Abstract
Background
Cancer stem cells are defined by their self-renewal and multipotential capabilities and are hypothesized to be the source of primary and recurrent cancers. The stem cell properties of self-renewal and pluripotency in embryonic stem cells and germ cells are regulated by Oct4A, a splice variant of the POU5F1 (Oct3/4) gene, while the function of the alternative splice variant, Oct4B, is unknown. Rare cells that express Oct4 were identified in several somatic cancers, however, the differential contributions of the Oct4A and Oct4B variants were not determined.
Methods
Oct4A expression and co-localization with lineage markers was performed with PCR and immunohistochemistry.
Results
Rare Oct4A expressing cells are present in human benign and malignant prostate glands and the number of Oct4A expressing cells increases in prostate cancers with high Gleason scores. Oct4A expressing cells were non-proliferative, and did not co-express markers of basal epithelial cell or luminal epithelial cell differentiation, or AMACR, a marker of prostate cancer epithelial cells. A subpopulation of the Oct4A expressing cells co-expressed Sox2, an embryonic stem cell marker, but did not express other putative stem cell markers, such as ABCG2, NANOG or CD133. The majority of Oct4A expressing cells co-expressed chromogranin A, and a subset of Oct4A expressing cells co-expressed synaptophysin, both markers of neuroendocrine differentiation.
Conclusion
The increased number of cells that expressed Oct4A in prostate cancer compared to benign prostate, and in cancers of increasing grade, suggests that Oct4A/Chromogranin A co-expressing cells represent neuroendocrine cells in prostate cancer.
Keywords: Neuroendocrine cell, Oct4, Prostate Cancer
Introduction
Oct4 (Oct3/4, POU5F1) is a transcription factor that is sufficient to maintain self-renewal and pluripotency in embryonic stem cells and primordial germ cells, with Oct4 expression lost upon differentiation in vitro and in vivo (1). The POU5F1 gene encodes two isoforms, POU5F1_iA (Oct4A) and POU5F1_iB (Oct4B). Oct4A and Oct4B are composed of 360 and 265 amino acids, respectively, of which 225 amino acids in the carboxy-terminal portion are common (2). Oct4B generally is localized in the cytoplasm, and its role is unknown. Oct4A is localized in the nucleus, and its expression appears associated with maintenance of an undifferentiated state in embryonic cells (3) and of stem cell properties in embryonic stem cells and primordial germ cells (1). Additionally, Oct4A expression is a diagnostic marker in germ cell tumors (4). Recent studies demonstrated Oct4 expression in benign skin and several somatic cancers, including breast, bladder, and retinoblastoma, however, these studies did not discriminate between Oct4A or Oct4B expression (5–8). It remains undetermined whether Oct4A expression also is a marker of adult stem cells (ASCs) and/or cancer stem cells (CSCs).
ASCs and CSCs are defined by their capacity to perpetuate themselves through self-renewal, and their generation of progeny that develop into the multiple differentiated cell phenotypes of the specific tissue or tumor (9). As suggested by Pierce, the many similarities between tumor formation and organogenesis suggest that CSCs functionally may be analogous to ASCs (10). CSCs were first identified in leukemia, and subsequently were demonstrated in solid tumors, such as breast and colon cancer (11–13). The hypothesis of the presence of CSCs in tumors was supported further by the observation that only a small subset of tumor cells were capable of regenerating the original tumor (14,15). While evidence of the presence and importance of CSCs is accumulating, the origin of CSCs remains unclear. There are several possible mechanisms for the development of CSCs: 1) malignant transformation of a benign ASC into a CSC that retains self-renewal and multipotent capabilities; 2) malignant transformation of a multipotent progenitor or transit/amplifying (T/A) cell into a CSC that acquires self-renewal potential through transformation; 3) malignant transformation of a differentiated cell into a CSC, with a re-acquisition of stem cell characteristics as part of the loss of differentiation. Elucidation of the origin of CSCs would enable design of therapeutic strategies that specifically target CSCs, eliminating the source of the tumor.
Benign prostate and prostate cancer (CaP) provide unique models to address the identity, localization and roles of ASCs and CSCs due to the fact that prostate luminal epithelial cells, and the majority of CaP cells, can be depleted selectively by androgen deprivation, leaving the stem cell compartment and stem cell niche intact. Studies in rodent prostate demonstrated that the prostate can undergo multiple rounds of castration-induced regression, and testosterone-induced regeneration, suggesting the stem cell compartment was not androgen dependent. Furthermore, the high likelihood of recurrence of CaP after androgen-deprivation therapy suggests that CSCs are present in primary CaP, and that they survive androgen deprivation and are the nidus of the lethal form of CaP (16).
Putative prostatic ASCs and CSCs have been identified by expression of markers such as: ABCG2, CD133, CD44, and α2β1 integrin (17–19), expression of which are lost with exit from the stem cell compartment. The progeny of prostate ASC are hypothesized to differentiate along luminal, basal and neuroendocrine (NE) lineages, with expression of stem cell markers replaced by expression of markers specific for the differentiated cell lineage. NE cells in the prostate are thought to originate from stem cells, not the neural crest, and are proposed to differentiate along a separate path from basal and luminal cells, possibly without a transition through a transit/amplifying compartment (20,21). Prostatic NE cells are widely scattered throughout the benign prostate (22), and NE cells in both benign and malignant prostate are hypothesized to regulate growth, survival, differentiation and secretory activity in prostate epithelial cells via secretion of neural peptides (23). The number of NE cells in prostate cancers in the peripheral zone is higher than in cancers in the transition zone, thus, NE cells may have increased stimulatory roles on epithelial cells within the peripheral zone that requires a higher concentration of NE cells (24,25). The increase in NE-differentiation is associated with tumor progression, stage and grade (26,27), and an expanded NE compartment correlates negatively with survival, particularly in recurrent CaP (28,29). While the prognostic importance of NE-differentiation is controversial, an expanded NE compartment may identify patients with potentially more aggressive cancers (30,31).
The present study examines Oct4A expression in benign prostatic hyperplasia (BPH) and CaP. A rare population of Oct4A expressing epithelial cells was observed in both BPH and CaP. Furthermore, the majority of Oct4A expressing cells co-expressed chromogranin A (ChrA) and a subset expressed synaptophysin, markers of NE differentiation. Oct4A positive cells were non-proliferative in both benign prostate and CaP, and did not co-express putative markers of prostate stem cells, of luminal or basal epithelial cell differentiation, or of CaP. This is the first report that Oct4A mRNA and protein is expressed in benign and malignant prostate tissue, and that the number of Oct4A expressing, NE-like, cells is increased in CaP compared to BPH.
