cisplatin.
Evoked potentials.
Auditory brainstem responses were measured before administration and 72 h after cisplatin administration, as described previously (Tanaka et al., 2003). Animals were tested with a stimulus intensity series that was initiated at 10 dB SPL and reached a maximum at 90 dB SPL. The stimulus intensity levels were increased in 10 dB increments, and the evoked ABR waveforms were observed on a video monitor. The auditory stimuli included tone bursts at 8, 16 and 32 kHz with a 5 ms plateau and a 1 ms rise/fall time presented at a rate of 5/s. Threshold was defined as the lowest intensity capable of evoking a reproducible, visually detectable response with two distinct waveforms and a minimum amplitude of 0.5 μV.
Morphological studies: scanning electron microscopy.
Immediately after completion of post-treatment ABRs, deeply sedated rats were killed, their cochleae harvested and processed as described previously (Kamimura et al., 1999). Sputter coated cochleae were then viewed and photographed with a Hitachi S-500 scanning electron microscope (Hitachi).
Processing of cochlea for immunohistochemistry.
Cochleae perfused with 4% paraformaldehyde were decalcified and sectioned, as described previously (Dunaway et al., 2003). Samples were incubated with TRPV1 antibody (1:100 titer) for 1 h at 37°C incubator. Secondary antibody used was goat anti-rabbit IgG conjugated to horse radish peroxidase at a 1:200 dilution. ABC staining system (Santa Cruz Biotechnology), which included a diaminobenzidine as a peroxidase substrate, was used for visualization of protein expression. Slides were counterstained with toluidine blue and imaged using Scion Imaging system. For immunofluorescence imaging, we used a fluorescein-conjugated goat anti-rabbit IgG. Slides were imaged and analyzed using an Olympus confocal microscope (Olympus America).
Hair cell count.
Hair cell counts were performed using a modified version of the method described previously (Korver et al., 2002). Two representative areas of the basal turn and hook portion were photographed. In each area, outer hair cells were counted in an area that was 10 pillar cell heads in length. The results are presented as the percent hair cell damage per cochlear turn.
Cell culture.
Immortalized organ of Corti cells derived from the mouse, UB/OC-1 cells, were obtained from Dr. Matthew Holley (Institute of Molecular Physiology, Addison Building, Western Bank, Sheffield, UK) and cultured in RPMI 1640 supplemented with 10% Fetalclone II serum (Hyclone) and penicillin-streptomycin. Cultures were grown at 33°C in an incubator with 10% CO2 or at 39°C in 5% CO2 (where indicated).
Oligonucleotides.
The rodent set of primers and siRNA were based on the homologous sequences in the rat and mouse cDNA sequences. The primers were purchased from Sigma Genosys. Purified siRNA duplexes were purchased from Qiagen. Rodent NOX3 (sense): 5′-GTGAACAAGGGAAGGCTCAT-3′ (antisense): 5′-GACCCACAGAAGAACACGC-3′, Rodent-GAPDH (sense): 5′-ATGGTGAAGGTCGGTGTGAAC-3′ (antisense): 5′-TGTAGTTGAGGTCAATGAAGG-3′, Rodent TRPV1 (sense): 5′-CAAGGCTGTCTTCATCATCC-3′, (antisense): 5-AGTCCAGTTTACCTCGTCCA-3′, Rodent Rac1 (sense): 5′-ATC AGTTACACGACCAAT GC-3′, (antisense): 5′-GGGAAAAGCAAATTAAGAAC-3′, Rodent gp-91 (sense) 5′-TAAAGGAGT GCCCAGTACCAA-3′, (antisense): 5′-AATCCCTTCTTCTTCATCTGA-3′, and Rat p22 (sense): 5′-ACAGGGGGCATCGTGGCTACT-3′, (antisense) 5′-GGACGTAGTAATTTCTGG TGA -3′
Rodent NOX3 siRNA.
Target sequence: 5′-AAGGTGGTGAGTCACCCATCT-3′. Rodent TRPV1 siRNA: Target sequence: 5′-GCGCATCTTCTACTTCAACTT-3′ modified from Christoph et al. (2006).
siRNA transfection.
RNAi human mouse starter kit (Qiagen) including the HiPerFect transfection reagent, and AllStars negative control siRNA was used for all transfections according to manufacturer's instructions. Briefly, the UB/OC-1 cells were transfected with 5 nm of either TRPV1, NOX3 or scrambled siRNA for 48 h and then treated accordingly.
RNA isolation.
RNA was isolated by adding 1 ml TRI reagent to 100 mg of cochlea or 0.5 ml TRI reagent per well of each six well plate. Tissues were homogenized in TRI reagent using a Polytron (setting 7, 15 s) and centrifuged at 12,000 × g for 10 min at 4°C. The clear supernatant was transferred to a fresh tube, 0.2 ml of chloroform was added, and the tube was shaken vigorously for 15 s and centrifuged at 12,000 × g for 15 min. RNA was extracted by washing the pellet with 0.5 ml ice-cold isopropanol followed by cold 75% diethylpyrocarbonate treated ethanol. The ethanol was removed and the tube was air dried briefly. The RNA pellet was resuspended in nuclease free water and RNA levels were determined using optical density readings corresponding to wavelengths of 260 nm, 280 and 320 nm using a spectrophotometer (Eppendorf BioPhotometer).
Real-time reverse transcriptase-PCR.
One microgram of total RNA was converted to cDNA using iScript cDNA Synthesis Kit (Bio-Rad). The reaction mixture was set up as follows: 1 μg of total RNA, 4 μl of iScript reaction mix, 1 μl of iScript reverse transcriptase, nuclease free water to bring the total volume to 20 μl. The reaction mix was incubated at 25°C for 5 min, 42°C for 30 min and 85°C for 5 min. This cDNA reaction mix was used for real-time PCR.
PCR was set up as follows: 2 μl of cDNA, 0.5 μl of each primer (50 pm stock) and 12.5 μl of the iQ SYBR Green Supermix reagent (Bio-Rad), adjusted to a total volume of 25 μl with DNase/RNase free water. Glyceraldehyde phosphate dehydrogenase (GAPDH) was used for normalization. Amplification and detection was performed with the Cepheid Smart Cycler Detection System. Negative control reactions were set up as above without any template cDNA. Cycling conditions were as follows: 95°C for 3 min followed by 50 cycles at 95°C for 15 s, 64°C for 30 s and 72°C for 30 s. On completion of amplification, melting curve analysis was performed by cooling the reaction to 60°C and then heating slowly to 95°C, according to the instruction of manufacturer (Cepheid Smart Cycler). The cycle number at which the sample reaches the threshold fluorescent intensity was termed the cycle threshold (Ct). The relative change in mRNA levels between untreated control (1) and treated sample (2) was measured using the following formula: 2(Ct Target gene1−Ct GAPDH1)−(Ct Target gene2−Ct GAPDH2) (Soong et al., 2001). Negative controls for both target gene and GAPDH were used for all reaction groups. Real-time PCR products were analyzed on a 2% agarose gel to verify the correct product sizes and visualization of the amplified product was effected using the dye SyBr Green I (Invitrogen). Gene specific primer pairs were used for the various reactions and mRNA expression levels were normalized to the levels of GAPDH.
Apoptosis detection.
