Abstract
A central unresolved issue in hepatitis C virus (HCV) infection is how the virus establishes chronic infection. Recent studies suggest that the liver microenvironment leads to apoptosis of activated T cells, which may be involved in the tolerance to liver allograft. Here, We report that murine hepatocytes expressing a transgene encoding the HCV structural proteins core, envelope 1 (E1) and envelope 2 (E2) enhance apoptosis of activated T cells. Unlike normal liver, which appears to selectively remove only activated CD8+ T cells, enhanced apoptosis was seen for both CD4+ and CD8+ T cells. Enhanced apoptosis of activated T lymphocytes was associated with upregulation of FasL by HCV transgenic hepatocytes and was specifically inhibited by anti-FasL blocking antibody. Increased apoptosis of activated T cells induced by HCV structural proteins could amplify the ability of the liver to down-modulate T cell responses, leading to attenuation of anti-viral responses and facilitating viral persistence.
Keywords: HCV, Apoptosis, Immune evasion, Lymphocytes, Hepatocytes, Fas-FasL, Viral persistence
Introduction
Hepatitis C virus (HCV) infection has become the leading cause of chronic hepatitis and liver failure in the United States (Liang et al., 2000). One striking feature of hepatitis C is its propensity to develop chronic infection in the majority of infected individuals. A variety of mechanisms for persistence have been suggested, including quasispecies diversity leading to immune escape, viral protein interference with host immune function, and failure to develop an effective immune response. In humans and chimpanzees infected by HCV, failure of HCV clearance appears to correlate with a less vigorous, more narrowly focused HCV-specific CD4+ and CD8+ T cells against viral antigens (Cooper et al., 1999; Diepolder et al., 1995; Lechner et al., 2000a; Missale et al., 1996; Takaki et al., 2000). Chronic HCV infection is also characterized by an unusually low frequency of HCV-specific T cells in the liver and peripheral blood (He et al., 1999), when compared with other chronic viral infections such as Epstein–Barr virus (EBV) and human immunodeficiency virus (HIV) infections (Schmitz et al., 2000; Tan et al., 1999). However, the mechanism underlying the relatively low frequency of HCV-specific T cell responses is not clear.
The Fas receptor (Fas, Apo-1/CD95) and its ligand (FasL, CD95L) are transmembrane proteins of the tumor necrosis factor family of receptors and ligands. Fas–Fas ligand (FasL) interaction is one of the main effector mechanisms of cytotoxic T lymphocytes (Kagi et al., 1994). Engagement of Fas by FasL triggers a cascade of subcellular events that result in apoptosis, which has a fundamental role in normal biology as well as pathophysiology in human (Depraetere and Golstein, 1997; Nagata, 1999). FasL was initially thought to be expressed only in cells of the lymphoid/myeloid series including T cells and natural killer cells. Recently, it has been shown that FasL is expressed by non-lymphoid cells, where it contributes to immune privilege. In the liver, the Fas–FasL system plays an important role in regulation of apoptosis of hepatocytes (Ogasawara et al., 1993) as well as in liver disease, including liver injury, viral hepatitis, cirrhosis, and HCC (Hahn et al., 2001; Toubi et al., 2001). Moreover, HCV core plays a role in the induction of FasL in HepG2 cell line and leads to apoptosis of the Jurkat T cell line (Ruggieri et al., 2003).
Recent studies have shown that the liver is a unique immunological organ that can downregulate cellular immune responses (Belz et al., 1998; Crispe, 1999; Huang et al., 1994). For example, allogeneic liver transplants are more easily accepted than other solid organs, and the presence of a liver allograft can facilitate tolerance to syngeneic organ grafts that normally would be promptly rejected (Kamada et al., 1981; Rao et al., 1996). The acceptance of a liver allograft is associated with apoptosis of host T cells in the liver (Meyer et al., 1999; Qian et al., 1997), suggesting that the liver can induce tolerance by inducing activated T cells to undergo apoptosis. In vitro, both Kupffer cells and hepatocytes can induce activated alloreactive T cells to undergo apoptosis (He et al., 1999). In vivo, the liver appears to trap and eliminate activated T cells, especially CD8+ T cells, via apoptosis during the induction of peripheral tolerance (Huang et al., 1994; Tagashira et al., 2000; John and Crispe, 2004; Murray and Crispe, 2004). In fact, it has been postulated that a physiologic role of the liver is to terminate immune responses (Crispe et al., 2000). Since the primary site of replication of HCV is the hepatocyte, we hypothesized that HCV may enhance the physiologic apoptosis of activated T cells, thus facilitating viral persistence.
Since HCV only infects human and chimpanzees, and it is difficult to achieve primary infection of hepatocytes in vitro, we utilized a murine system of HCV expression in the liver to model HCV and T cell interactions. We used a line of HCV transgenic (Tg) mice expressing HCV genotype 1b core, E1 and E2 proteins under the control of the murine albumin promoter, thus directing expression to hepatocytes and do not spontaneously develop hepatocellular injury or inflammation (Kawamura et al., 1997).