Material and methods
Clinical specimens
De-identified human prostate, prostate cancer, kidney, and seminoma tumor specimens were obtained from the tissue archive in the Pathology Resource Network at Roswell Park Cancer Institute (RPCI), Buffalo, NY, in accordance with the National Institute of Health (NIH) guidelines for the use of human subjects in research, after review by the Internal Review Board of RPCI.
RT-PCR
Total RNA was isolated from non-tumor prostate tissue and CaP tissue obtained by radical prostatectomy using the RNeasy kit, and the RNA was treated with RNase-free DNase I (Qiagen, Chatsworth, CA). Total RNA (2 μg) was used as a template for cDNA synthesis. Reverse transcription was carried out using SuperScript III according to the manufacturer’s instructions (InVitrogen, Carlsbad, CA). PlatinumR PCR Super Mix (InVitrogen) was used to perform PCR amplification reactions with specific primers: Oct4A (32) (Sense: Oct4_F_P 5′-GATGGCGTACTGTGGGCCC-3′; sense: Oct4_F 5′-AGCCCTCATTTCACCAGGCC-3′; antisense: Oct4_R 5′-TGGGACTCCTCCGGGTTTTG -3′ and GAPDH (Sense: 5′-GTCTTCACCACCATGGAGAAG-3′; antisense: 5′-CAAAGTTGTCATGGATGACCTTGG-3′). PCR products were resolved on 2% agarose gels and visualized using SYBR Green I (InVitrogen).
Immunohistochemistry (IHC)
Paraffin-embedded human tissues were sectioned (5μm), de-paraffinized, rehydrated through graded alcohol washes, and antigens unmasked by boiling in citrate buffer pH 6.0. Co-localization analyses were performed by immunohistochemistry (IHC) using the EnVision™ G/2 Double Stain System according to the manufacturer’s instructions (Dako). For characterization of protein expression in human tissue, slides were incubated with primary antibodies against: Oct4 (1/500, SC9081; 1/100, SC8628), androgen receptor (AR) (1/100, SC816), p63 (1/200, SC8431, Santa Cruz Biotechnology, Santa Cruz, CA), cytokeratin (CK)5 (1/100, MAB3224), ABCG2 (1/25, MAB4146, Chemicon, Temecula, CA), Ki-67 (1/200, 556003, Biosciences, San Jose, CA), alpha-methylacyl-CoA racemase (AMACR) (1/100, 39-4000), Synaptophysin (1/400, 18-0130, Zymed, Carlsbad, CA), CD133 (1/10, 130-090-422, Miltenyi Biotec, Auburn, CA), Nestin (1/300, MAB1259), SOX2 (1/50, MAB2018, R&D systems, Minneapolis, MN), CD56 (1/25, 3576, Cell signaling, Danvers, MA), Bombesin (1/300, NCL-Bomp, Novocastra Laboratories, Newcastle, UK), ChrA (1/1200, A0430), prostate specific antigen (PSA) (1/50, M0750, Dako, Santa Barbara, CA), NANOG (1/80, 14-5769, eBiosience, San Diego, CA), ChrA (1/100, MS-381-P1, Thermo Fisher Scientific, Waltham, MA). Following incubation with the primary antibody, tissue sections were incubated at room temperature for 2 hours with the appropriate secondary antibody, AlexaFuor 594-conjugated anti-rabbit or anti-mouse antibody (1/400, InVitrogen, Carlsbad, CA), or horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse antibody (1/100, Dako) followed by 3,3′-diaminobenzidine (DAB) enzymatic development or co-localization analyses performed using the EnVision™ G/2 Double Stain System (Dako). Sections were counterstained using hematoxylin, mounted with Cytoseal 60 (Richard- Allan scientific, Kalamazoo, MI), and analyzed using a Zeiss Axioskop-2plus microscope. Control experiments included omission of primary antibody as a negative control, and tissues known to express the protein of interest (human seminoma and kidney) as a positive control. In addition, incubation with blocking peptide (1/10, sc-8628 P, Santa Cruz Biotechnology, Santa Cruz, CA) for 2 hours at room temperature was performed prior to immunostaining with Oct4 antibody (1/100, SC8628). The number of Oct4A positive cells in BPH and CaP specimens were determined by analyzing twenty random 20X fields of each specimen. The number of Oct4A+/ChrA−, Oct4A−/ChrA+, and Oct4A+/ChrA+ cells in BPH and CaP specimens were determined by analysis of six 20X fields of each specimen. Statistical analysis was performed using unpaired Student’s t-Test.
Results
Oct4A Expression in Prostate and CaP
Oct4A mRNA expression in clinical specimens of human prostate tissue harvested from radical prostatectomy specimens was analyzed using RT-PCR (Figure 1). Each prostate surgical specimen was evaluated by a pathologist, and total RNA was isolated from areas designated as non-tumor and tumor. Extracted RNA was treated with RNase-free DNase I to avoid amplification of genomic DNA contamination, particularly non-transcribed pseudogenes of the POU5/Oct4 family (32). PCR primers specific for Oct4A were used that did not recognize known pseudo-genes of Oct4 that have been found to be transcribed in many cancers (33). Oct4A mRNA was expressed in all benign prostate and prostate cancer tissue samples analyzed with both primers for Oct4A, and no apparent differences in mRNA levels were found between matched non-tumor and tumor specimens for individual patients.
Figure 1. Oct4A mRNA expression in human prostate tissue.
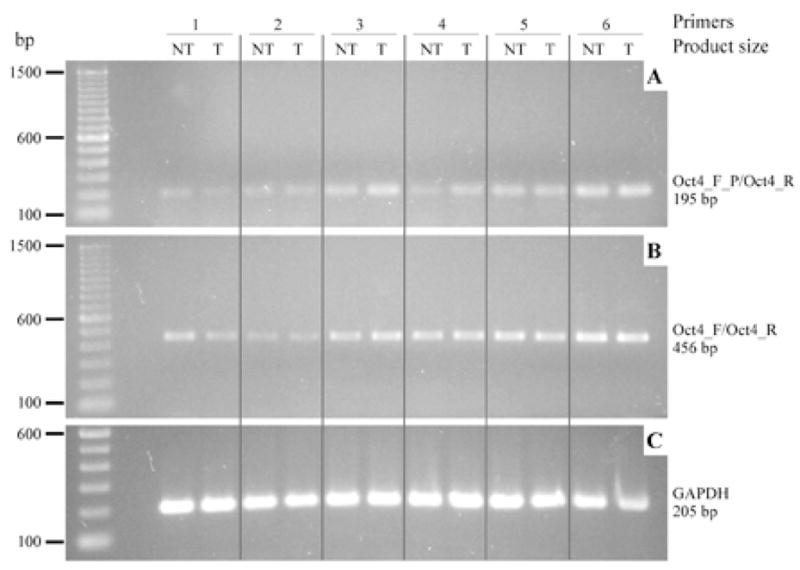
RT-PCR performed on cDNA synthesized from RNAs isolated from human prostate specimens representing tumor and non-tumor samples, each set represents 1 patient specimen. RT-PCR primers: (A) Oct4_F_P/Oct4_R, (B) Oct4_F/Oct4_R and (C) GAPDH specific primers.