For detecting apoptosis in UB/OC-1 cells, apoptotic cells were visualized using colorimetric TdT-FragEL DNA fragmentation detection kit performed according to the manufacturer's instructions (EMD Biosciences). Briefly, the cells were treated with cisplatin in the presence of other reagents for 24 h. After the treatments, the cells were washed with cold 1× PBS and fixed with 4% paraformaldehyde. Fixed cells were permeabilized with 20 μg/ml of proteinase K for 5 min, washed with 1× TBS and placed in 1× TdT equilibrating buffer for 20 min. Cells were then incubated in labeling reaction buffer containing FragEL TdT labeling reaction mix and TdT enzyme for 60 min at 37°C in a humidified chamber. After one wash with 1× TBS and 10 min incubation in blocking buffer, cells were incubated for another 30 min with 1× peroxidase streptavidin conjugate in a humidified chamber at room temperature. After rinsing with 1× TBS, diaminobenzidine (DAB) solution was applied to the individual coverslips for 15 min. Finally, the cells were counterstained with methyl green, mounted on glass slide and visualized by light microscope. Nonapoptotic cells appeared blue-green while apoptotic cells were dark brown in color. The percentage of apoptotic cells were assessed by analysis of digitized images from 12 or more microscopic fields from TIFF files using Adobe Photoshop CS2 (Adobe Systems).
Calcium imaging.
UB/OC-1 cells were transfected with siRNA for ∼48 h, or treated with various drugs for 30 min, then pretreated with cisplatin for 30 min, followed by incubation with Fluo-4AM dye for 30 min. Ca2+ imaging was performed using an Olympus confocal microscope (Olympus America) at a wavelength of 488 nm. To test the effect of extracellular Ca2+ on cisplatin-induced rise in intracellular Ca2+, cells were treated with 1.0 mm ethylene glycol-bis(β-aminoethylether) N,N,N′,N′-tetraacetic acid (EGTA) and the response to cisplatin measured.
ROS generation.
Imaging of ROS generation was done as described by Puntambekar et al. (2005). Briefly, UB/OC-1 cells were pretreated with different agents or transfected with siRNAs and then incubated with H2DCFDA dye for 30 min. H2DCFDA fluorescence was detected by confocal microscopy, 30 min after cisplatin administration.
Western blot analysis.
Cochleae or UB/OC-1 cells were homogenized in ice-cold 50 mm Tris HCl, 10 mm MgCl2 and 1 mm EDTA in the presence of protease inhibitors mixture (Sigma). The whole tissue/cell lysates were then used for Western blotting. After transfer to nitrocellulose membranes, blots were probed with a polyclonal primary TRPV1 antibody, followed by a horse radish peroxidase-tagged secondary antibodies and visualized by chemiluminescence detection (Pierce Biotechnology).
Statistical analyses.
Statistical significance differences among groups were performed using Student's t tests and ANOVA, followed by Tukey's post hoc test.
Results
Cisplatin treatment induces TRPV1 expression in the rat cochlea
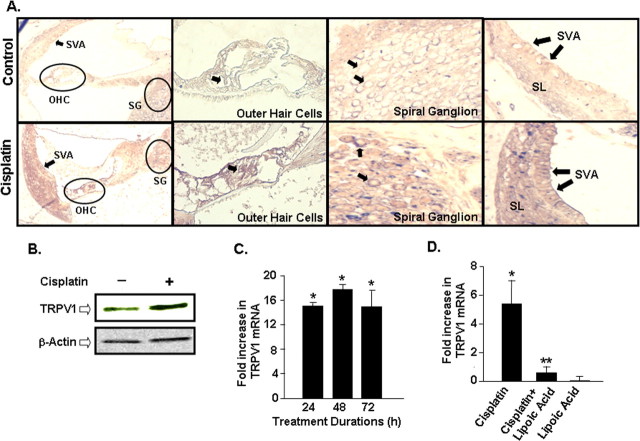
For these studies, male Wistar rats (200–250 g) were administered cisplatin (13 mg/kg) by i.p. infusion over a 30 min period. ABR measures, determined 72 h later, indicated elevations in thresholds by 20–40 dB over an 8–32 kHz frequency range, as observed previously (Kamimura et al., 1999; Tanaka et al., 2003), indicative of significant hearing loss produced by this dose of cisplatin. Cochleae obtained from animals 72 h after cisplatin administration were fixed, decalcified, sectioned and processed for TRPV1 immunoreactivity, using a polyclonal antibody (Puntambekar et al., 2005), and visualized by light microscopy or confocal microscopy (for visualizing immunofluorescence). Immunolabeling, depicted as red-brown staining, was observed in organ of Corti (inner and outer hair cells), supporting cells, spiral ganglion cells and, to a lesser extent, in stria vascularis (Fig. 1A). No significant immunoreactivity was observed in the absence of any primary antibody or in Ig-depleted antibody preparations, showing specificity of the primary antibody (data not shown). Furthermore, reduced immunolabeling was observed in the cochlea 2 d after round window application of TRPV1 siRNA, indicating specificity of the TRPV1 antibody (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Elevations in TRPV1 immunoreactivity in these regions were observed after cisplatin treatment (Fig. 1A; supplemental Fig. 1, available at www.jneurosci.org as supplemental material), and was substantiated by Western blotting studies of whole cochlear lysates for the TRPV1 protein (∼95 kDa band) (Fig. 1B). Quantitation of the TRPV1 bands on Western blots showed an increased expression of this protein averaging 193 ± 15% of control (n = 5, p < 0.05). The increases in TRPV1 immunoreactivity were associated with a 15 ± 2-fold increase in TRPV1 mRNA observed 24 h after cisplatin treatment, with no further elevations by 48 and 72 h. These increases in TRPV1 mRNA were 18 ± 1 and 15 ± 3-fold by 48 and 72 h, respectively, of cisplatin administration (Fig. 1C). The induction of TRPV1 preceded morphological changes in outer hair cells, which were generally observed 72 h after cisplatin administration (Ford et al., 1997; Whitworth et al., 2004; Mukherjea et al., 2006) and were associated with ROS generation (Kopke et al., 1997). To determine the involvement of ROS in the induction of TRPV1 in the cochlea, as demonstrated previously in vitro (Puntambekar et al., 2005), we tested whether the antioxidant, lipoic acid, could alter cisplatin-induced TRPV1 expression in vivo. Although the level of induction of TRPV1 by cisplatin (5.5 ± 1.5-fold) in these animals was smaller than that obtained in the previous study, it was completely abolished in rats pretreated with lipoic acid (Fig. 1D). The fold induction in animals pretreated with lipoic acid, followed by cisplatin, or lipoic acid alone were 0.6 ± 0.6 and 0.2 ± 0.1, respectively. These results suggest that lipoic acid inhibits cisplatin-mediated as well as the basal expression of TRPV1.
Figure 1.
Increased TRPV1 expression in organ of Corti after cisplatin administration in vivo. A, Rats were administered vehicle or cisplatin (13 mg/kg, i.p.), and cochleae were harvested after 3 d, decalcified in CAL-EX II over 3 weeks, sectioned and stained with a polyclonal antibody for TRPV1 and counterstained with toluidine blue. Increased immunostaining was observed in cisplatin treated samples, compared with vehicle-treated controls, in the outer hair cells (OHC) and spiral ganglion cells (SG) (arrows). Labeling also appears increased in the stria vascularis (SVA) (arrows). SL represents the spiral ligament. Magnification, 100 ×. B, Increased TRPV1 protein expression is seen in whole cochlear homogenates of rats treated with cisplatin for 72 h compared with untreated control cochleae. Western blot shown is the representative of five different experiments with identical results. C, Time course of cisplatin-mediated increase in TRPV1 mRNA in the rat cochlea shows significant increase in TRPV1 mRNA as early as 24 h compared with vehicle-treated control cochleae. The levels of TRPV1 mRNA were determined using real-time PCR. Asterisk indicates statistically significant increase from vehicle-treated controls (p < 0.05). D, Inhibition of cisplatin-induced TRPV1 expression in the rat cochlea by lipoic acid. Rats were pretreated with vehicle or lipoic acid (50 mg/kg, i.p.) before the administration of cisplatin (13 mg/kg, i.p.) and cochleae were obtained 24 h later for the isolation of RNA and real-time PCR. Single asterisk indicates statistically significant increase from control, while double asterisks indicate statistically significant suppression of the cisplatin-response (n = 5, p < 0.05).
