Abstract
Interest in constructing a reliable 3-dimensional (3D) collagen culture platform in microfabricated systems is increasing as researchers strive to investigate reciprocal interaction between extra cellular matrix (ECM) and cells under various conditions. However, in comparison to conventional 2-dimensional (2D) cell culture research, relatively little work has been reported about the polymerization of collagen type I matrix in microsystems. We, thus, present a study of 3D collagen polymerization to achieve reproducible 3D cell culture in microfluidic devices. Array-based microchannels are employed to efficiently examine various polymerization conditions, providing more replicates with less sample volume than conventional means. Collagen fibers assembled in microchannels were almost two-times thinner than those in conventional gels prepared under similar conditions, and the fiber thickness difference influenced viability and morphology of embedded human mammary fibroblast (HMF) cells. HMF cells contained more actin stress fibers and showed increased viability in 3D collagen matrix composed of thicker collagen fibers. Relatively low pH of the collagen solution within a physiological pH range (6.5~8.5) and pre-incubation at low temperature (~ 4 °C) before polymerization at 37 °C allow sufficient time for molecular assembly, generating thicker collagen fibers and enhancing HMF cell viability. The results provide the basis for improved process control and reproducibility of 3D collagen matrix culture in microchannels, allowing predictable modifications to provide optimum conditions for specific cell types. In addition, the presented method lays the foundation for high throughput 3D cellular screening.
Keywords: Collagen polymerization, microchannel, 3D cell culture, Array-based microsystem
Introduction
It is now well known that cellular function in 2D and 3D systems is considerably different due to the limited interaction between cells and their microenvironment in 2D culture systems [1, 2]. 3D in vitro cellular models provide enhanced interaction not only among cells but also with ECMs, more closely mirroring the morphology and phenotype of cells in vivo. In solid tumors, cancer cells in vivo exist in a 3D tumor mass, thus cancer growth, invasion, and metastasis are mainly governed by the complex interactions between cells and their microenvironment [3, 4]. For instance, Wang et al has shown that antibodies against β1-Integrin changed the behavior of breast cancer cells in 3D culture but not in 2D culture [5]. Various 3D cell culture systems such as ex-vivo culture, cellular multilayer, hollow-fiber bioreactor, matrix-embedded culture, multicellular tumor spheroid have been developed in attempts to mimic in vivo microenvironment in vitro [6-9]. Among those, matrix-embedded culture is a widely used method both in micro and macro systems due to its simplicity and versatility, and thus, is our focus.
The ECM consists of many different polymers. Collagen type I is one of the most abundant polymers in ECMs in vivo, and it is widely used for both micro and macro scale 3D cell culture. Because of its hierarchical structure, the physical properties of collagen are influenced by polymerization conditions such as pH, temperature, and polymerization rate [10-12]. Collagen molecules are mostly acid-soluble, consisting of homogeneous collection of thin rod shaped molecules (~ 1.5 nm wide, ~300 nm long) before polymerization, and they generate heterogeneous cross-linked structures when the conditions are adjusted to near physiological values (i.e., pH of 6.5 - 8.5 and temperature of 20 - 37 °C). Collagen polymerization goes through two phases: a nucleation phase during which molecular assembly occurs, and a rapid growth phase during which cross-linking takes place. The final thickness of collagen fibers (>200nm wide) is determined during the nucleation phase, where lower pH and lower curing temperature provide a longer nucleation phase, generating thicker collagen fibers [13, 14]. Because collagen is widely used and its polymerization process well understood, we have chosen to focus on collagen for this study.
From a technology perspective, the miniaturization of 3D culture systems holds the promise of enhanced efficiency and functionality. As numerous factors are involved in stimulating or inhibiting cross-talk between cells and their microenvironment, the use of microsystems may be beneficial to examine the factors with less required time, effort, and sample. For example, an adaptable hydrogel array for 3D cell culture has been realized using microfabricated multiwells to study the influence of various ECM parameters on cell behavior with enhanced throughput [15]. In addition, unique geometries and structures have been created in microsystems, mimicking 3D tissues in vivo. For example, using microfluidic patterning and contraction of biopolymers, Tan et al have constructed two- and three-layer cell-matrix structures that mimics in vivo tissue such as blood vessels [16]. Additionally, 3D in vitro hepatic tissue models and microfluidic scaffolds have been established by incorporating improved microflow control within complex 3D structures [17-19]. Physical properties of microflow (e.g. laminar flow) have also been employed to partition and compartmentalize 3D co-culture systems [20]. Although these novel techniques improve functionality over conventional 3D systems, their operational difficulty and poor reproducibility limits practical use. Thus, there is a need for more robust and high throughput 3D culture methods. Microfluidics has the potential to fill that need. However, it is important to consider the inherent physical differences between microchannels and canonical open well formats (e.g. surface area to volume ratio, small volumes, materials) and how these differences influence the resultant ECM characteristics. It is only by understanding the interplay between the physics of the microscale and the polymerization process, that one can develop an optimal process for microchannel 3D culture.