To identify the population of cells within the prostate that expressed Oct4A, IHC was performed in BPH (Figure 2A) and CaP (Figure 2B–D) tissue sections obtained from radical prostatectomy specimens with cancers of Gleason grades ranging from 5 to 10. Cytoplasmic and nuclear expression of Oct4A was observed in cells residing within the basal compartment of both benign prostate and CaP tissues using a rabbit polyclonal antibody against amino acids 1–134 of Oct4A that does not recognize Oct4B (Figure 2A–D). The total number of Oct4A positive cells was quantified in 20 fields from each specimen, and compared using unpaired Student’s t-Test (Figure 2G). The number of Oct4A expressing cells was lower in BPH specimens compared to the number in the matched CaP specimens. CaPs with a Gleason score >6 demonstrated a significantly increased number of Oct4A expressing cells (p ≤ 0.001) compared to BPH, suggesting that Oct4A positive cells may have a role during CaP progression. However, there was not a significant correlation between the number of Oct4A-positive cells in a prostate specimen and Gleason score for the small number of specimens in this study. Tissue specimens from human seminomas were analyzed as positive controls for Oct4 expression to verify the specificity of the Oct4A antibody (Figure 2E–F). A nuclear pattern of Oct4 expression was observed in seminoma tissue, consistent with previously published data (34). In order to determine whether the cytoplasmic localization detected in prostate specimens (Figure 2) was associated with the Oct4A isoform, immuno-co-localization with a second Oct4 antibody (SC8628) was performed in prostate and seminoma tissue in the presence (Figure W1 A1, B1) and absence (Figure W1 A2, B2) of a specific peptide analogue of the amino-terminal portion of Oct4A. Detection of Oct4A nuclear expression was lost in seminoma tissue in the presence of the blocking peptide (Figure W1B). Similarly, detection of Oct4 in both the nucleus and cytoplasm in prostate tissue was lost when the antibody was incubated with the blocking peptide prior to incubation with tissue.
Figure 2. Oct4A expression in human prostate tissue.
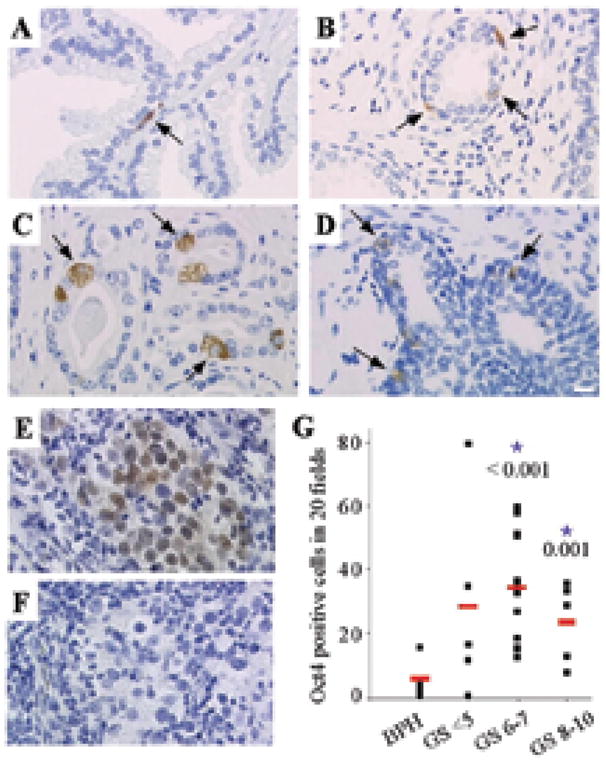
Oct4A expression in (A) BPH; (B) CaP Gleason Score 5; (C) CaP Gleason Score 6; (D) CaP Gleason Score 9. White bar: 10μM. (E) Oct4A expression in human seminoma as a positive control. (F) Human seminoma lacking Oct4 primary antibody as a negative control. (G) Oct4A quantification in different Gleason Score samples. The total number of Oct4A positive cells in 20 fields for each specimen versus Gleason Score. Statistical analysis of Oct4A expressing cells quantified in prostate specimens comparing different Gleason Scores and BPH: the red line represents the mean. * p-Value < 0.05.
Co-localization of Oct4A and Stem Cell, Cancer, and Proliferation Markers in Human Prostate
To determine whether Oct4A expressing cells had a stem cell phenotype, IHC analyses were performed to detect co-expression of Oct4A and the putative prostate tissue stem cell markers CD133, CD34 and ABCG2, or with the embryonic stem cell markers Sox2 and NANOG. A small fraction of Oct4A expressing cells co-expressed Sox2 (Figure 3A1–A2), however, not all Sox2 expressing cells co-expressed Oct4A (Figure 3A3). In contrast, Oct4A expression did not co-localize with expression of the putative stem cell markers NANOG (Figure 3B1), ABCG2 (Figure 3C), CD133 (Figure 3D1), or CD34 (data not shown). Importantly, neither NANOG nor CD133 expression were detected in any prostate cancer specimens examined. However, NANOG expression was present in seminoma specimens (Figure 3B2), and CD133 expression was present in benign human kidney specimens (Figure 3D2), tissues previously described as positive controls for NANOG and CD133 expression, respectively (7,35). Co-expression of Oct4A and ABCG2 was not observed (Figure 3C) even though cells individually expressing each of these markers were present in CaP tissue. Finally, co-expression of Oct4A and AMACR was examined to determine whether CaP cells that express AMACR also expressed Oct4A, suggesting the presence of CSC-like cells committed to a cancer cell lineage. However, Oct4A expressing cells that co-expressed AMACR were not observed (Figure 4A). Since the majority of Oct4A positive cells did not express putative stem cell markers, and were negative for expression of the CaP cell marker AMACR, Oct4A positive cells were examined for co-expression of the proliferation marker Ki-67 to determine if Oct4A expressing cells represented a T/A cell. Analysis for co-expression of Oct4A and Ki-67 demonstrated that Oct4A positive cells were not proliferative (Figure 4B), suggesting that Oct4A expressing cells were not in the T/A compartment.
Figure 3. Oct4A co-expression with markers for putative stem cells in human prostate tissue.