In this paper, we report studies of the influence of HCV structural proteins expressed in these hepatocytes on apoptosis of activated T cells. We demonstrate that hepatocytes expressing these structural proteins enhance apoptosis of activated CD4 and CD8 T cells in vitro and in vivo, which is mediated by increased expression of Fas ligand in Tg hepatocytes.
Results
Expression of HCV core/E1/E2 induces apoptosis of activated T cells
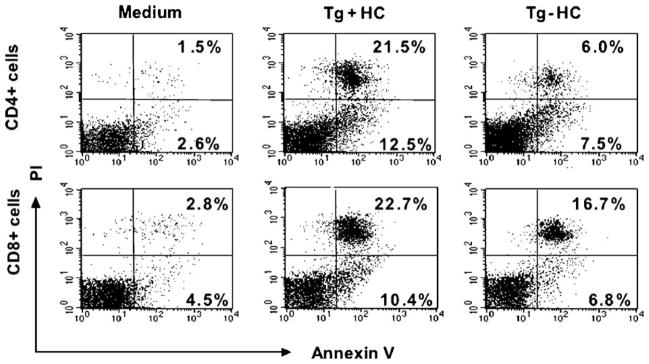
To study the influence of hepatocytes expressing HCV core, E1 and E2 proteins on cell death of activated T cells, we co-cultured Tg+ hepatocytes with sex and MHC-matched, activated T cells that were isolated from non-Tg littermates. Hepatocytes isolated from non-Tg littermates were used as controls. Apoptosis of activated T cells was studied by three-color FACS analysis. To this end, lymphocytes that were recovered from co-culture with hepatocytes were stained with FITC-conjugated anti-mouse CD4 or CD8 antibody and then with PE-conjugated Annexin V and propidium iodide (PI). In the representative experiment shown in Fig. 1, co-culture of Con-A activated CD4+ T cells with HCV Tg+ hepatocytes increased early apoptotic events from 7.5% to 12.5% (AV+/PI−, right lower quadrants), whereas apoptosis of activated CD8+ T cells increased from 6.8% to 10.4%. We chose to analyze Annexin V+/PI− cells as a more specific marker of early apoptosis in lymphocytes than all AV+ cells (Eray et al., 2001; McCloskey et al., 1998). However, note that in this representative example, if we compare total AV+, there is 34% AV+ CD4+ cells in HCV Tg+ mice compared to 13.5% in Tg− controls; for CD8+ T cells, there is 33.1% AV+ cells in HCV Tg+ compared to 23.5% in Tg− controls.
Fig. 1.
FACS analysis of apoptosis in CD4+ and CD8+ T cells. In this representative experiment, FACS analysis was performed on activated T cells after staining with FITC-conjugated anti-CD4 or anti-CD8 monoclonal antibody, PE-conjugated annexin V and propidium iodide (PI), 15 h after co-culture with HCV transgenic (Tg+ HC) or non-transgenic (Tg− HC) hepatocytes. Shown is a representative FACS profiles after gating on CD4+ (upper panel) or CD8+ (lower panel) T cells. A representative experiment is shown.
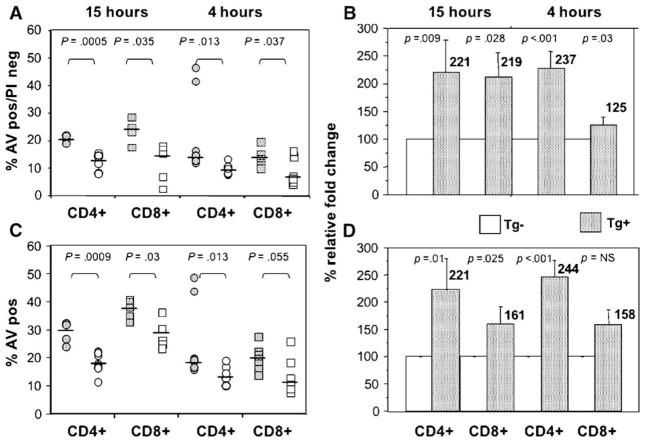
As shown in Fig. 2, multiple experiments confirm that apoptosis of both CD4+ and CD8+ T cells was significantly increased after co-culture with HCV Tg+ hepatocytes for 4 and 15 h, as compared with T cells co-cultured with non-Tg hepatocytes. After only 4 h of co-culture, there was a statistically significant difference in early apoptosis that was apparent between T cells co-cultured with HCV Tg hepatocytes and those co-cultured with non-Tg hepatocytes (Fig. 2). The percentage of annexin V+/PI− cells was increased from a median value of 9.5% in those CD4+ T cells cultured with the non-Tg hepatocytes to a median of 14.6% (Fig. 2A) when cultured with Tg hepatocytes. For CD8+ T cells, the median value of cells in early apoptosis was 6.8% after co-culture with non-Tg hepatocytes and 12.5% after co-culture with HCV Tg hepatocytes. Apoptosis was also significantly increased in both activated CD4+ and CD8+ T cells 15 h after co-culture with transgenic hepatocytes expressing the HCV structural proteins. The median percentage of annexin V+/PI− cells was increased from a median value of 14% in those CD4+ T cells cultured with the non-Tg hepatocytes to a median of 21% (Fig. 2A) when cultured with Tg hepatocytes. For CD8+ T cells, the median value of cells in early apoptosis was 15.6% after co-culture with non-Tg hepatocytes and 21% after co-culture with HCV Tg hepatocytes. As shown in Fig. 2B, if the relative fold increase in early apoptotic (AV+/PI−) cells is compared, there is a statistically significant increase for both time points for both CD4+ and CD8+ cells.