We have noted that the condition-dependent manner of collagen polymerization becomes more pronounced in microsystems due to surface area to volume ratio, volume, and material. To construct a reliable 3D culture platform for a broad range of applications, reproducibility of the base culture system is essential. Therefore, in this work, we have investigated various parameters involved in collagen polymerization in microchannels and characterized the polymerization process to enhance reproducibility of the system. Human mammary fibroblast (HMF) cells are used as a model cell type because, based on our initial experimental evidence, they are susceptible to the mechanical properties of the 3-D collagen matrix. The length of the nucleation phase of polymerization is known to be one of the critical determinants of fiber diameter. We control the nucleation phase by varying temperature and pH. The large surface area to volume ratio of a microchannel leads to more rapid temperature changes and faster termination of the nucleation phase. Therefore, the gel condition before warming is determinative of final structure after polymerization. Array-based microchannels are used to efficiently investigate the process parameters. Viability tests and stress fiber analysis of HMF cells are performed to measure cellular responses to the 3D microenvironment.
Materials and Methods
I. Cell culture and collagen sample preparation
Human mammary fibroblast (HMF) cells were cultured in DMEM supplemented with 10% calf serum (CS), 2mM L-glutamine, and penicillin/streptomycin. All cultures were maintained at 37 °C in a humidified atmosphere containing 5% CO2[21].
For collagen sample preparation, the cells were trypsinized, added to culture media, counted and centrifuged (300g, 3 min). Cells were resuspended in culture media at a concentration of 6×106 cells/ml. Collagen was prepared at a concentration of 1.6 mg/ml initially by neutralizing an acidic collagen solution (3.41 mg/ml, BD™ Collagen I, rat tail, BD Biosciences). Two different neutralization methods (HEPES and NaOH solution) were used. To neutralize collagen using 100mM HEPES buffer, the buffer was first prepared in a 2X PBS solution and mixed with same amount of acidic collagen solution (1:1 ratio). The concentration was adjusted by adding culture media. A basic solution (5N NaOH) was used as another neutralizing method: 4X PBS was added (one-quarter of the total volume) to make 1X PBS after neutralization, and culture media was added to adjust the collagen concentration. NaOH solution was carefully added to adjust the pH. Cells and culture media were added to the neutralized gel to achieve a final collagen concentration of 1.3 mg/ml and a cell concentration of 1×106 cells/mL (approximately 1200 cells/channel). The pH of the collagen solution was measured after neutralization using a pH meter (Accument Basic, Fisher Scientific), and the gel solution was kept in an ice chamber during measurement. To apply an additional nucleation phase before channel loading, the neutralized sample was kept at 4 °C for specific time intervals.
II. Device fabrication and operation
Devices were fabricated using soft lithography. Two layers of SU8-100 (Microchem Corp.) were spun and exposed individually and developed to generate a mold for 200 μm height fluidic channel and 400 μm deep fluid injection ports on a Silicon wafer. Polydimethylsiloxane (PDMS, Sylgard 184 Silicon Elastomer Kit, Dow Corning) was molded over the SU-8 master and sandwitched between transparency film and weights to allow access to the ports[22]. The fabricated PDMS channels are autoclaved and bonded to polystyrene cell culture dishes (TPP AG). The system was composed of arrays of identically shaped straight channels (W: 0.8mm, L: 4mm, H: 0.2mm). For multiphoton and confocal laser scanning microscopy, PDMS channels were bonded to a glass bottom culture dish.
Before channel loading, the samples were kept in an ice chamber, and 2 μl of collagen sample was injected by pipetting. Sixteen channels were used for each experimental condition. Collagen samples were well-mixed to obtain uniform cell density in each channel. After the loading process was completed, the channel was placed in a water-containing plastic chamber to prevent evaporation and incubated at 37 °C in a humidified atmosphere containing 5% CO2 for 6 min to polymerize the sample. Afterwards, culture media was added to both inlet and outlet ports, and was replaced every other day.
IV. Viability assay and immunofluorescent staining of collagen fibers
Viability of cultured HMF was assessed 3 days after loading. Collagen gels in microchannels were washed three times with phosphate-buffered saline (PBS) and an appropriate concentration (4⌈M Calcein AM and 2⌈M Ethidium homodimer-1 in 1xPBS) of viability assay reagent was added (LIVE/DEAD® Viability/Cytotoxicity Kit for mammalian cells, Invitrogen) and incubated for 30 min at room temperature. Finally, the gels were washed three times with PBS.