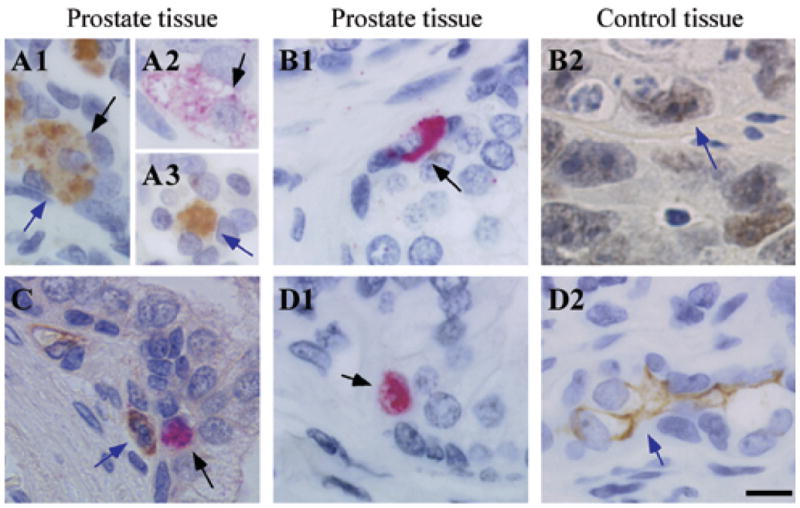
(A) Co-expression of Oct4A (Permanent red) and Sox2 (DAB); (B1) Oct4A (Permanent red) and NANOG (DAB); (C) Oct4A (Permanent red) and ABCG2 (DAB); (D1) Oct4A (Permanent red) and CD133 (DAB) in prostate tissue. (B2) NANOG (DAB) in seminoma tissue; (D2) CD133 (DAB) in human kidney tissue. Black bar: 10μM. Black arrows indicate Oct4A staining and blue arrows indicate expression of secondary markers.
Figure 4. Oct4A co-expression with markers for CaP and proliferation in human prostate tissue.
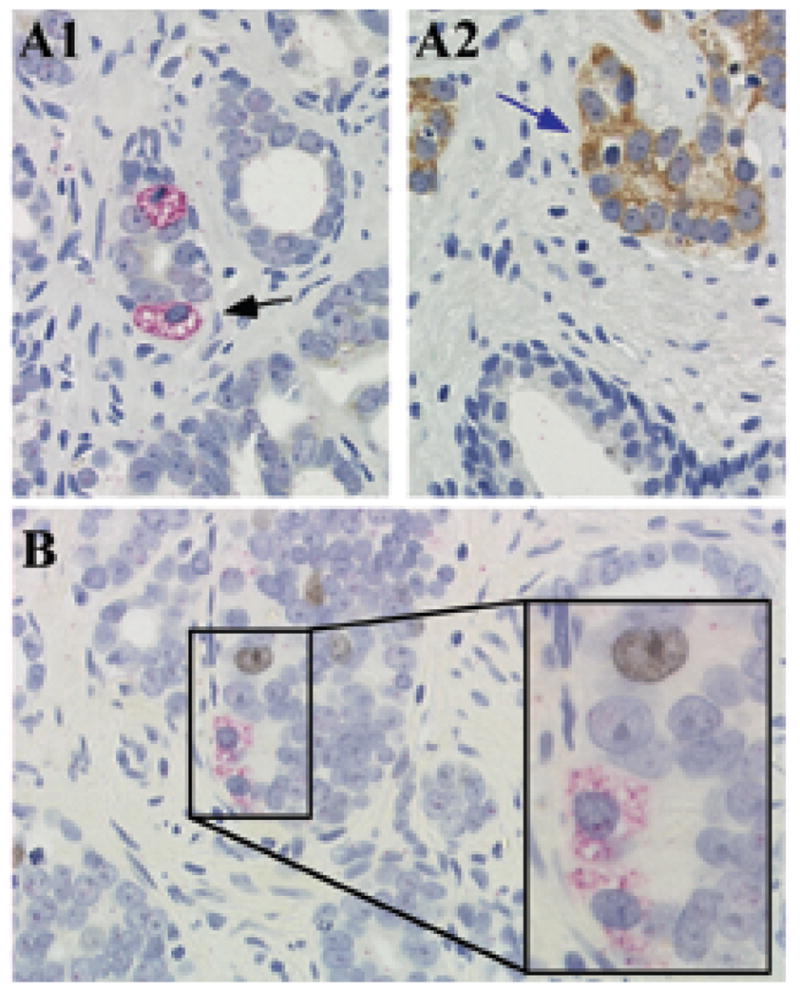
(A) Oct4A (Permanent red) and AMACR (DAB); (B) Oct4A (Permanent red) and Ki67 (DAB) in prostate tissue.
Oct4A and Differentiation Marker Co-Expression in Human Prostate
Since Oct4A expressing cells did not co-express putative markers of stem or T/A cells, Oct4A expressing cells were characterized for co-expression of markers of commitment to basal cell (p63, CK5, and CK15) and luminal cell (AR and PSA) differentiation lineages. Commitment to the basal epithelial lineage was evaluated by analysis of co-expression of Oct4A and the prostate basal cell markers p63 (Figure 5A), CK5 (Figure 5B), and CK15 (data not shown). Because both nuclear and cytoplasmic cellular localization of Oct4A was observed, and expression of many of the markers of stromal and epithelial differentiation is cytoplasmic, a dual-staining protocol was developed: immunofluorescent staining (Alexa 594) was utilized in conjunction with DAB colorimetric co-staining Oct4A positive cells did not co-express any of the basal cell markers examined, suggesting that Oct4A expressing cells do not represent a subset of basal cells. Furthermore, consistent with cellular morphology and localization within the prostate glands, Oct4A expressing cells did not co-express the luminal epithelial cell markers AR and PSA (Figure 5C, 5D).
Figure 5. Oct4A co-expression with differentiation markers for basal, luminal, and neuroendocrine lineages in human prostate tissue.