Cisplatin induces NOX3 expression in the rat cochlea
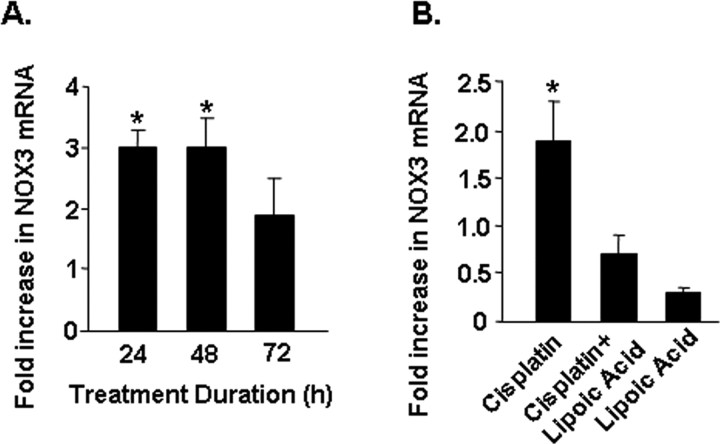
The NOX3 subunit of NADPH oxidase represents the gp91phox homolog of this enzyme which is predominantly expressed in the cochlea, and which is induced by cisplatin in organotypic cultures (Bánfi et al., 2004). Results shown in Figure 2A indicate a statistically significant increase in NOX3 mRNA in the cochlea by cisplatin which was maximal by 24 h and did not show any further change by 48 and 72 h. The fold increases observed at 24, 48 and 72 h were 3.0 ± 0.3, 3.0 ± 0.5 and 1.9 ± 0.6, respectively. The increase in NOX3 by cisplatin was attenuated by lipoic acid, implicating ROS in its induction (Fig. 2B). In addition, lipoic acid significantly reduced the basal expression of NOX3, implicating ROS in the basal regulation of this gene. The fold increases by cisplatin, cisplatin + lipoic acid and lipoic acid alone were 1.9 ± 0.4, 0.7 ± 0.2 and 0.3 ± 0.1, respectively. Other NADPH oxidase isoforms, such as Rac1, gp91 and p22, were also induced by cisplatin, with increases in expression being 478 ± 85, 1000 ± 102 and 81 ±-fold, respectively. The high fold induction of these latter transcripts over that observed for NOX3 might reflect their low basal expression in the cochlea compared with NOX3.
Figure 2.
Cisplatin increased the expression of NADPH oxidase in the cochlea. A, Time course of induction of NOX3 mRNA by cisplatin. Rats were administered cisplatin (13 mg/kg) and were killed at different time points at which their cochleae were dissected out and processed for RNA and real-time PCR for NOX3. B, Lipoic acid (50 mg/kg, i.p.) abolished cisplatin-mediated increase in NOX3 mRNA. Asterisk indicates statistically significant change compared with vehicle-treated controls (p < 0.05, n = 5).
Cisplatin upregulates the expression of TRPV1, NOX3 and other NADPH subunits in UB/OC-1 cells
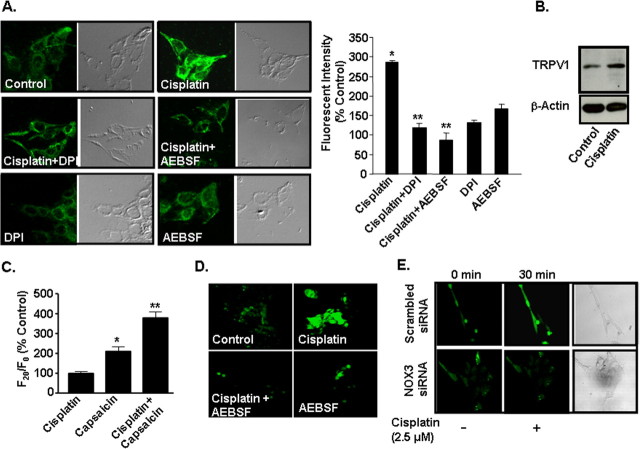
To further study the role of ROS generated via NADPH oxidases in the induction of TRPV1 in the cochlea, we performed in vitro experiments using the organ of Corti transformed hair cell line, UB/OC-1 (Rivolta et al., 1998; Mukherjea et al., 2006). Cisplatin (2.5 μm) increased TRPV1 immunolabeling over vehicle-treated cells to 287 ± 2% of control (Fig. 3A). Pretreatment of these cultures with either 100 μm AEBSF (Diatchuk et al., 1997) or 10 μm DPI (O'Donnell et al., 1993), inhibitors of NADPH oxidase, attenuated the increases observed with cisplatin to 120 ± 12 and 87 ± 15% of control, respectively (Fig. 3A), implicating ROS in this process. The levels of TRPV1 immunoreactivity were respectively 132 ± 9% and 168 ± 18% of control in cells treated with DPI or AEBSF alone. These increases in TRPV1 protein expression were confirmed by Western blotting (Fig. 3B), which showed that cisplatin increased the levels to 171 ± 29% of control. In addition, real-time PCR showed increases in TRPV1 transcripts of 3.9 ± 0.3-fold.
Figure 3.
Cisplatin increased TRPV1 expression through activation of NADPH oxidase in UB/OC-1 cells. A, UB/OC-1 cells were pretreated with vehicle, DPI (10 μm) or AEBSF (100 μm) for 30 min, followed by 2.5 μm cisplatin for 24 h. TRPV1 protein was assessed by immunolabeling using a polyclonal primary antibody and a fluorescein-labeled secondary antibody and visualized by confocal microscopy at a 200-fold magnification. Cisplatin increased TRPV1 immunoreactivity in UB/OC-1 cells by 24 h and this response was blocked by DPI and AEBSF. Graphical representation of fluorescence intensity is quantitated by the confocal microscope fluoview software. Asterisk (*) indicates statistically significant increase from control, while ** indicates statistically significant reduction compared with the cisplatin-treated group (p < 0.05, n = 3). The differential interference contrast images of the fluorescent cells are shown in the right panels. Cisplatin treatment for 24 h increased TRPV1 protein (B) in UB-OC1 cells. These studies were repeated at least three times. C, Increase in capsaicin-mediated Ca2+ influx in cells pretreated with cisplatin (2.5 μm) over vehicle-treated controls for 24 h. Asterisk (*) indicates statistically significant increase from vehicle-treated controls, while ** indicates statistically significant difference (p < 0.05, n = 3) from cells exposed to vehicle, followed by capsaicin. D, Cisplatin increased ROS generation in UB/OC-1 cells. Cells were treated with cisplatin (2.5 μm) for 30 min and ROS generation was determined by H2DCFDA fluorescence. Pretreatment of cells with AEBSF (100 μm) attenuated the increase in ROS generation produced by cisplatin. E, Knockdown of NOX3 by siRNA attenuated cisplatin-mediated ROS generation. Cells were transfected with a scrambled siRNA sequence or a NOX3 siRNA (5 nm) for 48 h and then challenged with cisplatin for 30 min to determine ROS generation. ROS images are presented of cells before and after 30 min exposures to cisplatin. Similar findings were observed in three separate experiments.