For immunofluorescent staining of collagen fibers, collagen gels without cells were injected, and the gels were fixed in 4% paraformaldehyde in PBS for 15 min at room temperature and washed 3 times with PBS. Collagen gels were then treated with 0.1M glycine in PBS at 4 °C for 30 min to reduce autofluorescence followed by PBS washing (3X). The channels were blocked with 3% Fetal Bovine Serum (FBS) for 1 hr at 4 °C and incubated with primary antibody (2 μg/ml, Rabbit polyclonal Collagen I antibidy, abcam) at 4 °C overnight. After washing with PBS (4X), the secondary antibody (1:200, Alexa 488-conjugated anti-rabbit, Invitrogen) was added and incubated at 4 °C overnight followed by PBS washing (4X). Lastly, mounting media (90% glycerol in 100mM Tris) was injected into each channel.
V. Image acqusition and analysis
Images for viability assays were acquired on an inverted microscope (IX70, Olympus) using the SPOT imaging system (Diagnostic Instruments, Inc.). Image J software (1.38x, NIH) was used to quantify the number of live and dead cells in each 3D collagen gel. All Multiphoton laser scanning microscopy (MPLSM) and Second Harmonic Generation (SHG) imaging was done on an Optical Workstation that was constructed around a Nikon Eclipse TE300 [23-27]. A Tsunami Ti:sapphire laser driven by a Millenia 8 W pump laser (Spectra Physics, Mountain View, CA) excitation source producing around 100 fs pulse widths and tuned to 890 nm was utilized to generate both Multiphoton excitation and SHG. The beam was focused onto the sample with a Nikon 40X Plan Apo oil-immersion lens (numerical aperture (NA) = 1.4). All SHG imaging was detected from the back-scattered SHG signal [23,25,26], and the presence of collagen confirmed by filtering the emission signal. We used a 464 nm (cut-on) long pass filter to isolate the emission from autofluorescence from the conserved 445 nm SHG emission. A 445 nm (narrow-band pass) filter was therefore used to isolate the SHG emission. Fiber thickness was measured using the line-drawing tool of Image J software. Confocal microscopy (Biorad MRC 1024 confocal scanning laser microscopy on an inverted Nikon Eclipse TE300) was used to image immunofluorescently labeled collagen, and a cross-sectional image was taken to measure gel thickness.
Results and Discussion
We first describe physical differences in microchannels affecting collagen polymerization, and present relevant experimental observations. In the following two sections, parameters are quantitatively examined by varying pH and temperature to characterize the polymerization process and thus, enhance reproducibility of the system. Lastly, potential applications such as collagen fiber alignment and high throughput analysis are discussed.
I. Collagen polymerization in microchannels
Collagen polymerization in conventional systems has been studied for decades, while collagen polymerization in microsystems has only recently emerged. Importantly, microchannels present some inherent physical differences such as surface area to volume ratio, total volume, and materials that can influence polymerization. High surface area to volume ratio coupled with the small volume in microsystems enhance the heat transfer efficiency, causing increased sample warming rate in microchannels [28]. In addition to generic influences such as pH, temperature, and ionic strength, sample evaporation and gel contraction are considered as substantial factors in microsystems that need to be controlled carefully during culture periods as they may lead to osmotic and mechanical stress to the embedded cells. Microchannels used in this work were fabricated using polydimethylsiloxane (PDMS), and they were adhered to a polystyrene (PS) culture dish. We have observed that collagen gels were more adherent to the PS surface or collagen molecule coated glass surface and substantial delamination from the PDMS surfaces occur creating fluid paths that simplify media exchange and staining protocols (Figure 1). The degree of gel contraction typically depends on collagen concentration, mechanical strength, and cell density [16].
Figure 1.
Cross-sectional image of collagen gel in a PDMS microchannel showing contraction from top and side walls of a PDMS channel (red lines). The channel height is 200 μm and the height of contracted gel is 167.25 μm.
As mentioned, the large surface area to sample volume ratio in a microchannel causes the sample to heat up rapidly, leading to rapid termination of collagen polymerization at 37 °C. In our process, collagen gels were kept in an ice chamber during the channel loading process and then placed promptly in a 37 °C incubator for polymerization. The increased warming rate of gels in microchannels shortens the nucleation phase, producing thinner fibers than those in conventional gels prepared using similar polymerization conditions. Previously, we observed that when gels embedded with HMF cells were loaded into 8 channels, the majority of the cells remained round and eventually died (Figure. 2A); which was confirmed after LIVE/DEAD staining, while they looked more stretched and viable when gels were loaded into 32 channels (Figure. 2B). Moreover, collagen fibers were visible in 32 channels, but not in 8 channels. All conditions were kept the same for the 32 compared to the 8 channel configuration, except gel loading time. The loading time for 32 channels was 4 times longer than 8 channels, thus the collagen sample remained at low temperature longer prior to 37 °C incubation in the case of 32 channels. This observation suggested that the structure of the assembled fibrils was affected by the different incubation times at cold and warm temperatures. This was not observed in the multiwells because the slower warming rate of gels in multiwells provided a longer nucleation phase at 37 °C to generate an appropriate thickness of fibers for cell culture. Thus, we propose that an additional nucleation phase time at low temperature is beneficial for the gels in microsystems to obtain a similar fibril property to those in conventional gels. In addition, it is known that a relatively low pH of the collagen solution extends the nucleation phase time and produces thicker collagen fibers [13, 14]. Based on these observations and knowledge, we next carried out a series of quantitative experiments to understand and optimize the microchannel polymerization process - specifically the effects of pH and temperature during the pre-incubation and polymerization steps.