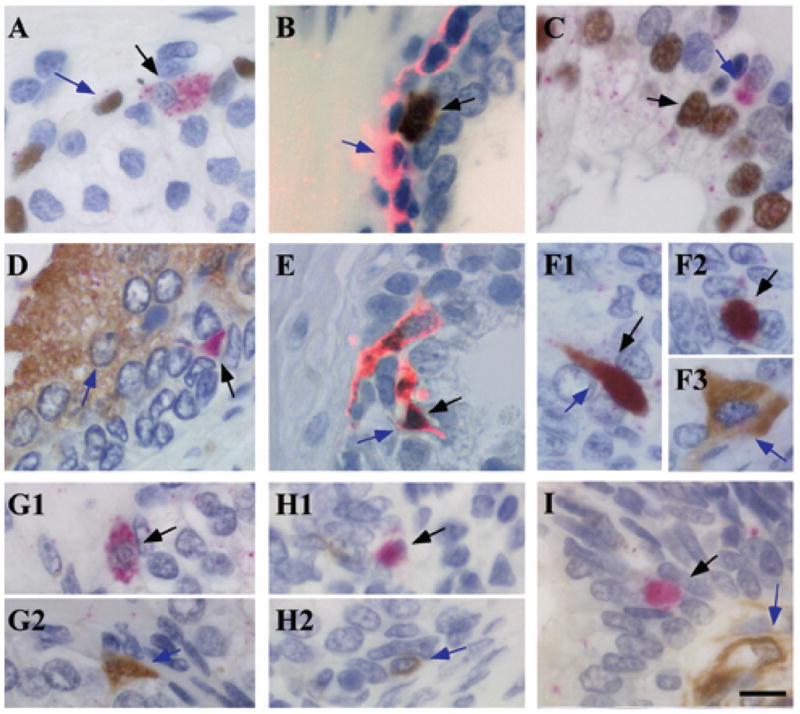
(A) Co-expression of Oct4A (Permanent red) and p63 (DAB); (B) Oct4A (DAB) and cytokeratin 5 (alexa 594); (C) Oct4A (Permanent red) and AR (DAB); (D) Oct4A (Permanent red) and PSA (DAB); (E) Oct4A (DAB) and chromogranin A (alexa 594); (F) Oct4A (Permanent red) and synaptophysin (DAB); (G) Oct4A (Permanent red) and bombesin (DAB); (H) Oct4A (Permanent red) and CD56 (DAB); (I) Oct4A (Permanent red) and nestin (DAB). Black bar: 10μM. Black arrows indicate Oct4A expression and blue arrows indicate expression of differentiation markers.
Oct4A expressing cells were evaluated for co-expression of markers associated with commitment to the NE differentiation lineage. The majority of Oct4A expressing cells co-expressed the NE marker ChrA (Figure 5E), with Oct4A−/ChrA+ and Oct4A+/ChrA− cells observed infrequently. Immunofluorescence staining for ChrA was utilized in conjunction with DAB-based colorimetric staining for Oct4A for evaluation of co-expression because of the strong cytoplasmic expression of ChrA and the low levels of Oct4 expression. As controls of the specificity and sensitivity of the dual staining technique, immunofluorescence and IHC analyses were performed in the presence of only the Alexa 594-conjugated secondary antibody (fluorescence), and in presence (Figure W2A) and absence (Figure W2B) of the primary antibody for ChrA (Figure W2A-B). The control demonstrated that immuno-reaction of the anti-rabbit Alexa 594-conjugated secondary antibody occurred only in the presence of anti-ChrA primary antibody, indicating the Alexa 594-conjugated secondary antibody was not recognizing the Oct4 primary antibody (Figure 5E). In addition, staining performed simultaneously in prostate and seminoma tissue demonstrated co-expression of ChrA and Oct4A in prostate (Figure W2C), however, co-expression was not observed in seminoma (Figure W2D). To confirm co-localization of Oct4 and ChrA, additional antibodies against Oct4 and ChrA were analyzed (Figure W3A–B). Co-localization of Oct4 and ChrA with antibodies developed in different species demonstrated the identical staining pattern, and indicated the staining was not due to non-specific cross-reaction between primary and secondary antibodies.
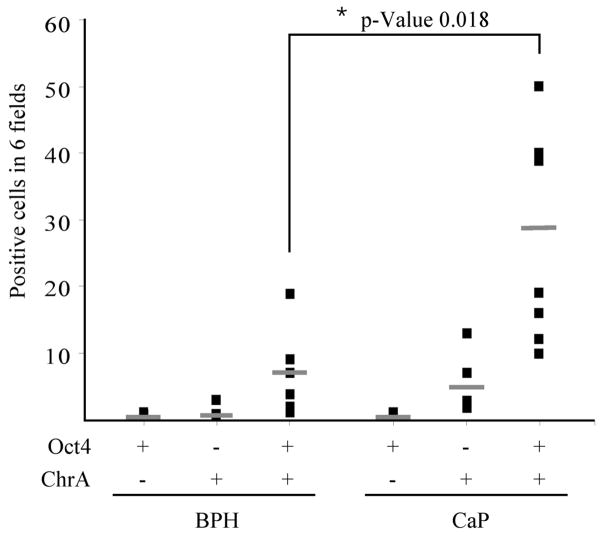
ChrA is a sensitive marker of the NE lineage and is used frequently to detect the NE phenotype in prostate cancers. Co-localization of Oct4 with additional markers of NE differentiation was examined to determine the maturation of the NE phenotype associated with NE-differentiation. Approximately 50% of Oct4A positive cells co-expressed synaptophysin (Figure 5F), a marker of the NE lineage that may identify more mature NE cells compared to ChrA expression. However, Oct4A expressing cells did not co-express the NE markers bombesin or CD56, NE-differentiation markers expressed normally by only a small proportion of prostate neuroendocrine cells, possibly the most mature NE cells (Figure 5G–H) (26,36). Furthermore, Oct4A positive cells did not co-express the NE marker nestin (Figure 5I). However nestin was expressed in the endothelial cells, as previously reported (37,38). To determine whether the increase in the number of NE cells was correlated to the increase in the number of Oct4A expressing cells, the total number of Oct4A+/ChrA−, Oct4A−/ChrA+, and Oct4A+/ChrA+ cells were quantified in 6 low-magnification microscopic fields of six BPH and seven CaP specimens, and were analyzed using unpaired Student’s t-Test (Figure 6). No significant differences were found in the number of Oct4A+/ChrA− and Oct4A−/ChrA+ cells in BPH specimens compared with CaP. However, the number of Oct4A+/ChrA+ cells increased significantly in CaP compared to BPH (p=0.018). Therefore, CaP is characterized by an increased population of Oct4A expressing cells that co-expressed the NE lineage markers ChrA and/or synaptophysin.
Figure 6. Oct4A and ChrA quantification in CaP compared to BPH.
Total number of Oct4A+/ChrA−, Oct4A−/ChrA+, and Oct4A+/ChrA+ cells in 6 fields for each specimen. n = 6 BPH and n = 7 CaP. Statistical analysis of Oct4A+/ChrA−, Oct4A−/ChrA+, and Oct4A+/ChrA+ cells quantification comparing CaP and BPH. Lines represent the mean. * p-Value = 0.018.