To determine whether the increase in TRPV1 observed after cisplatin treatment is associated with a functional increase in TRPV1 activity, cells were treated with vehicle or with cisplatin (2.5 μm) for 24 h. The media were removed and replaced with fresh media without cisplatin and the cells were then exposed to capsaicin (1 μm) to induce plasma membrane Ca2+ influx. Figure 3C indicates a statistically significant increase in Ca2+ influx in 20 s in vehicle-treated cells (∼2-fold) which was significantly enhanced in cells pretreated with cisplatin (∼4-fold increase).
UB/OC-1 cells treated with cisplatin showed a robust increase in ROS generation, which was abolished in cells pretreated with AEBSF (100 μm), implicating NADPH oxidase activation (and possibly of NOX3) in this process (Fig. 3D). Reduction in NOX3 expression by siRNA also reduced cisplatin-mediated ROS generation, compared with cells treated with a scrambled siRNA sequence (Fig. 3E). Inhibition of NOX3 expression by NOX3 siRNA is demonstrated in Figure 5.
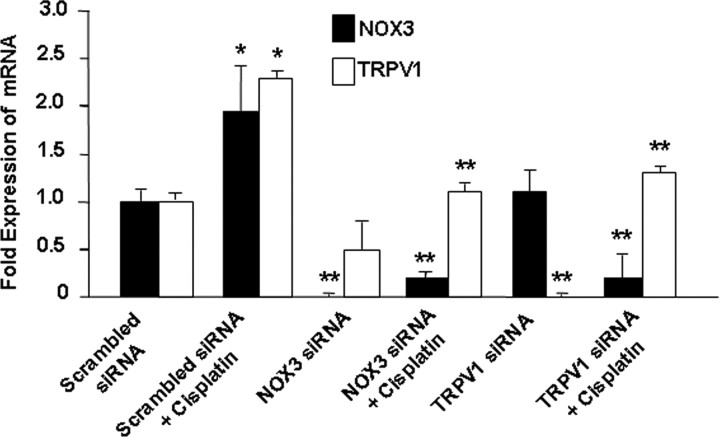
Figure 5.
TRPV1 and NOX3 siRNAs reduced TRPV1 and NOX3 expression in UB/OC-1 cells. UB/OC-1 cells were transfected with a scrambled siRNA sequence or siRNAs against NOX3 or TRPV1 for 48 h, followed by exposure to 2.5 μm cisplatin for an additional 24 h. Cisplatin significantly increased the expression of TRPV1 and NOX3 but the expression of both of these genes were suppressed in cells pretreated with NOX3 siRNA. Transfection of cells with siRNA against TRPV1 reduced the basal expression of TRPV1 but not NOX3 and also reduced cisplatin-stimulated expression of both NOX3 and TRPV1. Asterisk (*) indicates statistically significant difference from no cisplatin treatment, while ** indicates statistically significant reductions in the basal or cisplatin responses (p < 0.05, n = 4).
Cisplatin-induced ROS generation was followed by increases in mRNA encoding NOX3, gp91 and Rac1 by 2.7 ± 0.4, 2.9 ± 0.2 and 2.0 ± 0.2-fold, respectively. The increases in expression of these genes were inhibited by DPI (10 μm), indicative of a role for NADPH oxidase activity in the induction of these subunits. The addition of DPI alone did not significantly alter the expression of these genes from control. These results suggest that the initial generation of ROS mediated by NADPH oxidase promotes de novo synthesis of NADPH oxidase subunits in vitro and subsequently enhances ROS generation.
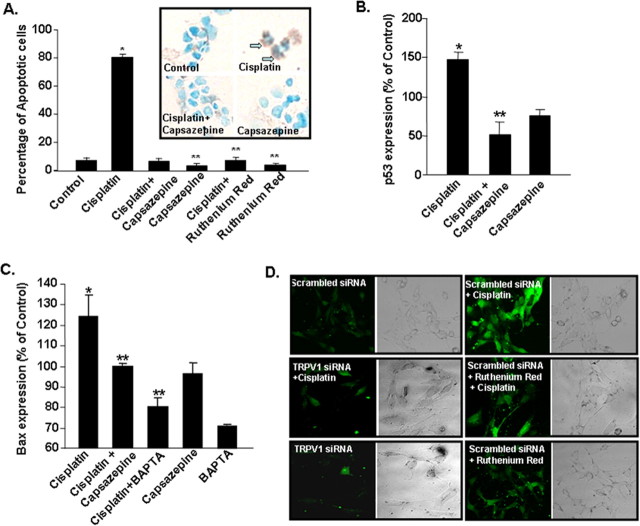
Cisplatin increases apoptosis in UB/OC-1 cells
UB/OC-1 cells grown under normal culture conditions showed 7 ± 1% of apoptotic cells, determined by DNA strand breaks using a TdT-FragEL DNA Fragmentation Detection Kit (EMD Biosciences) (Fig. 4A). Treatment of UB/OC-1 cells with cisplatin (20 μm) for 24 h increased the percentage of apoptotic cells to 80 ± 2%, as depicted by dark brown DAB stained cells (Fig. 4A, inset, arrows). When cells were treated for 30 min with either capsazepine (10 μm) or ruthenium red (20 μm), inhibitors of TRPV1, and then exposed to cisplatin (20 μm), the percentage of apoptotic cells were reduced to 6 ± 1% and 7 ± 2%, respectively (Fig. 4A). Neither capsazepine nor ruthenium red, added alone, altered cell apoptosis, compared with vehicle-treated control groups. The percentage of apoptotic cells were 3 ± 1 and 4 ± 1%, after the addition of capsazepine or ruthenium red alone. The increase in apoptosis by cisplatin was associated with increases in proapoptotic proteins, such as p53, to 147 ± 9% of control (Fig. 4B) and Bax to 124 ± 11% of control (Fig. 4C). Cisplatin-induced increases in p53 and Bax were attenuated by capsazepine (10 μm), implicating TRPV1 activation in the apoptosis. The addition of capsazepine alone did not significantly alter the levels of p53 or Bax. UB/OC-1 cells pretreated with 2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA-AM) (10 μm) for 30 min to chelate intracellular Ca2+ before administration of cisplatin showed a reduction in Bax protein (Fig. 4C), implicating Ca2+ accumulation (presumably via influx through TRPV1 channels) in mediating apoptosis induced by cisplatin. Cisplatin increases intracellular Ca2+ in UB/OC-1 cells, but this response was blunted in cells in which the activity of TRPV1 was reduced by ruthenium red or after reduction of the expression of TRPV1 by siRNA (Fig. 4D). Furthermore, the rise in intracellular Ca2+ was abrogated upon depletion of extracellular Ca2+ using EGTA (1.0 mm) (data not shown). These studies implicate the TRPV1 channel in mediating the rise in intracellular Ca2+ by cisplatin. Together, these findings suggest apoptosis induced by cisplatin is likely mediated through activation of TRPV1, followed by increases in intracellular Ca2+ accumulation and induction of the apoptotic pathway. We also observed cisplatin-induced Ca2+ influx in cells stably expressing TRPV1, which was inhibited by ruthenium red (data not shown).
Figure 4.