Figure 2.
HMF cells loaded into 8 channels (A), and into 32 channels (B). Scale bar represents 100 μm.
II. Influence of pH variance and neutralizing methods
As mentioned before, changes in the pH of the collagen solution lead to changes in the physical properties of collagen fibers. The neutralization of an acidic collagen solution is usually achieved by either adding a small volume of basic solution (e.g. 5N NaOH) or a larger volume of neutral buffer solution (e.g., HEPES buffer). Colorimetric indication commonly follows to identify the approximate pH value of the target solution. Because dispensing error becomes relatively greater as the volume decreases [29], the likelihood for increased variance in microchannel experiments is significant. For microscale experiments, the amount of sample required for one experiment is a few hundred microliters and the amount of basic solution for neutralizing is very small (e.g. 0.1-2 μl for about 300 μl sample), leading to volume dispensing errors and inconsistent pH values from sample to sample. To explore the effect of pH changes on gel properties, we carefully prepared collagen solutions of various pH (pH 7.1 ~ 8.3), using two neutralizing methods mentioned above.
Figure 3A shows second harmonic generation (SHG) images of the collagen gels that were prepared under various pH conditions. SHG is a non-linear optical method that occurs for certain ordered molecules. Because collagen is one of the strongest harmonophores, SHG has been widely used to image collagen [10, 23-27, 30]. For these images, other factors remained consistent except pH value. As expected, thicker collagen fibers were generated in the lower pH solution. Moreover, fibers cured in the microsystem were almost half as thick as those in conventional systems (Figure 3B). The measured fiber thickness from the SHG images is about an order of magnitude larger than other reported values measured from electron microscope images, perhaps because the samples for electron microscope were fixed and dehydrated, while those used for SHG imaging were still hydrated. While the SHG data is not a direct measurement of fiber thickness, the data we obtained are useful to compare the thickness between micro and macro systems and to observe the trend of fiber thickness over a range of pH values.
Figure 3.
(A) Second harmonic generation images of collagen gel at different pH level in microchannels and in multiwells. Scale bar represents 5 μm. (B) Measured collagen fiber thickness at different pH. Standard deviation is used for error bars. (C) HMF viability at different pH level. Error bars represent the standard error of 16 samples.
The effect of pH on cell viability was determined using Human Mammary Fibroblast (HMF) cells embedded and cultured in the collagen matrix. The collagen-cell mixture was injected into microchannels and viability was examined at 3 days. While the collagen gels were polymerized under various pH values, a constant pH of 7.4 was used for cell culture. Figure 3C shows that the viability changed at different pH values. Lower pH gels led to higher viability and the viability dropped significantly in pH 8.3 gels. The viability changes corresponded to changes in the fiber thickness, suggesting that fibril structure influences cell survival. Maximum viability was achieved in the gel polymerized at pH 7.4 suggesting that the combination of proper pH value and collagen structure increases cell viability significantly.
III. Influence of low temperature pre-incubation of collagen solution
Low temperature pre-incubation provides a longer nucleation phase, which results in thickening of the collagen fiber structure. In the previous section, pH 8.3 gel neutralized by 5N NaOH solution produced thin fibers and low viability in microsystems. We used that gel condition as a starting point and pre-incubated the solution for different times to increase fiber thickness and determine how that affects the viability of cells. A one hour pre-incubation at 4 °C resulted in a 1.3 fold increase in fiber thickness, as determined by SHG imaging (Figure 4A). Differences between micro and macro systems similar to those described above were again observed. Interestingly, the fibers prepared by NaOH were shorter and straighter, while the fibers generated in HEPES buffer were longer and contained more curves. Moreover, these conditions differed in the amount of gel contraction. Figure 4B and 4C show cross-sectional confocal images of fluorescently labeled empty collagen gels in microchannels to examine the degree of gel contraction from the top surface. Figure 4B is prepared without pre-incubation and Figure 4C is with one hour pre-incubation. Gels constructed with thin fibers show considerable contraction from the top surface (channel height is 250 um, gel thickness is ~ 30 um.), while gels with thick fibers show only slight contraction (gel thickness is ~236 um). Tian et al have indicated that β1 integrin regulates fibroblasts viability during collagen matrix contraction by regulating Akt expression, and have seen that fibroblasts undergo apoptosis during contraction of collagen matrices [31]. Although we have not performed further experiments to understand the mechanism details, our results are consistent with Tian et al and, thus, the mechanisms involved are likely similar.
Figure 4.