Fig. 2.
Increased apoptosis of activated CD4+ and CD8+ T cells after co-culture with transgenic hepatocytes expressing HCV core, E1 and E2 proteins. Apoptosis of activated T cells was studied by 3-color FACS analysis, 4 or 15 h after co-culture with HCV transgenic (Tg+, stippled) or non-transgenic (Tg−, open) hepatocytes. (A) T cells in the early phase of apoptosis (annexin V-positive, PI-negative) are shown. Increased apoptosis of activated T cells after co-culture with HCV transgenic (HCV Tg+) compared to non-transgenic (HCV Tg−) hepatocytes was seen at both 4 and 15 h and was seen for both CD4+ (circles) and CD8+ (squares) cells. Data of five to eight independent experiments were analyzed by Mann–Whitney U test. (B) The relative increase of early apoptotic cells increases after co-culture with Tg hepatocytes. In this example, data for Tg hepatocytes were normalized to the appropriate Tg− control. Results for both CD4+ and CD8+ remain statistically significant at 4 and 15 h. (C) All annexin V+ cells, showing increased apoptosis of both CD4+ and CD8+ T cells at 15 h, and an increase in CD4+ AV+ cells at 4 h. (D) Relative increase in all AV+ cells.
When all annexin V+ cells were analyzed (Fig. 2C), the differences remained statistically significant, with the exception of the difference between CD8+ cells at 4 h. As expected, the absolute values for all AV+ increased over time. For example, in the case of CD4+ T cells, the median value of AV+ cells after 15 h of co-culture was 17.3% after co-culture with non-Tg hepatocytes and 28.5% after co-culture with HCV Tg hepatocytes. For CD8+ T cells, the median value of 30% of cells after co-culture for 15 h increased to 37% after co-culture with HCV Tg hepatocytes. Similarly, there is an increase in the relative degree of apoptosis if all AV+ cells are measured, with the exception of CD8+ cells at the early time point (Fig. 2D). The data suggest that CD8+ T cells at 4 h are primarily still in the early phases of apoptosis and have not progressed to later stages. Non-activated T cells had very low levels of apoptosis, and there is no difference between Tg+ and Tg− for non-activated cells (data not shown). Taken together, the data suggest that the expression of HCV structural proteins in hepatocytes increases the apoptosis of activated CD4+ and CD8+ T cells.
Increased apoptosis of activated T cells is associated with upregulation of Fas ligand expression on HCV transgenic hepatocytes
Activated T cells may be induced to undergo apoptosis by a variety of death-inducing molecules, including FasL (also known as CD95 ligand), TNFα (Crispe, 1999), galectin-1 (Baum et al., 1995), and TNFα related apoptosis inducing ligand (TRAIL, also known as Apo-2 ligand) (Jeremias et al., 1998). To study whether any of these death-inducing molecules is responsible for increased apoptosis of activated T cells induced by hepatocytes expressing HCV proteins, we studied and compared the expression of these molecules between HCV Tg+ and non-Tg hepatocytes. We performed Western blotting analysis to study the expression of FasL, TRAIL, and galectin-1 using specific antibodies against these molecules. The level of FasL expression was increased in HCV Tg hepatocytes relative to their non-Tg counterparts (Fig. 3A). The findings were similar for total RNA using RT-PCR (Fig. 3B), as well as analysis of cell surface FASL expression using flow cytometry (Fig. 3C). To measure the concentration of TNFα in the supernatants of co-culture of hepatocytes and activated T cells, we used ELISA. No statistically significant difference was observed between HCV Tg+ hepatocytes and non-Tg hepatocytes at either 4 or 15 h of co-culture (data not shown). Expression of either TRAIL or galectin-1 was not observed in Tg or non-Tg hepatocytes (data not shown). After co-culture with HCV-expressing hepatocytes, there was no change in FasL expression on lymphocytes (Fig. 3D).
Fig. 3.