Cisplatin induces UB/OC-1 cell apoptosis through activation of TRPV1. A, Cells were pretreated with vehicle, capsazepine (10 μm) or ruthenium red (20 μm) for 30 min, followed by vehicle or cisplatin (20 μm) for 24 h. Cisplatin produced significant increase in apoptotic cells (indicated by arrows pointing to dark brown cells), which was reduced by capsazepine or ruthenium red. These agents, added alone, did not produce any change in cell apoptosis (blue green staining). Asterisk (*) indicates a statistically significant increase in apoptosis compared with control, while ** indicates statistically significant protection versus cisplatin (p < 0.05, n = 3). Cisplatin increased the levels of proapoptotic proteins p53 (B) and Bax (C) by 24 h, which were reversed by capsazepine. Cells pretreated with BAPTA-AM showed reduced cisplatin-mediated Bax protein induction. The results show the mean ± SEM of three independent experiments. D, siRNA against TRPV1 reduced cisplatin-mediated Ca2+ influx in UB/OC-1 cells. Cells were pretreated with a scrambled siRNA (control) or TRPV1 siRNA (5 nm) for 48 h, followed by cisplatin (2.5 μm) for 30 min, and intracellular Ca2+ levels were determined using Fluo-4AM by confocal microscopy. Other cells were treated with ruthenium red 30 min before cisplatin and then used for measuring Fluo-4AM fluorescence. Both TRPV1 siRNA and ruthenium red reduced cisplatin-mediated Fluo-4AM fluorescence, indicating reduction in Ca2+ influx. The right panels show the differential interference contrast images of the fluorescent cells (magnification, 200 ×).
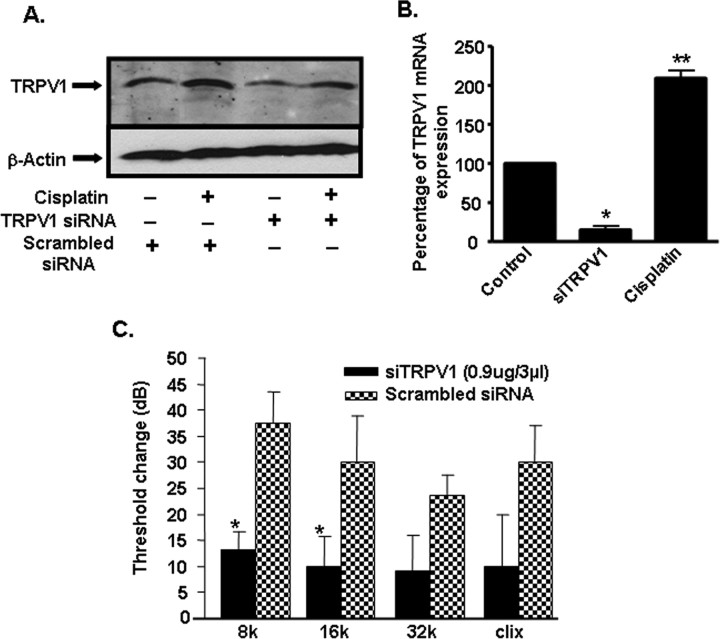
siRNAs suppress the expression of NOX3 and TRPV1 in UB/OC-1 cultures
Since NOX3 serves as a major source of ROS production in the cochlea (Bánfi et al., 2004), we next determined whether selective reductions in NOX3 by siRNA decrease the expression of TRPV1. In these cultures, cisplatin increased the expression of NOX3 by 2.0 ± 0.5-fold. The administration of NOX3 siRNA reduced basal and cisplatin-stimulated expression of NOX3 by 24 h. NOX3 RNA was undetectable in control cells pretreated with NOX3 siRNA. In addition, cisplatin-stimulated NOX3 expression was also reduced to 0.2 ± 0.1-fold by NOX3 siRNA. Assessment of TRPV1 mRNA indicates that NOX3 siRNA significantly reduced cisplatin-induced TRPV1 expression, without altering its basal expression (Fig. 5). Based on the previous data using inhibitors of NADPH oxidase (in vitro) and lipoic acid (in vivo), these data implicate NOX3 as a major regulator of TRPV1 expression in UB/OC-1 cultures. Cells pretreated with siRNA against TRPV1 showed a significant reduction in the basal and cisplatin-induced TRPV1 expression. The basal and cisplatin-stimulated levels of TRPV1 were 0.1 ± 0.1 and 0.5 ± 0.3-fold, compared with cells administered a scrambled siRNA sequence. In addition, TRPV1 siRNA reduced cisplatin-stimulated NOX3 expression but not the basal expression of NOX3. The levels of NOX3 were 1.1 ± 0.4 and 0.2 ± 0.3-fold versus a scrambled siRNA sequence, under basal conditions and after cisplatin administration (Fig. 5). These data suggest cross- regulation of NOX3 expression via TRPV1 activation.
A previous study (Rivolta et al., 2002) has reported differences in gene expression of UB/OC-1 cells grown at the permissive condition (33°C, 10%CO2) versus differentiation condition (39°C, 5%CO2). We examined the basal and cisplatin-stimulated expression of TRPV1 under these two growth conditions. We observed that the expression of TRPV1 (as determined by real-time PCR) was suppressed by 75 ± 6% when cells were cultured at 39°C (5% CO2) versus 33°C (10% CO2). These results are presented in supplemental Figure 2, available at www.jneurosci.org as supplemental material, which shows real-time PCR products of TRPV1. In addition, the levels of GAPDH were reduced by 70 ± 8% in cultures maintained at 39°C versus 33°C. When cells were treated with cisplatin for 24 h, the induction of TRPV1 expression was also blunted in cultures at 39°C versus 33°C. No change in the ability of TRPV1 siRNA to decrease the expression of this protein was observed at 33°C versus 39°C (data not shown).
siRNAs suppress the expression of NOX3 and TRPV1 in rats and protect against cisplatin ototoxicity
To determine whether TRPV1 siRNA is able to reduce cisplatin-induced hearing loss, ABRs were measured in rats which were pretreated with scrambled or TRPV1 siRNA by round window application. These rats were then administered vehicle or cisplatin (13 mg/kg) by i.p. injections 48 h later. Post-treatment ABRs were performed 72 h after administration of cisplatin. Using cyanine-3 labeled scrambled siRNA, we showed that round window application delivered the fluorescent siRNA into cochlear structures, including the organ of Corti by 3 d after administration (the earliest time examined) and that the signal persisted for ∼10 d, with no significant change in ABR thresholds (data not shown). Round window application of TRPV1 siRNA reduced the basal and cisplatin-induced TRPV1 protein levels in the cochlea (Fig. 6A; supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Real-time PCR, performed to determine the level of TRPV1 mRNA in the cochlea, indicated an 85 ± 5% decrease in expression after administration of TRPV1 siRNA and examining the cochlea on day 2 (Fig. 6B), compared with rats administered a scrambled siRNA sequence. The induction of TRPV1 in cochleae obtained from rats treated with cisplatin alone was 2.1 ± 0.1-fold. In the rats treated with a scrambled siRNA, cisplatin increased ABR thresholds by 38 ± 6, 30 ± 9, 24 ± 4 and 30 ± 7 dB at testing frequencies of 8, 16, 32 kHz or clicks, respectively, within 72 h (Fig. 6C), indicative of hearing loss. However, in rats pretreated with round window application of TRPV1 siRNA (0.9 μg/3 μl), cisplatin-induced shifts in ABR thresholds observed at 8 and 16 kHz were significantly reduced to 13 ± 3 and 10 ± 6, respectively (Fig. 6C). Cisplatin-induced ABR shifts observed at 32 kHz and with clicks, while showing substantial reductions in the siRNA-pretreated group (being 9 ± 7 and 10 ± 10 dB, respectively), were not statistically different from the rats pretreated with a scrambled siRNA sequence (Fig. 6C).