(A) Second harmonic generation images of collagen gel neutralized by 5N NaOH (pH 8.3). Top two images are without incubation and bottom two images are after 1hr incubation at 4 °C. (B) Cross-sectional image of collagen gel neutralized by 5N NaOH. Taken by a confocal fluorescent microscope. Scale bare is 100 μm. (C) Cross-sectional image of collagen gel in (B) after 1hr incubation at 4 °C. Scale bar is 100 μm. (D) Fiber thickness increase after 1hr incubation at 4 °C. Standard deviation is used for error bars. (E) Viability increase after 1hr incubation at 4 °C. Standard error of 16 samples is used for error bars. *p<0.05 compared with no incubation.
Cells that interact with extracellular matrices organize their actin cytoskeleton and adhesions in ways that relate to the physical properties of the matrix [32-34]. Thus, it was notable that stress fibers of HMF cells were enhanced when cultured on the thicker collagen fibers prepared by pre-incubation of the collagen solution at 4 °C, and this also corresponded to cell viability (Figure 4D and 4E). Higher cell viability and more stress actin fibers in HMFs were achieved as incubation time was increased (Figure 5). Cells can be introduced into the collagen mixture before or after low-temperature incubation. We have compared cells incubated with the gel for 75 min at 4°C, with those added to the gel after the incubation, and no significant difference in morphology and viability was observed. Yeung et al, have also found that fibroblasts express more stress fibers on stiffer surfaces while they remain round shaped on softer surfaces [35]. Cell viability dropped when the gel was pre-incubated for 135 min at 4 °C and cell morphology became similar to that of cells cultured on a 2D surface. These data suggest that there is an optimum fiber thickness for stable 3D cell culture with suitable cell morphology. Once a proper gel structure was achieved, consistent cell viability was maintained over a 9 day culture period, which is sufficient for many cellular assays (Figure 6A). It is known that HMF cells proliferate slowly in 3D collagen matrices [36], so the generation of new cells is negligible. However, to confirm this, we found that the total cell number changed little over 9 days of culture (Figure 6B).
Figure 5.
(A) HMF viability according to various gel incubation times at 4 °C after neutralization. Viability is examined after LIVE/DEAD cell staining (Calcein-AM for live cells and Ethidium homodimer-1 for dead cells). (B) 3D cultured HMF in the gel with 30 min incubation time, expressing numerous dead cells (red) and a few live cells (green). (C) 3D culture with 75 min incubation. (D) 3D culture with 135 min incubation. Scale bare is 50 μm.
Figure 6.
HMF viability for 9 days culture period (A) and total cell number (B). Error bars represent the standard error of 15 samples. *p<0.05 compared with day 1.
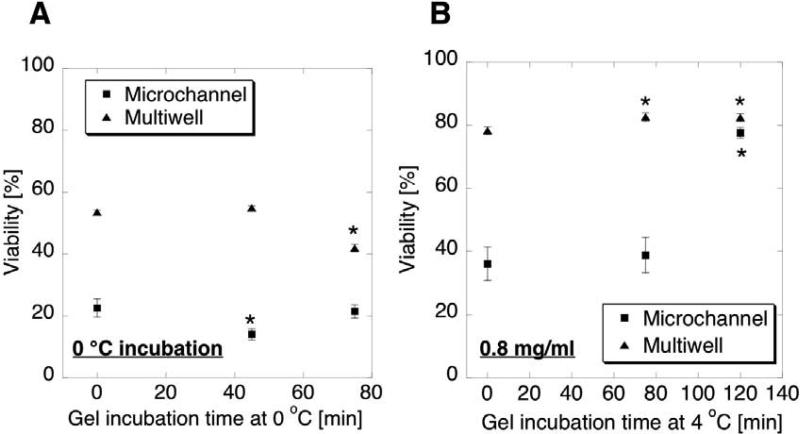
The pre-incubation temperature and the collagen concentration also affected the gel structure and cell viability. Pre-incubation was done at both 4 °C and 0 °C, with 0 °C producing lower viability of cells for both micro and macro systems (Figure 7A). A low temperature environment is known to slow the polymerization process, so a longer lag-time at 0 °C may be needed. However, it may not be desirable to keep the live cells at 0 degree C for extended periods. A lower concentration of collagen generates a softer 3D ECM, which in our system correlated to reduced cell viability and a rounded morphology. Figure 7B shows that viability in 0.8 mg/ml collagen concentration was close to that in 1.3 mg/ml concentration when 0.8 mg/ml gel is prepared with longer pre-incubation (~ 2hr) than 1.3 mg/ml gel (75 min).
Figure 7.
(A) HMF viability in collagen gels pre-incubated at 0 °C. (B) HMF viability in collagen gels of 0.8 mg/ml concentration. Error bars represent the standard error of 13 samples for A and 16 samples for B. *p<0.05 compared with 0 min.