Increased expression of Fas ligand in HCV transgenic hepatocytes expressing structural proteins. (A) Cell lysates (200 μg) were subjected to Western blot analysis using specific polyclonal rabbit anti-mouse Fas ligand antibodies. The level of cellular murine beta actin is shown as an internal control for comparison. One representative experiment is shown. (B) Induction of mRNA for FasL by HCV structural proteins. Whole-liver tissue from Tg− and Tg+ mice was harvested; RNA was extracted and analyzed by reverse transcription PCR for FasL transcripts. Results for GAPDH mRNA are shown as controls. Results are a representative experiment of 3 individual experiments. (C) Differences in surface expression of FasL analyzed by flow cytometry. Freshly isolated HC were stained with 0.5 μg/ml FasL. Tg+ HC (thick line) had increased expression of FasL compared to Tg− HC (dashed line), which had only minimal increase compared to the isotype control (thin dashed line). One representative experiment of three is shown. (D) Activated lymphocytes show no difference in surface FasL expression after co-culture with either Tg− or Tg+ hepatocytes.
Enhanced apoptosis of activated T cells induced by HCV transgenic hepatocytes is Fas–FasL dependent
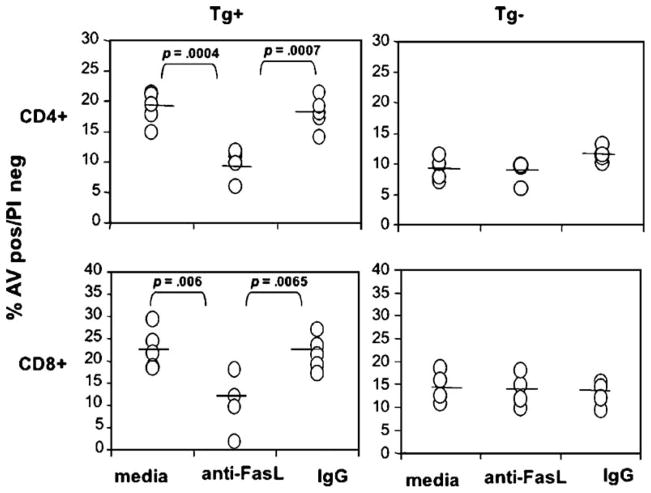
To determine whether Fas–FasL interaction is indeed responsible for enhanced apoptosis of activated T cells induced by hepatocytes expressing HCV proteins, we performed blocking experiments using polyclonal rabbit anti-mouse FasL antibodies. Purified normal rabbit IgG was used as a control. As shown in Fig. 4, the addition of 10 μg/ml anti-FasL antibodies decreased apoptosis of activated CD4+ and CD8+ T cells induced by Tg+ hepatocytes expressing HCV structural proteins compared to cells incubated with Tg hepatocytes and either media or the isotype control antibody. Similar findings were observed for alloreactive T cells (data not shown). It is important to note that anti-FasL antibodies had no effect on apoptosis of activated CD4+ or CD8+ T cells induced by non-transgenic hepatocytes (Fig. 4), suggesting that basal apoptosis of activated T cells induced by hepatocytes in the absence of HCV is mediated by death-induced molecule(s) other than those mentioned above. These consistent with a previous report that Fas-deficient mice do not have different kinetics of T cell apoptosis within the liver (Mehal and Crispe, 1998), so that physiologic depletion of activated T cells within the normal liver is not dependent on Fas–FasL interaction.
Fig. 4.
HCV induces apoptosis through Fas–FasL cascade. (A) Anti-Fas ligand antibodies inhibit enhanced apoptosis of activated CD4+ and CD8+ T cells induced by transgenic hepatocytes expressing HCV core, E1 and E2 proteins. Apoptosis of activated T cells was studied by 3-color FACS analysis 15 h after co-culture with HCV transgenic hepatocytes (Tg+ HC), in the presence of 10 μg/ml rabbit anti-mouse Fas ligand polyclonal antibodies (Ab) or purified rabbit IgG (IgG) as an isotype control or media (Med). Results are based on binding of annexin V after gating on viable CD4+ and CD8+ cells. Shown are means and standard deviations of 5 experiments.