Figure 6.
siRNA against TRPV1 reduced cisplatin-induced ototoxicity in rats. A, siRNA against TRPV1 suppressed the basal and cisplatin-stimulated TRPV1 protein levels in the cochlea assessed 24 h after cisplatin administration. This is a representative of three independent experiments showing similar responses. B, Cochlear mRNA obtained from rats administered TRPV1 siRNA and assessed 48 h later indicated a ∼85% reduction in basal TRPV1 mRNA. Asterisk (*) indicates statistically significant reductions in the cochleae treated with TRPV1 siRNA versus those treated with a scrambled siRNA (p < 0.05, n = 5). Double asterisk (**) indicates statistically significant induction by cisplatin. C, Pretreatment ABRs were determined in the rats which were then administered either a scrambled siRNA sequence in one ear or 0.9 μg siRNA against TRPV1 by round window application in the other ear. Cisplatin (13 mg/kg, i.p) was administered 48 h later and post-treatment ABRs were determined after an additional 72 h period. Cisplatin produced a shift in ABR thresholds (determined by comparing pre- and post-treatment ABR thresholds) of ∼25–40 dB over an 8–32 kHz frequency range in the ears of animals pretreated with the scrambled siRNA. However, statistically significant reductions in ABR thresholds (p < 0.05, n = 5) were obtained at 8 and 16 kHz frequency range and a trend for protection observed at the 32 kHz range and for clicks (clix) in rats pretreated with siRNA against TRPV1.
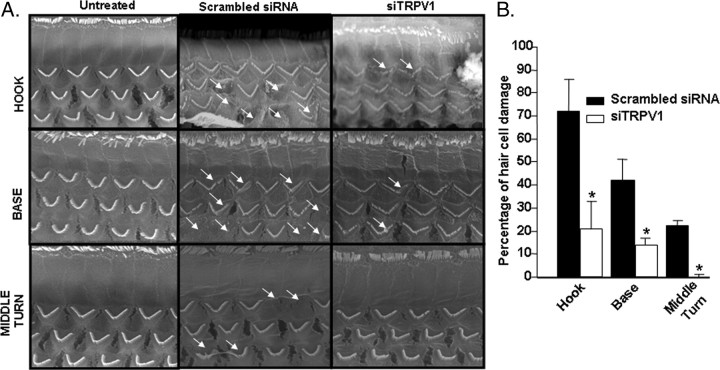
Morphological assessment of the outer hair cells by scanning electron microscopy (SEM) indicated significant damage to, or loss of, hair cells in rats pretreated with scrambled siRNA and administered cisplatin (13 mg/kg, i.p.) in the hook, basal and middle turns of the cochlea (Fig. 7A), averaging 72 ± 4, 42 ± 9 and 22 ± 3%, respectively. However, pretreatment with TRPV1 siRNA resulted in significant reductions in the percentage of hair cell loss to 21 ± 12, 14 ± 21 and 0% loss in the hook, base and the middle turns, respectively (Fig. 7B). The morphology of the outer hair cells in naive rats is depicted on the left (Fig. 7A). No significant change in outer hair cell morphology was observed after the administration of TRPV1 siRNA alone, without the addition of cisplatin (data not shown).
Figure 7.
TRPV1 siRNA protects against cisplatin-induced outer hair cell damage. A, Rats were pretreated with either a scrambled siRNA sequence or with siRNA against TRPV1 by round window application for 48 h. This was followed by cisplatin administration (13 mg/kg, i.p.). The cochleae were collected 72 h later and were processed for scanning electron microscopy. TRPV1 siRNA protected against cisplatin-mediated damage and loss of cochlear outer hair cells. The scanning electron microscopy of samples collected 72 h after cisplatin administration indicate damage to or loss of outer hair cells in cochleae pretreated with scrambled siRNA sequence, with greatest effects obtained in the hook, followed by the base and the middle turn (arrows). Cochleae obtained from rats pretreated with siRNA against TRPV1 showed statistically significant reductions in hair cell loss in all three regions examined. B, The percentage of outer hair cell damage in A is presented in graphical format. Asterisk (*) indicates statistically significant reductions in the cochleae treated with TRPV1 siRNA versus those treated with a scrambled siRNA (p < 0.05, n = 5).
Discussion
This study implicates TRPV1 in cisplatin-induced ototoxicity and shows the potential therapeutic benefit of targeting this protein for knockdown in the treatment of cisplatin ototoxicity. We provide evidence that cisplatin generates ROS in the cochlea, resulting primarily from activating the NOX3 isoform of NADPH oxidase. While previous studies demonstrated that ROS promotes lipid peroxidation and damage to the cochlea, we show that ROS could also activate and increase the expression of TRPV1. This would contribute to cell death by increasing the influx of Ca2+ into the cell through these channels. ROS also serve as a positive feedback regulator of NOX3 expression, thereby promoting further increases in ROS generation and induction of TRPV1. We propose that the coordinate activation and/or induction of these proteins could contribute significantly to cisplatin ototoxicity.
Cisplatin enhances ROS generation in cochlear tissue explants (Clerici et al., 1996; Kopke et al., 1997). The increase in ROS generation is likely due to depletion of reduced glutathione and antioxidant enzymes and/or direct activation of ROS generating systems, such as NADPH oxidase. The former mechanism requires conversion of cisplatin intracellularly into a monohydrated complex (van den Berg et al., 2006). This monohydrated complex form of cisplatin is believed to mediate the therapeutic and side effects of cisplatin (Clerici et al., 1996). The reductions in antioxidant enzymes could be explained by direct binding of cisplatin to sulfhydryl groups within these enzymes, to depletion of copper and selenium which are essential cofactors for regulating the activity of these enzymes, to inactivation of these enzymes by lipid peroxides and to depletion of NADPH, essential for the activity of glutathione peroxidase and glutathione reductase (Rybak et al., 2007). These findings form the basis for focusing on antioxidant therapy in the treatment of cisplatin ototoxicity. Recent studies have provided evidence that the NOX3 isoform of NADPH oxidase is the primary source of ROS generation by cisplatin in cochlear explants (Bánfi et al., 2004). We have confirmed these findings in this study by showing that NOX3 is an essential component of cisplatin-mediated ROS generation in UB/OC-1 cells and that NOX3 is induced by cisplatin in the cochlea in an ROS-dependent manner. As such, NOX3 could serve as the major source for ROS production and lipid peroxidation in the cochlea.
Our data also support a contribution of ROS to enhanced activity of TRPV1, thereby mediating additional toxicity via increasing intracellular Ca2+ accumulation in these cells. ROS contribute to the activation of TRPV1 channels in vagal lung afferent fibers (Ruan et al., 2005) and in vagal and sympathetic afferents in the heart (Schultz and Ustinova, 1998). The mechanism of ROS activation of TRPV1 is not clear, but may involve modification of cysteine thiols on the receptors as observed for nitric oxide (Yoshida et al., 2006). While our data suggest that NOX3 is a major source of ROS generation in the cochlea, other likely sources of ROS also exist. These include other subunits of NADPH oxidase present in the cochlea, such as gp91phox and Rac1, and xanthine oxidase, which would be activated by a rise in hypoxanthine, derived from the metabolism of endogenous adenosine (Linden, 1994). A rise in adenosine could accompany increased oxidative stress to cells in the inner ear. Adenosine is derived primarily from the metabolism of ATP and ADP, which are themselves modulators of TRPV1 currents through activation of P2 purinergic receptors (Moriyama et al., 2003). Reactive nitrogen species could also serve as another activator of TRPV1. Cisplatin increases inducible nitric oxide synthase immunoreactivity in the cochlea via an NF-κB dependent pathway (Watanabe et al., 2002). The resulting increase in NO production could mediate cochlear dysfunction, since inhibition of NOS by N-nitro-l-arginine methyl ester reduced the toxicity of cisplatin (Watanabe et al., 2000). NO is able to mediate cysteine S-nitrosylation and activation of a number of TRP channels, including TRPV1 (Yoshida et al., 2006), as described above.