IV. Potential applications : fiber alignment, platform for high throughput analysis
We have shown that the collagen fiber thickness and polymerization rate are controllable via pH and temperature of the collagen solution, providing a reproducible microscale 3D culture platform. Here we demonstrate how the control process can be employed to achieve collagen fiber alignment and provide a platform for high throughput studies. Thick collagen fibers can be aligned along the flow direction in a microchannel, and a slow polymerization rate can provide consistent gel characteristics over many replicates.
Collagen fiber alignment was obtained by controlling fiber thickness of collagen gel. If the gel was pre-incubated at 4 °C in a vial before injection, thick fibers produced during the incubation period are affected by flow induced during injection, causing fiber and cell alignment (Figure. 8A). However, if the gel was incubated after loading, the fiber alignment was not achieved (Figure. 8B) as molecules were assembled after injection. Vacuum- or pH-induced fiber alignment methods have been recently developed [37, 38], but the advantage of the alignment method presented here is the simplicity of the operation, as only low temperature incubation and pipette injection are used. However, further work examining the effect of the channel geometry and diameter need to be completed to enhance the reproducibility of this alignment method. It has been known that the collagen matrix around cancer cells is highly aligned and also affects cancer cell invasion and migration [23]. Therefore, the ability to produce an aligned matrix in a high throughput platform could enhance our ability to carcinoma cell behavior.
Figure 8.
(A) Aligned HMFs in microfluidic channel. Cells and collagen are injected after 75min incubation at 4°C. Viability=83%±2.95, (B) Cells and Collagens are injected prior to 4°C incubation, and thus the molecules’ self-assembly occurs in microchannels, resulting in a random arrangement of fibers. Viability=79%±4.99, Scale bars represent 300 μm.
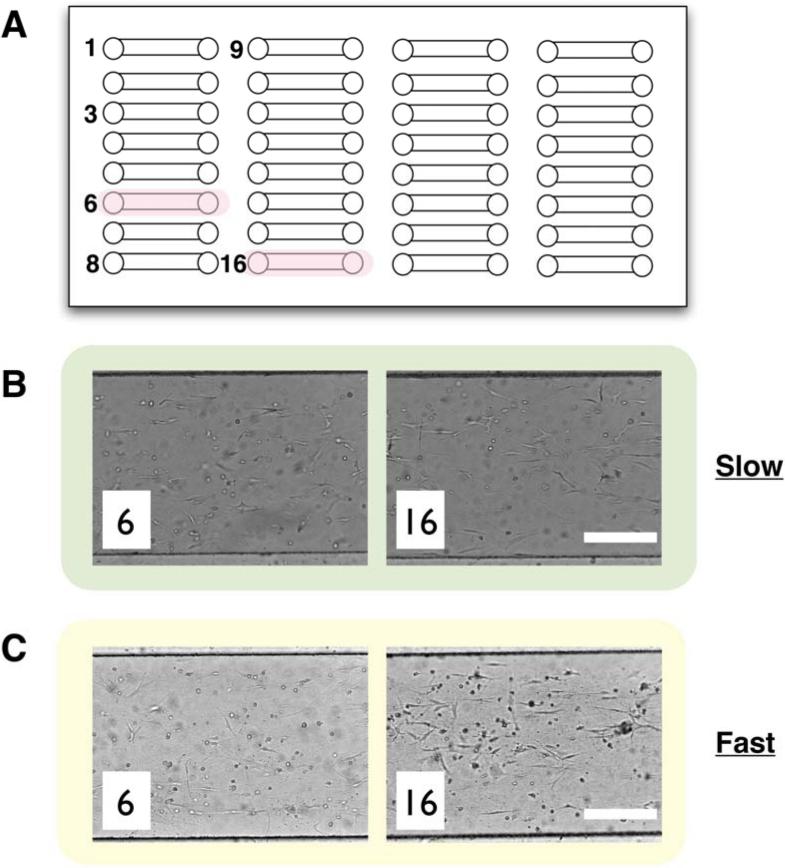
When considering the use of the microchannel arrays for high throughput studies, one needs to consider the sensitivity of process parameters within the context of a high throughput work flow. To illustrate the importance of process parameters, a simple array system was used (Figure 9A). Figure 9B and 9C represent the difference between slow and fast polymerization. For slow polymerization, the sample was kept at low temperature (~4 °C), while it was kept at room temperature to cause fast polymerization during loading. As can be seen from the figures, the gel condition was uniform during the loading process under slow polymerization conditions and variable under fast polymerization conditions. (Figure 9C). Thus, as we move towards miniaturized high throughput 3D culture systems [39], it will be important to carefully consider the interplay between the inherent process limitations of high throughput automated systems and the influence of process parameters and microchannel structure on the resultant gel characteristics.
Figure 9.
(A) Illustration showing an example of array-based microchannels used in this work. The number indicted the injection order. Gels in pink-labeled channels are used for comparisons. (B) Gels in channel # 6 and #16. Slow polymerization is controlled by lowering the temperature during injection. (C) Gels injected under the fast polymerization condition. A gel sample is kept at room temperature to increase the rate. Scale bars represent 300 μm.