Discussion
This study is the first to demonstrate expression of Oct4A mRNA and protein in human benign prostate tissue and CaP, and that the number of Oct4A expressing cells is increased in CaP compared to benign prostate. We demonstrated that Oct4A mRNA was expressed in all the non-tumor and tumor samples analyzed, and no detectable differences were found at the mRNA expression level, consistent with the low number of NE-like cells. Expression of Oct4 mRNA was demonstrated previously in bladder and prostate carcinomas. Oct4 mRNA expression was increased in bladder carcinoma compared to benign bladder tissue, however, there was not a significant correlation between the expression level of Oct4 and tumor grade or stage (6). Low levels of Oct4 mRNA were detected in normal prostate and CaP tissues, however, when Oct4 expression was corrected for levels of CK18 expression (number of prostate epithelial cells), the level of Oct4 mRNA was decreased slightly in CaP compared to normal prostate (39). The apparent decrease of Oct4 expressing cells in high Gleason Grade CaP based on mRNA evaluation probably reflected a total increase in the epithelial cell compartment in these aggressive malignancies rather than a decrease in NE cells. Additionally, apparent levels of Oct4 expression measured only by RT-PCR may be confounded by artifacts generated by pseudo-gene transcripts resulting in false positive expression (32). Six highly conserved pseudo-genes for Oct4 (Oct4-pg1 to Oct4-pg6) have been indentified by nucleotide BLAST (basic local alignment sequence tool) searches against mRNA transcripts (40). Two of the six pseudogenes, Oct4-pg1 and Oct4-pg5, have been identified as being transcribed in some cancer cell lines and cancer tissues (33). Two forward primers designed by Liedtke et al. that do not recognize any pseudo-genes currently identified were used in our studies (32). We demonstrated that Oct4A mRNA was expressed in all the non-tumor and tumor samples analyzed, and no detectable differences were found at the mRNA expression level, consistent with the low number of NE-like cells.
The localization of Oct4A expressing cells within glandular structures in the area anticipated to house the stem cell “niche” was suggestive, however, Oct4A expressing cells did not co-express candidate markers for ASCs or CSCs, or markers of commitment to the differentiated lineage of prostate luminal or basal lineage, or of cancer epithelial cells. Additionally, it is possible that prostate stem cells do not express CD133, ABCG2, or NANOG; thus Oct4A cannot be ruled out as a prostate stem cell marker. Oct4A expressing cells did not demonstrate proliferative activity, indicating that they did not represent cells within a T/A compartment, the immediate progeny of the ASCs/CSCs that are responsible for population expansion before commitment to differentiation. Rather, the small population of Oct4A expressing cells in CaP co-expressed markers of immature/primitive NE cells, suggesting these cells might represent stem cell progeny committed to the NE lineage. NE cells in prostate are intriguing since they do not depend on androgen for survival and are likely to survive androgen deprivation therapy, and they may play an important role in advanced CaP through the secretion of growth and survival factors (41). Importantly, the extent of NE-differentiation in CaP and metastases is correlated with increased stage, Gleason grade, microvessel density, and proliferation and survival of cancer cells, particularly in recurrent CaP (41,42). Consequently, identification of Oct4A expression as an early marker of commitment to a NE lineage and phenotype in CaP may provide insight into the mechanism of NE-differentiation in prostate cancer and their role in the evolution of androgen-independent CaP.
The transcriptional regulatory function of Oct4A suggests the protein should be localized in the nucleus of the cell. The Oct4A isoform has been described to have predominantly a nuclear localization pattern while the Oct4B isoform has a cytoplasmic localization pattern (3,43). However, the detection of both nuclear and cytoplasmic expression of Oct4A in this study is consistent with a previous report of Oct4 expression in bladder cancer (6). Our data demonstrate that both the nuclear and cytoplasmic staining in prostate are associated with Oct4A expression since pre-incubation of the antibody (sc-8628) with a blocking peptide (1/10, sc-8628 P, Santa Cruz Biotechnology, Santa Cruz, CA) homologous with a segment of the Oct4A protein blocked detection of Oct4 protein in both the nucleus and cytoplasm. Cytoplasmic localization of Oct4A could be due to post-translational regulation since Oct4 activity is regulated post-translationally by phosphorylation and/or ubiquitination (44–46). Alternatively, cytoplasmic localization of Oct4A in human cancer cells could be due to alteration of translocation of the transcription factor into the nucleus through a modulated process. Recent studies demonstrated that Oct4 was imported into the nucleus by importin-α1β and that a decrease in the importin-α1 activity immediately reduced nuclear localization of Oct4 (47). Overall, cytoplasmic localization of Oct4A observed in human prostate could be due to a failure in translocation, post-translational regulation or mutation in the protein coding sequence; however, further analyses are necessary to confirm these hypotheses.
Oct4 expression has been observed in benign skin and several non-germ cell cancers, including: breast, bladder, and retinoblastoma (5–8). In addition, ectopic expression of Oct4 resulted in dysplastic growth that was dependent on continuous Oct4 expression in multiple epithelial tissue, such as fore-stomach, intestine, and skin (48). Furthermore, induction of human pluripotent stem cells was reported by three independent groups using different combination of factors, including: Oct4, Sox2, Myc, Klf4, Nanog and Lin28 (49–51). Considering the role of Oct4 as a pluripotentcy factor, and a possible role in the etiology of cancer, Oct4 was investigated as a marker for CSCs.
Oct4 expression has been reported previously in benign prostate and cancer cell lines as evidence of the presence of ASC and CSC compartments. A subpopulation of telomerase-immortalized prostate epithelial cells that demonstrated stem cell properties expressed Oct4 protein (52). Similarly, subpopulations of CaP cells that were capable of reconstitution of the original prostate tumor in vivo expressed Oct4 mRNA in culture (53). These observations suggested that Oct4 is a marker for prostate ASCs and CSCs. However, our data that the rare cells in human prostate tissue that express Oct4A co-express NE markers, but not putative stem cell markers, raises questions about the validity of comparing putative stem cell markers in long term cultured prostate cell lines and prostate tissue.
Although the origin of NE cells, and the mechanism responsible for the increase in NE cells in advanced CaP are unknown, it has been proposed that NE cells present in CaP are different in their biochemical properties and origins than NE cells in benign prostate (22). Proposed mechanisms for the increased number of NE cells in advanced CaP include: differentiation of the progeny of CSCs to the NE lineage, and/or trans-differentiation of CaP epithelial cells into NE cells. NE cells in benign prostate glands express CK5 (54), which was not co-expressed in the Oct4A and ChrA expressing cells in CaP, suggesting these cells do not represent benign NE cells. Previous studies suggested that NE cells in CaP express AMACR (55), however, in this study, AMACR did not co-localize with Oct4A, contraindicating that NE cells represent a trans-differentiated CaP cell. However, the increased numbers of Oct4A+/ChrA+ in CaP, in combination with the lack of expression for putative markers of stem cells, cellular proliferation, and luminal (AR, PSA) or basal cell differentiation (CK5, p63), suggests that Oct4 expression represents a marker of NE cells in CaP.
Conclusion
An increased NE cell population in CaP often is associated with more aggressive disease and recurrence after androgen-deprivation therapy, suggesting that NE cells play a crucial role in CaP progression (23,56). The characterization of molecular and cellular mechanisms that determine NE-differentiation during CaP progression is critical to identification of possible therapeutic targets for inhibition of expansion of the NE compartment. Our discovery that NE cells express Oct4A could provide a new key for exploring further the mechanism of NE-differentiation and identifying new targeted therapy.