Apoptosis of activated T cells occurs in vivo
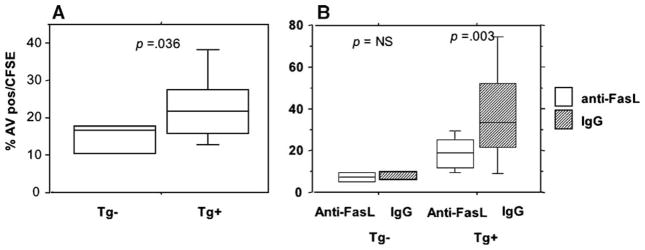
In order to confirm that apoptosis of activated T cells is accelerated in vivo in HCV transgene positive (Tg+) compared to non-Tg mice, we performed adoptive transfer of concanavalin A activated T cells into Tg+ mice and their non-Tg littermates (Fig. 5). Splenocytes from non-Tg littermates were activated with conA and labeled with supravital dye CFSE in order to track them in vivo. These labeled cells were infused into the portal circulation of HCV Tg+ and non-Tg littermates. Sixteen hours after infusion, intrahepatic lymphocytes were harvested and analyzed for the degree of apoptosis among CFSE+ cells. As shown in Fig. 5A, the degree of apoptosis was significantly higher among the intrahepatic lymphocytes of HCV Tg+ animals. Resting T cells from HCV non-Tg mice were also CFSE labeled and adoptively transferred into HCV Tg+ and non-Tg mice. CFSE cells were readily detected in the spleen, with few cells present in the liver; there was no difference in apoptosis or cell number between HCV Tg+ and non-Tg mice (data not shown). This observation is consistent with prior published data, suggesting that there is no apoptosis within the liver of resting T cells (Huang et al., 1994). Similarly, rare CFSE+ cells were detected within splenocytes (<0.5% of cells), and there was no difference in the degree of apoptosis of CFSE+ cells (data not shown). In order to confirm that FasL was implicated in vivo, we incubated cells with antibody that blocks Fas–FasL interaction. This significantly reduced the apoptosis of CFSE+ cells following adoptive transfer in HCV Tg+ mice (Fig. 5B), whereas there was no significant difference in apoptosis following adoptive transfer into non-Tg mice.
Fig. 5.
Increased apoptosis of activated T cells after adoptive transfer into HCV Tg mice. Cells were activated with conA and given via intraportal injection as in the text. The degree of apoptosis in CFSE+ cells by AV staining was determined 16 h following adoptive transfer. Median values and intraquartile ranges (IQR) of 3 independent experiments with 2–3 mice in each group are shown are shown. (B) HCV Tg+ or non-Tg mice were treated with 20 μg/mouse of anti-FasL Ab (Ab-1, Oncogene Research) or the isotype control. Data (median (IQR)) for 3 HCV Tg+ and 3 non-Tg mice (difference not significant) in each of two independent experiments. Statistical significance by Mann–Whitney.
To examine whether the increased apoptosis of activated T lymphocytes by HCV Tg+ hepatocytes occurred via the conventional Fas-mediated pathway, we examined the activation of and necessity for specific caspases involvement in this pathway. Caspase-3 is a downstream, effector caspase that is activated in response to ligand-mediated Fas signaling, among others. After co-culture with either Tg+ or non-Tg hepatocytes, lymphocytes were harvested for measurement of caspase-3 activity by detection of the cleavage of colorimetric substrate of this caspase. As shown in Fig. 6, activation of caspase-3 in activated lymphocytes was significantly increased after 15 h of co-culture with Tg+ hepatocytes compared to non-Tg hepatocytes. These data demonstrate that expression of HCV proteins by hepatocytes mediates apoptotic signaling of activated T cells that is dependent on Fas–FasL interaction.
Fig. 6.

Caspase-3 activity of activated lymphocytes in HCV Tg+ and non-HCV Tg− mice. Caspase-3 activity was measured in lysates from cells by release of pNA from peptide substrates as described in Materials and methods. Results represent the average of three independent experiments.
Discussion
We have shown here that the expression of HCV core, E1 and E2 proteins in HCV Tg hepatocytes induces enhanced apoptosis of activated CD4+ and CD8+ T cells in vitro and in vivo. In our studies, there was an upregulation in FasL expression in the liver, consistent with published observations increased expression of both Fas and FasL mRNA in the liver in humans with chronic hepatitis C (Galle et al., 1995; Hiramatsu et al., 1994; Mita et al., 1994; Tagashira et al., 2000) and in hepatoma cells transfected with HCV core protein (Ruggieri et al., 2003). Our data therefore suggest that the weakness of T cell response and the relative rarity of virus-specific T cells in chronic HCV infection are related to increased apoptosis of activated, virus-specific T cells. Although we used polyclonally activated, non-HCV-specific T cells in our experiments, these findings need to be examined in future studies with HCV-specific cells.
We have shown here that both CD4+ and CD8+ T cells were induced by hepatocytes to undergo apoptosis in vitro. This differs from previous in vivo studies in non-Tg mice, which have shown that trapping and elimination of activated T cells are limited to activated CD8+ T cells and not CD4+ T cells (Belz et al., 1998; Crispe, 1999; Crispe et al., 2000; Emi et al., 1999; Huang et al., 1994). Following activation, CD8+ T cells appear in the periphery and spleen initially. As these CD8+ T cells disappear from the lymph nodes and spleen, they accumulate in the liver, where they undergo apoptosis. This apoptosis of activated T cells has been shown in two different systems, namely autoreactive cells in H-2k transgenic mice (Bertolino et al., 1995) and T cells isolated from TCR Tg mice injected with peptide (Mehal et al., 1999; Wack et al., 1997). This is specific for activated cells, as activated but not resting cells are selectively retained by the normal liver (Mehal et al., 1999). In a more physiologic model, during influenza infection in the mouse, where the infection is limited to the respiratory tract, the resolution phase of the infection is associated with accumulation of apoptotic, virus-specific CD8+ T cells in the liver, not in the lymph node or lung (Belz et al., 1998). Deletion of activated CD8+ T cells has been postulated to play a significant role not only in T cell homeostasis but also in the relative tolerance of liver allografts (Qian et al., 1997). For example, in a B10 (H-2b) to C3H (H-2k) transplant model, manipulation of the model to decrease apoptosis of infiltrating T cells in the liver led to an increase in rejection. The mechanism whereby the normal liver selectively retains activated CD8+ T cells is not entirely understood, but in normal liver tissue, this does not appear to be dependent on Fas–FasL interactions, as Fas-deficient mice do not have different kinetics of T cell apoptosis within the liver (Mehal and Crispe, 1998).