Conclusions
The importance of a 3D environment in building more relevant in vitro culture models is evident. However, the impact of 3D culture has been limited by the inability to perform this technique in a high throughput screening mode. In this paper, we have examined process related issues and challenges in moving 3D culture from canonical open well systems to microscale closed channel systems. We have shown that collagen polymerization in a microsystem is different from that in a canonical system mainly due to the large surface area to volume ratio in a microchannel causing increased rate of sample warming. Neutralization by HEPES buffer reduces volumetric error and provides a stable range of pH. Even with the high pH of the gel, the fiber thickness can be controlled by providing pre-incubation at low temperature allowing more nucleation of collagen molecules. Moreover, the use of an arrayed microchannel platform can facilitate rapid screening of process parameters leading to a highly reproducible HMF 3D culture with proper cell morphology and high viability. Furthermore, this 3D culture method opens broad possibilities for many other 3D cellular applications including co-culture, small molecule inhibitor screening, and matrix component screening as this system can be readily modified. Finally, the array-based approach integrates with existing HTS infrastructure via passive pumping, lowering the barriers to use.
Acknowledgements
The authors would like to thank Dr. Suzanne Ponik for the helpful discussion. This study was supported by NIH grant K25-CA104162, the Wisconsin Partnership Program and the DARPA Micro/nano Fluidics Fundamentals Focus Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smalley KSM, Lioni M, Herlyn M. Life isn't flat: Taking cancer biology to the next dimension. In Vitro cell Dev Biol-Animal. 2006;42:242–247. doi: 10.1290/0604027.1. [DOI] [PubMed] [Google Scholar]
- 2.Weaver VM, Peterson OW, Wang F, Larabell CA, Briand P, Damsky C, et al. Reversion of the Malignant Phenotype of Human Breast Cells in Three-dimensional Culture and In Vivo by Integrin Blocking Antibodies. J Cell Biol. 1997;137(1):231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bissell MJ, Radisky D. Putting tumors in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, et al. Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement memrane breast cultures: A different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padron JM, Wilt CLvd, Smid K, Smitskamp-Wilms E, Backus HH, Pizao PE, et al. The multilayered postconfluent cell culture as a model for drug screening. Crit Rev Oncol Hematol. 2000;36:141–157. doi: 10.1016/s1040-8428(00)00083-4. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988;240:177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- 8.Casciari JJ, Hollingshead MG, Alley MC, Mayo JG, Malspeis L, Miyauchi S, et al. Growth and Chemotherapeutic Response of Cells in a Hollow-Fiber. In Vitro Solid Tumor Model J Natl Cancer Inst. 1994;86:1846–1852. doi: 10.1093/jnci/86.24.1846. [DOI] [PubMed] [Google Scholar]
- 9.Berglund Å , Glimelius B, Bergh J, Brodin O, Fjällskog M-L, Hagberg H, et al. Selection of chemotherapy by ex vivo assessment of tumor sensitivity to cytotoxic drugs. Med Oncol. 2002;19:151–159. doi: 10.1385/MO:19:3:151. [DOI] [PubMed] [Google Scholar]
- 10.Raub CB, Suresh V, Krasieva T, Lyubovitsky Ji, Mih JD, Putnam AJ, et al. Noninvasive Assessment of Collagen Gel Microstructure and Mechanics. Using Multiphoton Microscopy Biophysical Journal. 2007;92:2212–2222. doi: 10.1529/biophysj.106.097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL. Tensile Mechanical Properties of Three-Dimensional Type I Collagen Extracellular Matrices With Varied Microstructure. Transactions of the ASME. 2002;124(214-222) doi: 10.1115/1.1449904. [DOI] [PubMed] [Google Scholar]
- 12.Silver FH, Freeman JW, Seehra GP. Collagen self-assembly and the development of tendon mechanical properties. Journal of Biomechanics. 2003;36:1529–1553. doi: 10.1016/s0021-9290(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 13.Wood GC, Keech MK. The Formation of Fibrils from Collagen Solutions. Biochem J. 1960;75:588–598. doi: 10.1042/bj0750588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McPherson JM, Wallace DG, Sawamura SJ, Conti A, Condell RA, Wade S, et al. Collagen Fibrillogenesis In Vitro: A Characterization of Fibril Quality as a Function of Assembly Conditions. Collagen Rel Res. 1985;5:119–135. doi: 10.1016/s0174-173x(85)80034-0. [DOI] [PubMed] [Google Scholar]