Supplementary Material
Acknowledgments
The authors thank the Pathology Resource Network at Roswell Park Cancer Institute for the archived tissues.
These studies were supported by the NCI Cancer Center Support Grant CA016056 to Roswell Park Cancer Institute; NCI CA77739 (GJS) and 2006–2007 Developmental Funds award from the Roswell Park Alliance Foundation (WJH).
References
- 1.Pesce M, Scholer HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem Cells. 2001;19(4):271–278. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- 2.Takeda J, Seino S, Bell GI. Human Oct3 gene family: cDNA sequences, alternative splicing, gene organization, chromosomal location, and expression at low levels in adult tissues. Nucleic Acids Res. 1992;20(17):4613–4620. doi: 10.1093/nar/20.17.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J, Kim HK, Rho JY, Han YM, Kim J. The human OCT-4 isoforms differ in their ability to confer self-renewal. The Journal of biological chemistry. 2006;281(44):33554–33565. doi: 10.1074/jbc.M603937200. [DOI] [PubMed] [Google Scholar]
- 4.Santagata S, Ligon KL, Hornick JL. Embryonic stem cell transcription factor signatures in the diagnosis of primary and metastatic germ cell tumors. The American journal of surgical pathology. 2007;31(6):836–845. doi: 10.1097/PAS.0b013e31802e708a. [DOI] [PubMed] [Google Scholar]
- 5.Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26(2):495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 6.Atlasi Y, Mowla SJ, Ziaee SA, Bahrami AR. OCT-4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int J Cancer. 2007;120(7):1598–1602. doi: 10.1002/ijc.22508. [DOI] [PubMed] [Google Scholar]
- 7.Ezeh UI, Turek PJ, Reijo RA, Clark AT. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer. 2005;104(10):2255–2265. doi: 10.1002/cncr.21432. [DOI] [PubMed] [Google Scholar]
- 8.Seigel GM, Hackam AS, Ganguly A, Mandell LM, Gonzalez-Fernandez F. Human embryonic and neuronal stem cell markers in retinoblastoma. Mol Vis. 2007;13:823–832. [PMC free article] [PubMed] [Google Scholar]
- 9.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 10.Pierce GB, Speers WC. Tumors as caricatures of the process of tissue renewal: prospects for therapy by directing differentiation. Cancer research. 1988;48(8):1996–2004. [PubMed] [Google Scholar]
- 11.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 14.Collins AT, Maitland NJ. Prostate cancer stem cells. Eur J Cancer. 2006;42(9):1213–1218. doi: 10.1016/j.ejca.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 15.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 16.Tang DG, Patrawala L, Calhoun T, Bhatia B, Choy G, Schneider-Broussard R, Jeter C. Prostate cancer stem/progenitor cells: identification, characterization, and implications. Mol Carcinog. 2007;46(1):1–14. doi: 10.1002/mc.20255. [DOI] [PubMed] [Google Scholar]
- 17.Huss WJ, Gray DR, Greenberg NM, Mohler JL, Smith GJ. Breast cancer resistance protein-mediated efflux of androgen in putative benign and malignant prostate stem cells. Cancer Res. 2005;65(15):6640–6650. doi: 10.1158/0008-5472.CAN-04-2548. [DOI] [PubMed] [Google Scholar]
- 18.Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117(Pt 16):3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 19.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 20.Kurita T, Medina RT, Mills AA, Cunha GR. Role of p63 and basal cells in the prostate. Development (Cambridge, England) 2004;131(20):4955–4964. doi: 10.1242/dev.01384. [DOI] [PubMed] [Google Scholar]
- 21.Litvinov IV, Vander Griend DJ, Xu Y, Antony L, Dalrymple SL, Isaacs JT. Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer research. 2006;66(17):8598–8607. doi: 10.1158/0008-5472.CAN-06-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan TC, Veeramani S, Lin MF. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocrine-related cancer. 2007;14(3):531–547. doi: 10.1677/ERC-07-0061. [DOI] [PubMed] [Google Scholar]
- 23.Abrahamsson PA. Neuroendocrine cells in tumour growth of the prostate. Endocrine-related cancer. 1999;6(4):503–519. doi: 10.1677/erc.0.0060503. [DOI] [PubMed] [Google Scholar]
- 24.Speights VO, Jr, Cohen MK, Riggs MW, Coffield KS, Keegan G, Arber DA. Neuroendocrine stains and proliferative indices of prostatic adenocarcinomas in transurethral resection samples. Br J Urol. 1997;80(2):281–286. doi: 10.1046/j.1464-410x.1997.00359.x. [DOI] [PubMed] [Google Scholar]
- 25.Van de Voorde WM, Van Poppel HP, Verbeken EK, Oyen RH, Baert LV, Lauweryns JM. Morphologic and neuroendocrine features of adenocarcinoma arising in the transition zone and in the peripheral zone of the prostate. Mod Pathol. 1995;8(6):591–598. [PubMed] [Google Scholar]
- 26.Cindolo L, Franco R, Cantile M, Schiavo G, Liguori G, Chiodini P, Salzano L, Autorino R, Di Blasi A, Falsaperla M, Feudale E, Botti G, Gallo A, Cillo C. NeuroD1 expression in human prostate cancer: can it contribute to neuroendocrine differentiation comprehension? Eur Urol. 2007;52(5):1365–1373. doi: 10.1016/j.eururo.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Grobholz R, Bohrer MH, Siegsmund M, Junemann KP, Bleyl U, Woenckhaus M. Correlation between neovascularisation and neuroendocrine differentiation in prostatic carcinoma. Pathol Res Pract. 2000;196(5):277–284. doi: 10.1016/S0344-0338(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 28.Bollito E, Berruti A, Bellina M, Mosca A, Leonardo E, Tarabuzzi R, Cappia S, Ari MM, Tampellini M, Fontana D, Gubetta L, Angeli A, Dogliotti L. Relationship between neuroendocrine features and prognostic parameters in human prostate adenocarcinoma. Ann Oncol. 2001;12 (Suppl 2):S159–164. doi: 10.1093/annonc/12.suppl_2.s159. [DOI] [PubMed] [Google Scholar]
- 29.Cheville JC, Tindall D, Boelter C, Jenkins R, Lohse CM, Pankratz VS, Sebo TJ, Davis B, Blute ML. Metastatic prostate carcinoma to bone: clinical and pathologic features associated with cancer-specific survival. Cancer. 2002;95(5):1028–1036. doi: 10.1002/cncr.10788. [DOI] [PubMed] [Google Scholar]