Resolution of acute HCV appears to be dependent on the coordinated activity of both CD4+ and CD8+ T lymphocytes in acutely infected chimpanzees and man, with chronic HCV infection marked by the failure to develop and sustain an effective T lymphocyte response (Diepolder et al., 1996; Grakoui et al., 2003; Missale et al., 1996; Shoukry et al., 2003; Takaki et al., 2000). Thus, the dominant cause of viral persistence might be the development of a weak immune response to viral antigens with a corresponding inability to eradicate HCV-infected cells. Moreover, in subjects who develop chronic infection the frequency of HCV-specific CTL displaying activation markers declines rapidly during the initial phase of the infection despite ongoing viremia, suggesting an active termination of the immune response (Lechner et al., 2000a). The weakness of virus-specific T cell responses is reflected in the unusually low frequency of HCV-specific T cells in patients with chronic HCV infection, as compared with other viral infections. For example, in chronic HIV infection and in latent EBV infection in humans, up to 10% of the T cells in peripheral blood are virus specific (Ogg et al., 1998, 1999; Tan et al., 1999; Tussey et al., 2000). In contrast, the frequency of HCV-specific CD8+ T cells detected by the same techniques is much lower in chronic HCV infection. In one study, He et al. showed that the frequency of HCV-specific CD8+ T cells was <0.01% in peripheral blood in more than half of the patients, as revealed by staining with two MHC/HCV peptide tetramers that detect CD8+ T cells specific for two epitopes in the NS3 protein (He et al., 1999). Although HCV-specific CD8+ T cells are relatively enriched in the liver compared with peripheral blood, they still only account for 1–2% of intrahepatic CD8+ T cells. Similar results were reported by Lechner et al., who found that in more than half of the patients with chronic HCV infection, virus-specific CD8+ T cells were not detectable in the peripheral blood by staining with MHC/HCV peptide tetramers (Lechner et al., 2000b). In the remaining patients where HCV-specific cells could be detected, the average percentage of HCV-specific CD8+ T cells was only 0.07% in peripheral blood lymphocytes. Possible mechanisms of this relatively low frequency of HCV-specific T cells responses include a failure to generate virus-specific T cell response due to impaired antigen presentation (Bain et al., 2001; Kanto et al., 1999). Here, we suggest that CD95L-positive hepatocytes increase apoptosis of activated effector T cells, which might thereby promote chronic infection.
There have been several conflicting reports on the role of HCV proteins in immunosuppression, with evidence both for (Large et al., 1999) and against (Liu et al., 2002; Sun et al., 2001) the role of HCV structural proteins in failure to clear other viral infections. We do not believe that the apoptosis of activated T cells observed in our model necessarily must result in an absolute failure of virus-specific immune responses. There was not an absolute elimination of either CD4+ or CD8+ T cells, consistent with observations that these mice do not have abnormalities in CD4+ or CD8+ cells compared to littermate controls (data not shown). However, multiple observations of increased apoptosis of T cells in patients with chronic HCV infection in both the periphery (Emi et al., 1999; Taya et al., 2000; Toubi et al., 2001) and liver (Nuti et al., 1998) are consistent with our hypothesis and support the physiologic relevance of these studies. Although HCV patients are not considered to be immunosuppressed, recent studies have shown defects in the memory T lymphocyte response against heterologous viruses (Lucas et al., 2004), an observation which could be related to increased apoptosis of T lymphocytes.
In summary, our results have shown that the HCV structural proteins increase the expression of FasL in hepatocytes and enhance the ability of hepatocytes to induce apoptosis of activated T cells. We propose that this apoptosis upon exposure to HCV-infected hepatocytes in the liver is one mechanism that contributes to the ineffective immune response against this virus. Increased apoptosis of activated T cells induced by hepatocytes expressing HCV proteins may amplify the ability of the liver to down modulate T cell responses, contributing to HCV persistence.