- 15.Jongpaiboonkit L, King WJ, Lyons GE, Paguirigan AL, Warrick JW, Beebe DJ, et al. An adaptable hydrogel array format for 3-dimensional cell culture and analysis. Biomaterials. 2008;29:3346–3356. doi: 10.1016/j.biomaterials.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan W, Desai TA. Microfluidic patterning of cellular biopolymer matrices for biomimetic 3-C structures. Biomedical Microdevices. 2003;5(3):235–244. [Google Scholar]
- 17.Hwa AJ, Sivaraman RCF, So PT, Samson LD, Stolz DB, Griffith LG. Rat liver sinusoidal endothelial cells survive without exogenous VEGF in 3D perfused co-cultures with hepatocytes. FASEB J. 2007;21:2564–2579. doi: 10.1096/fj.06-7473com. [DOI] [PubMed] [Google Scholar]
- 18.Choi NW, Cabodi M, Held B, Gleghorn JP, Bonassar LJ, Strook AD. Microfluidic scaffolds for tissue engineering. Nature Materials. 2007;6:908–915. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 19.Khetani SR, Bhatia SN. Microscale human human liver tissue for drug development. Nature Biotechnology. 2007;26(1):120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 20.Wong AP, Perez-Castillejos R, Love JC, Whitesides GM. Partitioning microfluidic channels with hydrogel to construct tunable 3-D cellular microenvironments. Biomaterials. 2008;29:1853–1861. doi: 10.1016/j.biomaterials.2007.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proia AD, Kuperwasser C. Reconstruction of human mammary tissues in a mouse model. Nature Protocols. 2006;1(1):206–214. doi: 10.1038/nprot.2006.31. [DOI] [PubMed] [Google Scholar]
- 22.Jo BH, Lerberghe LMV, Motsegood KM, Beebe DJ. Three-dimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer. Journal of Microelectromechanical Systems. 2000;9:76–81. [Google Scholar]
- 23.Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ. Contact Guidance Mediated 3D Cell Migration is Regulated by Rho/ROCK-dependent Matrix Reorganization. Biophys J. 2008;95:5374–5384. doi: 10.1529/biophysj.108.133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 25.Mohler W, Millard AC, Campagnola PJ. Second harmonic generation imaging of endogenous structural proteins. Methods. 2003;29:97–109. doi: 10.1016/s1046-2023(02)00292-x. [DOI] [PubMed] [Google Scholar]
- 26.Wokosin DL, Squirrell JM, Eliceiri KE, White JG. An optical workstation with concurrent, independent multiphoton imaging and experimental laser microbeam capabilities. Rev Sci Inst. 2003;74:193–201. doi: 10.1063/1.1524716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams RM, Zipfel WR, Webb WW. Interpreting second-harmonic generation images of collagen I fibrils. Biophys J. 2005;88:1377–1386. doi: 10.1529/biophysj.104.047308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satish G, Kandlikar SG, Dongqing Li, Stephane Colin, Michael R. King. Heat Transfer and Fluid Flow in Minichannels and Microchannels. Elsevier; 2006. [Google Scholar]
- 29.Warrick J, Meyvantsson I, Ju JI, Beebe DJ. High-throughput microfluidics: improved sample treatment and washing over standard wells. Lab Chip. 2007;7:316–321. doi: 10.1039/b613350a. [DOI] [PubMed] [Google Scholar]
- 30.Zoumi A, Yeh A, Tromberg BJ. Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. PNAS. 2002 August 20;99(17):11014–11019. doi: 10.1073/pnas.172368799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian B, Lessan K, Kahm J, Kleidon J, Henke C. β1 integrin regulates fibroblast viability during collagen matrix contraction through a phosphatidylinositol 2-kinase AKT/Protein Kinase B signaling pathway. J Cell Biol. 2002;277(27):24667–24675. doi: 10.1074/jbc.M203565200. [DOI] [PubMed] [Google Scholar]
- 31.Pelham RJ, Jr., Wang YL. Cell locomotion and fical adhesions are regulated by substrate flexibility. PNAS. 1997 December;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 33.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, et al. Effects of Substrate Stiffness on Cell Morphology, Cytoskeletal Structure, and Adhesion. Cell Motility and the Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 35.Su G, Blaine SA, Qiao D, Friedl A. Shedding of Syndecan-1 by Stromal Fibroblasts Stimulates Human Breast Cancer Cell Proliferation via FGF2 Activation. J Cell Biol. 2007;282(20):14906–14915. doi: 10.1074/jbc.M611739200. [DOI] [PubMed] [Google Scholar]
- 36.Koster S, Leach JB, Wong JY, Pfohl T. Microaligned collagen matrices by hydrodynamic focusing: Controlling the pH-induced self-assembly. Mater Res Soc Symp Proc. 2006;898E:0898–L0805-0821.0891-0896. [Google Scholar]
- 37.Lee P, Lin R, Moon J, Lee LP. Microfluidic alignment of collagen fibers for in vitro cell culture. Biomed Microdevices. 2006;8:35–41. doi: 10.1007/s10544-006-6380-z. [DOI] [PubMed] [Google Scholar]
- 38.Meyvantsson I, Warrick JW, Hayes S, Skoien A, Beebe DJ. Automated cell culture in high density tubeless microfluidic device arrays. Lab Chip. 2008;8:717–724. doi: 10.1039/b715375a. [DOI] [PubMed] [Google Scholar]