Although cisplatin-induced lipid peroxidation plays a major role in the damage and apoptosis of hair cells, it is likely that activation of TRPV1 also contributes significantly. We show that inhibition of TRPV1 in UB/OC-1 cells by capsazepine or ruthenium red blunted the cisplatin-induced apoptotic cell death. The proapoptotic effect of cisplatin likely results from increased Ca2+ influx, calcium overload and activation of caspases. Knockdown of TRPV1 expression by siRNA inhibited Ca2+ influx after cisplatin treatment (Fig. 4D), suggesting that this is the primary mechanism by which cisplatin increases intracellular Ca2+. A rise in intracellular Ca2+ by cisplatin (presumably via transmembrane influx) appears to be important for mediating cisplatin-induced apoptosis, since pretreating cells with BAPTA-AM to chelate intracellular Ca2+ reduced cisplatin-induced Bax expression, a proapoptotic protein (Fig. 4C). Similar mechanisms might mediate the loss of hair cell in the cochlea produced by cisplatin and explain the protective effect of TRPV1 siRNA administration in vivo.
The utility of siRNA is manifested by its prolonged presence in the cochlea after round window application. Others have observed that siRNA against different targets, such as the ApoB gene (Zimmermann et al., 2006), produced longer term reductions in these target proteins, compared with conventional drugs which target these proteins, and with minimal side effects. An additional advantage of our study is that the siRNA administration was localized to the cochlea where it produced significant inhibition of TRPV1 expression at this location. While it is clear from our in vitro studies that siRNA against TRPV1 and NOX3 could be effective against cisplatin ototoxicity, we chose TRPV1 siRNA for our in vivo ototoxicity studies since this protein represents the distal target of cisplatin-mediated hair cell damage. In addition, while NOX3 contributes a significant portion of cisplatin-induced ROS generation, it might mediate some normal physiological functions in the cochlea, which could be antagonized by siRNA. Furthermore, as described below, TRPV1 might contribute to the entry of cisplatin into the cell. Thus, knockdown of TRPV1 by siRNA would be expected to also decrease drug entry into the hair cells. siRNA against TRPV1 was also observed to reduce cisplatin-mediated ROS generation (data not shown) and induction of NOX3 expression (Fig. 5), suggesting benefits beyond TRPV1 knock down. Together, these findings strongly support the utility of TRPV1 siRNA in the treatment of cisplatin ototoxicity.
An interesting observation is that mechanotransducer TRP channels in hair cells could serve as permeation channels for the entry of aminoglycoside antibiotics into hair cells (Gale et al., 2001). These channels also gate the entry of small styryl dyes such as FM1–43 into hair cells. Permeation of aminoglycoside antibiotics is reduced by FM1–43, suggesting competition of these two compounds from the mechanotransducer TRP channels. Recent studies indicate that the TRPV1 channels also serve as the entry port of FM1–43, which can be used to label cells expressing these channels (Meyers et al., 2003). Entry of these dyes into hair cells occurs through the hair bundles present on the apical portions of these cells (Meyers et al., 2003). Recent results from our laboratory show that pretreatment of UB/OC-1 cells with cisplatin for a time period associated with TRPV1 activation (∼30 min), followed by FM1–43 challenge, led to enhanced intracellular accumulation of the dye (data not shown). This finding provides additional evidence that cisplatin activates TRPV1 channel.
The mechanism underlying the induction of TRPV1 is not clear at present. Certainly, as described above, ROS represent key signals in the induction of this gene. A previous study concerning the induction of TRPV1 in DRG neurons showed that one target of ROS is p38 mitogen activated protein kinase, which increased this receptor protein via a post-transcriptional mechanism (Ji et al., 2002). We have also shown a similar role of p38 mitogen activated protein kinase mediating the increase in TRPV1 in DRG neurons and PC-12 cells by nerve growth factor (NGF) (Puntambekar et al., 2005). However, unlike these two reports, we have observed induction in TRPV1 mRNA by cisplatin, thereby implicating a different or additional signaling pathway. A recent report suggests that the rat TRPV1 P1 and P2 promoters contain Sp1 transcription factor binding sites which might serve as sites for regulation by NGF (Xue et al., 2007). Interestingly, NGF promotes ROS generation through a Rac1-dependent activation of NADPH oxidase (Suzukawa et al., 2000), which could serve as the mechanism underlying Sp1 activation. As such, it is reasonable to propose that cisplatin-mediated increases in ROS could lead to a similar induction in TRPV1 expression via Sp1 sites on the gene.
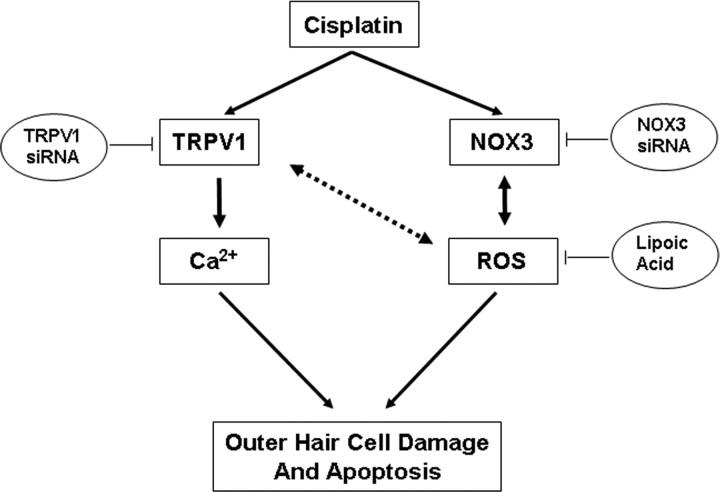
In summary, the present data indicate that the cochlear TRPV1 could serve as a sensor of cisplatin-induced oxidative stress and a mediator of cochlear damage. Induction of TRPV1 clearly results from ROS generation from NOX3 in the cochlea. In this regard, cisplatin regulates ROS generation acutely and chronically, through activation and induction, respectively, of NADPH oxidase isoforms. Increases in TRPV1 expression could exacerbate the toxicity of cisplatin through increasing intracellular Ca2+. A proposed model describing such an involvement of NOX3 and TRPV1 in cisplatin ototoxicity is presented in Figure 8. Overall, our data provide some novel targets for treating cisplatin ototoxicity, by inhibition of NOX3 and other NADPH oxidase isoforms and inhibition of TRPV1 channel activity.
Figure 8.
Proposed model of TRPV1 and NOX3 interaction in mediating cisplatin ototoxicity. Cisplatin administration increases NOX3 and TRPV1 expression in the cochlea. These changes in turn increase ROS and intracellular Ca2+ accumulation via NOX3 and TRPV1, respectively. Increased ROS leads to further TRPV1 and NOX3 activation and induction (bidirectional arrows), which contribute to further increases in Ca2+ influx into the cell and finally to apoptosis of cells expressing these receptors, such as outer hair cells and spiral ganglion cells. The increased NOX3 expression, through increased ROS generation, may promote feedback induction of TRPV1 expression and activity. In addition, TRPV1 activity may contribute to ROS generation through increased NOX3 activity. Antioxidants, such as lipoic acid, scavenge ROS and therefore abrogate the induction and activation of TRPV1, NOX3 and apoptosis mediated by cisplatin. Targeting TRPV1 and NOX3 for knockdown using the respective siRNAs for these mRNAs would also be expected to decrease the influx of Ca2+ into the cell, ROS generation and apoptosis.