- 30.Krijnen JL, Bogdanowicz JF, Seldenrijk CA, Mulder PG, van der Kwast TH. The prognostic value of neuroendocrine differentiation in adenocarcinoma of the prostate in relation to progression of disease after endocrine therapy. The Journal of urology. 1997;158(1):171–174. doi: 10.1097/00005392-199707000-00054. [DOI] [PubMed] [Google Scholar]
- 31.Nelson EC, Cambio AJ, Yang JC, Ok JH, Lara PN, Jr, Evans CP. Clinical implications of neuroendocrine differentiation in prostate cancer. Prostate cancer and prostatic diseases. 2007;10(1):6–14. doi: 10.1038/sj.pcan.4500922. [DOI] [PubMed] [Google Scholar]
- 32.Liedtke S, Enczmann J, Waclawczyk S, Wernet P, Kogler G. Oct4 and its pseudogenes confuse stem cell research. Cell stem cell. 2007;1(4):364–366. doi: 10.1016/j.stem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Suo G, Han J, Wang X, Zhang J, Zhao Y, Zhao Y, Dai J. Oct4 pseudogenes are transcribed in cancers. Biochemical and biophysical research communications. 2005;337(4):1047–1051. doi: 10.1016/j.bbrc.2005.09.157. [DOI] [PubMed] [Google Scholar]
- 34.Jones TD, Ulbright TM, Eble JN, Baldridge LA, Cheng L. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol. 2004;28(7):935–940. doi: 10.1097/00000478-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166(2):545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma. Prostate. 1999;39(2):135–148. doi: 10.1002/(sici)1097-0045(19990501)39:2<135::aid-pros9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 37.Lobo MV, Arenas MI, Alonso FJ, Gomez G, Bazan E, Paino CL, Fernandez E, Fraile B, Paniagua R, Moyano A, Caso E. Nestin, a neuroectodermal stem cell marker molecule, is expressed in Leydig cells of the human testis and in some specific cell types from human testicular tumours. Cell Tissue Res. 2004;316(3):369–376. doi: 10.1007/s00441-003-0848-4. [DOI] [PubMed] [Google Scholar]
- 38.Klein T, Ling Z, Heimberg H, Madsen OD, Heller RS, Serup P. Nestin is expressed in vascular endothelial cells in the adult human pancreas. J Histochem Cytochem. 2003;51(6):697–706. doi: 10.1177/002215540305100601. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann MJ, Muller M, Engers R, Schulz WA. Epigenetic control of CTCFL/BORIS and OCT4 expression in urogenital malignancies. Biochem Pharmacol. 2006;72(11):1577–1588. doi: 10.1016/j.bcp.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 40.Pain D, Chirn GW, Strassel C, Kemp DM. Multiple retropseudogenes from pluripotent cell-specific gene expression indicates a potential signature for novel gene identification. The Journal of biological chemistry. 2005;280(8):6265–6268. doi: 10.1074/jbc.C400587200. [DOI] [PubMed] [Google Scholar]
- 41.Yuan TC, Veeramani S, Lin FF, Kondrikou D, Zelivianski S, Igawa T, Karan D, Batra SK, Lin MF. Androgen deprivation induces human prostate epithelial neuroendocrine differentiation of androgen-sensitive LNCaP cells. Endocr Relat Cancer. 2006;13(1):151–167. doi: 10.1677/erc.1.01043. [DOI] [PubMed] [Google Scholar]
- 42.Huss WJ, Gregory CW, Smith GJ. Neuroendocrine cell differentiation in the CWR22 human prostate cancer xenograft: association with tumor cell proliferation prior to recurrence. Prostate. 2004;60(2):91–97. doi: 10.1002/pros.20032. [DOI] [PubMed] [Google Scholar]
- 43.Cauffman G, Liebaers I, Van Steirteghem A, Van de Velde H. POU5F1 isoforms show different expression patterns in human embryonic stem cells and preimplantation embryos. Stem cells (Dayton, Ohio) 2006;24(12):2685–2691. doi: 10.1634/stemcells.2005-0611. [DOI] [PubMed] [Google Scholar]
- 44.Brehm A, Ohbo K, Scholer H. The carboxy-terminal transactivation domain of Oct-4 acquires cell specificity through the POU domain. Molecular and cellular biology. 1997;17(1):154–162. doi: 10.1128/mcb.17.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei F, Scholer HR, Atchison ML. Sumoylation of Oct4 enhances its stability, DNA binding, and transactivation. The Journal of biological chemistry. 2007;282(29):21551–21560. doi: 10.1074/jbc.M611041200. [DOI] [PubMed] [Google Scholar]
- 46.Xu HM, Liao B, Zhang QJ, Wang BB, Li H, Zhong XM, Sheng HZ, Zhao YX, Zhao YM, Jin Y. Wwp2, an E3 ubiquitin ligase that targets transcription factor Oct-4 for ubiquitination. The Journal of biological chemistry. 2004;279(22):23495–23503. doi: 10.1074/jbc.M400516200. [DOI] [PubMed] [Google Scholar]
- 47.Yasuhara N, Shibazaki N, Tanaka S, Nagai M, Kamikawa Y, Oe S, Asally M, Kamachi Y, Kondoh H, Yoneda Y. Triggering neural differentiation of ES cells by subtype switching of importin-alpha. Nat Cell Biol. 2007;9(1):72–79. doi: 10.1038/ncb1521. [DOI] [PubMed] [Google Scholar]
- 48.Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121(3):465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 49.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451(7175):141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 51.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, NY) 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 52.Li H, Zhou J, Miki J, Furusato B, Gu Y, Srivastava S, McLeod DG, Vogel JC, Rhim JS. Telomerase-immortalized non-malignant human prostate epithelial cells retain the properties of multipotent stem cells. Exp Cell Res. 2007 doi: 10.1016/j.yexcr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007;67(10):4807–4815. doi: 10.1158/0008-5472.CAN-06-4608. [DOI] [PubMed] [Google Scholar]
- 54.Hudson DL. Epithelial stem cells in human prostate growth and disease. Prostate cancer and prostatic diseases. 2004;7(3):188–194. doi: 10.1038/sj.pcan.4500745. [DOI] [PubMed] [Google Scholar]
- 55.Huang J, Yao JL, di Sant’Agnese PA, Yang Q, Bourne PA, Na Y. Immunohistochemical characterization of neuroendocrine cells in prostate cancer. The Prostate. 2006;66(13):1399–1406. doi: 10.1002/pros.20434. [DOI] [PubMed] [Google Scholar]
- 56.Humez S, Monet M, Legrand G, Lepage G, Delcourt P, Prevarskaya N. Epidermal growth factor-induced neuroendocrine differentiation and apoptotic resistance of androgen-independent human prostate cancer cells. Endocrine-related cancer. 2006;13(1):181–195. doi: 10.1677/erc.1.01079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.