Materials and methods
Mice
The line of HCV transgenic (Tg+) mice carrying a transgene that encodes the core, E1 and E2 proteins of type 1b HCV has been described previously (Kawamura et al., 1997). The expression of the transgene is under the control of a liver-specific murine albumin promoter. This line of HCV transgenic mice was originally created in FVB background and bred to BALB/c mice to generate (FVB × BALB/c) F1 mice (H-2q/d). Transgenic mice were typed by PCR as previously described (Kawamura et al., 1997). Age- and sex-matched littermates negative for expression of HCV (Tg−) were used as a control.
Isolation and culture of murine hepatocytes
Murine hepatocytes were isolated by in situ perfusion via the portal vein with collagenase IV solution (Gibco BRL), according to standard techniques as described (Klaunig et al., 1981). Cells were washed three times in RPMI supplemented with 10% FCS, 1% penicillin and streptomycin, and 2 mM glutamine before being plated into Primaria 24- or 6-well plates in William’s medium supplemented with insulin (10 μg/ml), EGF (50 ng/ml) and glucagon (10 μg/ml), as described (Enat et al., 1984). Non-adherent cells, including contaminating lymphocytes, were removed by washing 4 h after plating, and the hepatocytes were then cultured for 24 to 48 h.
Activation of T cells
Splenic T cells were isolated from non-transgenic controls, and cultured in vitro for 24 h in the presence of 5 μg/ml Concanavalin A (Con A) in RPMI medium supplemented with 10% FCS, penicillin (100 U/ml), streptomycin (100 μg/ml), HEPES buffer, and L-glutamine (2 mM). Gradient centrifugation was performed over Ficoll-Hypaque immediately before use to maximize the viability of activated T cells used in co-culture. Freshly isolated ex-vivo splenic T cells were used as resting T cell controls. In addition, cells were also activated by co-culture of splenic T cells from C57/B6 mice (H-2b) with non-transgenic splenocytes (H-2q/d) for 48–72 h.
Co-culture of activated T cells and primary cultured hepatocytes
Four million activated, unfractionated T cells were co-cultured with adherent HCV transgenic or non-transgenic hepatocytes. After incubation for indicated periods of time, lymphocytes were harvested by pipetting. Hepatocytes remain adherent after pipetting. After one wash in PBS, viable lymphocytes were counted using a hemacytometer. Trypan blue was used to exclude non-viable cells. In some experiments, co-cultures were performed by adding antibody, 10 μg/ml anti-FasL (Ab-1, Oncogene Research Products, Boston, MA), or the isotype control antibody, and then apoptosis was measured in CD4+ and CD8+ T cells.
Staining of apoptotic cells by annexin V
0.1–0.5 × 106 recovered lymphocytes were stained for 30 min in U-bottomed 96 plates with FITC-conjugated anti-CD4 (BD Pharmingen, San Diego, CA) or anti-CD8 monoclonal antibody (BD Pharmingen, San Diego, CA), dissolved in PBS containing 1% FCS and 0.1% sodium azide. Thirty minutes before FACS analysis, cells were then stained with PE-conjugated annexin V (BD Pharmingen, San Diego, CA) and propidium iodide (PI; BD Pharmingen, San Diego, CA), as suggested by the manufacturer. During FACS analysis, a live acquisition gate was placed on lymphocytes based on FSC/SSC profile. Side and forward angle light scattering was used to electronically gate the cells of choice and to exclude debris. Ten thousand events within the gate region were collected for each sample. Rare contaminating hepatocytes were excluded on the basis of FSC/SSC. The percentages of CD4+ and CD8+ T cells and the percentages of CD4+ and CD8+ T cells that were annexin V and/or PI positive were measured by FACScan using CellQuest software (BD, San Jose, CA). The absolute numbers of viable CD4+ and CD8+ T cells were calculated by multiplying the total number of viable lymphocytes recovered from individual co-cultures with the percentages of CD4+ and CD8+ T cells, respectively.
Adoptive transfer and in vivo activation
Splenocytes from non-Tg littermates were activated with conA as above. Forty-eight hours later, five to ten million CFSE-labeled cells were injected via the portal circulation into HCV Tg mice as described (Huang et al., 1994). Non-Tg littermates were used as controls. Sixteen hours after injection, the animals were sacrificed, and liver and spleen lymphocytes were harvested. The degree of apoptosis of CFSE labeled cells was analyzed as described above. In some experiments, HCV Tg+ or non-Tg mice were treated with 20 μg/mouse of anti-FasL Ab (Ab-1, Oncogene Research) or the appropriate isotype control prior to adoptive transfer of cells.
Western blotting analysis
200 μg of cell lysates of freshly isolated transgenic and non-transgenic hepatocytes were electrophoresed in 10% SDS-PAGE and transferred to nitrocellulose membrane. Immunodetection was performed using the enhanced chemiluminescence technique (NEN Life Science Products, Boston, MA). The following specific antibodies were used in the study: polyclonal rabbit anti-mouse Fas ligand antibodies (Ab-1, Oncogene Research Products, Boston, MA), polyclonal anti-mouse β-Actin, polyclonal rabbit anti-serum to galectin-1 (gift of Dr. Linda Baum; University of California, Los Angeles, CA), and a monoclonal antibody against TRAIL (clone N2B2; gift of Dr. Hideo Yagita, Juntendo University, Japan). Mouse beta actin (clone C-11, Santa Cruz) was used as a control, and expression of FasL was normalized to beta actin. Densitometry was performed using the NIH Image software downloaded from the NIH web site.