Footnotes
This work was supported by National Institutes of Health Grant DC02396 (L.P.R.), a grant from the National Organization for Hearing Research, and Southern Illinois University School of Medicine Excellence in Academic Medicine Award to V.R. We thank Drs. Z. Nie and P. A. Randazzo at the National Cancer Institute–National Institutes of Health (Bethesda, MD) for generously providing us with plasmid vectors expressing RacQL and RacN17.
References
- Bánfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Christoph T, Grünweller A, Mika J, Schäfer MK, Wade EJ, Weihe E, Erdmann VA, Frank R, Gillen C, Kurreck J. Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun. 2006;350:238–243. doi: 10.1016/j.bbrc.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Clerici WJ, Hensley K, DiMartino DL, Butterfield DA. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res. 1996;98:116–124. doi: 10.1016/0378-5955(96)00075-5. [DOI] [PubMed] [Google Scholar]
- Diatchuk V, Lotan O, Koshkin V, Wikstroem P, Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem. 1997;272:13292–13301. doi: 10.1074/jbc.272.20.13292. [DOI] [PubMed] [Google Scholar]
- Dunaway G, Mhaskar Y, Armour G, Whitworth C, Rybak LP. Migration of cochlear lateral wall cells. Hear Res. 2003;177:1–11. doi: 10.1016/s0378-5955(02)00767-0. [DOI] [PubMed] [Google Scholar]
- Ford MS, Nie Z, Whitworth C, Rybak LP, Ramkumar V. Up-regulation of adenosine receptors in the cochlea by cisplatin. Hear Res. 1997;111:143–152. doi: 10.1016/s0378-5955(97)00103-2. [DOI] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1–43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci. 2001;21:7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Kamimura T, Whitworth CA, Rybak LP. Effect of 4-methylthiobenzoic acid on cisplatin-induced ototoxicity in the rat. Hear Res. 1999;131:117–127. doi: 10.1016/s0378-5955(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Kopke RD, Liu W, Gabaizadeh R, Jacono A, Feghali J, Spray D, Garcia P, Steinman H, Malgrange B, Ruben RJ, Rybak L, Van de Water TR. Use of organotypic cultures of Corti's organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am J Otol. 1997;18:559–571. [PubMed] [Google Scholar]
- Korver KD, Rybak LP, Whitworth C, Campbell KM. Round window application of D-methionine provides complete cisplatin otoprotection. Otolaryngol Head Neck Surg. 2002;126:683–689. doi: 10.1067/mhn.2002.125299. [DOI] [PubMed] [Google Scholar]
- Linden J. Purinergic systems. In: Siegel G, Agranoff B, Albers R, Molinoff P, editors. Basic neurochemistry. New York: Raven; 1994. pp. 401–416. [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1–43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23:6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjea D, Whitworth CA, Nandish S, Dunaway GA, Rybak LP, Ramkumar V. Expression of the kidney injury molecule 1 in the rat cochlea and induction by cisplatin. Neuroscience. 2006;139:733–740. doi: 10.1016/j.neuroscience.2005.12.044. [DOI] [PubMed] [Google Scholar]
- O'Donnell BV, Tew DG, Jones OT, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntambekar P, Mukherjea D, Jajoo S, Ramkumar V. Essential role of Rac1/NADPH oxidase in nerve growth factor induction of TRPV1 expression. J Neurochem. 2005;95:1689–1703. doi: 10.1111/j.1471-4159.2005.03518.x. [DOI] [PubMed] [Google Scholar]
- Rivolta MN, Grix N, Lawlor P, Ashmore JF, Jagger DJ, Holley MC. Auditory hair cell precursors immortalized from the mammalian inner ear. Proc Biol Sci. 1998;265:1595–1603. doi: 10.1098/rspb.1998.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivolta MN, Halsall A, Johnson CM, Tones MA, Holley MC. Transcript profiling of functionally related groups of genes during conditional differentiation of a mammalian cochlear hair cell line. Genome Res. 2002;12:1091–1099. doi: 10.1101/gr.225602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan T, Lin YS, Lin KS, Kou YR. Sensory transduction of pulmonary reactive oxygen species by capsaicin-sensitive vagal lung afferent fibres in rats. J Physiol. 2005;565:563–578. doi: 10.1113/jphysiol.2005.086181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak LP, Kelly T. Ototoxicity: bioprotective mechanisms. Curr Opin Otolaryngol Head Neck Surg. 2003;11:328–333. doi: 10.1097/00020840-200310000-00004. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Ramkumar V. Ototoxicity. Kidney Int. 2007;72:931–935. doi: 10.1038/sj.ki.5002434. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Ustinova EE. Capsaicin receptors mediate free radical-induced activation of cardiac afferent endings. Cardiovasc Res. 1998;38:348–355. doi: 10.1016/s0008-6363(98)00031-5. [DOI] [PubMed] [Google Scholar]
- Soong R, Beyser K, Basten O, Kalbe A, Rueschoff J, Tabiti K. Quantitative reverse transcription-polymerase chain reaction detection of cytokeratin 20 in noncolorectal lymph nodes. Clin Cancer Res. 2001;7:3423–3429. [PubMed] [Google Scholar]
- Southall MD, Li T, Gharibova LS, Pei Y, Nicol GD, Travers JB. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J Pharmacol Exp Ther. 2003;304:217–222. doi: 10.1124/jpet.102.040675. [DOI] [PubMed] [Google Scholar]
- Suzukawa K, Miura K, Mitsushita J, Resau J, Hirose K, Crystal R, Kamata T. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J Biol Chem. 2000;275:13175–13178. doi: 10.1074/jbc.275.18.13175. [DOI] [PubMed] [Google Scholar]
- Tanaka F, Whitworth CA, Rybak LP. Influence of pH on the ototoxicity of cisplatin: a round window application study. Hear Res. 2003;177:21–31. doi: 10.1016/s0378-5955(02)00771-2. [DOI] [PubMed] [Google Scholar]
- van den Berg JH, Beijnen JH, Balm AJ, Schellens JH. Future opportunities in preventing cisplatin induced ototoxicity. Cancer Treat Rev. 2006;32:390–397. doi: 10.1016/j.ctrv.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Hess A, Michel O, Yagi T. Nitric oxide synthase inhibitor reduces the apoptotic change in the cisplatin-treated cochlea of guinea pigs. Anticancer Drugs. 2000;11:731–735. doi: 10.1097/00001813-200010000-00010. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Inai S, Jinnouchi K, Bada S, Hess A, Michel O, Yagi T. Nuclear-factor kappa B NF-κB-inducible nitric oxide synthase (iNOS/NOS II) pathway damages the stria vascularis in cisplatin-treated mice. Anticancer Res. 2002;22:4081–4085. [PubMed] [Google Scholar]
- Whitworth CA, Ramkumar V, Jones B, Tsukasaki N, Rybak LP. Protection against cisplatin ototoxicity by adenosine agonists. Biochem Pharmacol. 2004;67:1801–1807. doi: 10.1016/j.bcp.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Xue Q, Jong B, Chen T, Schumacher MA. Transcription of rat TRPV1 utilizes a dual promoter system that is positively regulated by nerve growth factor. J Neurochem. 2007;101:212–222. doi: 10.1111/j.1471-4159.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- Zheng J, Dai C, Steyger PS, Kim Y, Vass Z, Ren T, Nuttall AL. Vanilloid receptors in hearing: altered cochlear sensitivity by vanilloids and expression of TRPV1 in the organ of corti. J Neurophysiol. 2003;90:444–455. doi: 10.1152/jn.00919.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Röhl I, Seiffert S, Shanmugam S, Sood V, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]