RNA isolation and RT-PCR
Liver tissue from Tg+ or non-Tg littermates was harvested, and total RNA was isolated using the TriZol reagent (Invitrogen, Inc. Carlsbad, California) according to the manufacturer’s instructions. For RT-PCR, 2 μg of total RNA was treated for 15 min at room temperature with 2 U of amplification grade DNase I (Invitrogen, Inc. Carlsbad, California) to remove the genomic DNA. The cDNA was generated from 2 μg of total RNA using Ready-To-Go First-Strand Beads (Amersham Biosciences, Piscataway, NJ). RT-PCR amplifications of cDNA were carried out using the PTC-100 Thermal Controller (MJ Research, Inc. Waltham, MA) as follows: 5 min at 94 °C, 35 cycles of 1 min at 94 °C, 1 min at 55 °C, and 2 min at 72 °C extension, followed by 10 min at 72 °C. The PCR products were analyzed on 1.5% agarose gel, stained with ethidium bromide, and visualized with UV light. The sense primer for FasL was 5′-TCATCTTGGGCTCCTCCAGGGTCAG-3′, and the antisense primer was 5′-GGCTTTGGTTGGTGAACT-CACGGAG-3′ (nucleotides 185–483), which gave a 298-bp PCR product.
Flow cytometric analysis of FasL expression
For analysis of surface expression of FasL on hepatocytes, a total of 1 × 106 freshly isolated hepatocytes were stained with an optimal dilution of PE-anti-mouse Fas Ligand antibody (MFL3 clone, Pharmingen, San Diego, CA). After several washes with PBS, hepatocytes were incubated in blocking solution (PBS containing 5% normal mouse serum) at 4 °C for 30 min and then with PE-conjugated anti-FasL at 4 °C for 30 min. Fluorochrome-conjugated isotype-matched Abs were used as controls for non-specific binding. In all experiments, cells were taken from either Tg+ or non-Tg littermates as controls. Data were acquired on a FACScan flow cytometer. Data were analyzed using CellQuest software (BD Biosciences, San Jose, CA). Similar experiments were performed to analyze the expression of FasL and Fas on lymphocytes. In these experiments, lymphocytes were activated as above, then co-cultured with either Tg+ or non-Tg hepatocytes. FasL and Fas expressions were measured as above.
Caspase-3 activity
Following co-culture with either Tg+ or non-Tg hepatocytes, lymphocytes were collected, purified using Ficoll-Hypaque centrifugation, and the pellet was lysed by addition of lysis buffer. The lysates of 2 × 106 cells were incubated on ice for 10 min and centrifuged at 10,000 × g for 1 min. The supernatant was collected and used as cell lysate. The activity of caspase-3 was measured using caspase-3 colorimetric Assay kit (R&D Systems, Inc. Minneapolis, MN) according to the manufacturer. Briefly, lysates were incubated with caspase-3 colorimetric substrate (DEVD-pNA) for 2 h at 37 °C in a 96-well flat bottom microplate. Upon cleavage of the substrate, the liberated chromophore pNA is spectrophotometrically detected. The pNA light emission was quantified using a microtiter plate reader at 405 nm. The level of caspase-3 enzymatic activity in the cell lysate is directly proportional to the color reaction.
ELISA
The concentration of tumor necrosis factor α (TNF α) was measured using a TNF α ELISA kit (Endogen, Woburn, MA) according to manufacturer’s specifications.
Statistical analysis
Statistical analysis was performed using the StatView software (SAS Institute Inc., Cary, NC). Analysis was performed using student t test for parametric data and Mann–Whitney test for non-parametric, as appropriate, and Kruskal–Wallis for comparison of multiple groups.
Acknowledgments
We thank Drs. Linda Baum and Hideo Yagita for providing reagents. This work was supported by grants from the NIH to E.S. (AI43478) and M.K. (AI43478 and AI AI50752). L.H. was supported by an NIH training grant (DK075331-4) and a Settleman and Levine postdoctoral fellowship from the American Liver Foundation.
References
- Bain C, Fatmi A, Zoulim F, Zarski JP, Trepo C, Inchauspe G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–524. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- Baum LG, Pang M, Perillo NL, Wu T, Delegeane A, Uittenbogaart CH, Fukuda M, Seilhamer JJ. Human thymic epithelial cells express an endogenous lectin, galectin-1, which binds to core 2 O-glycans on thymocytes and T lymphoblastoid cells. J Exp Med. 1995;181:877–887. doi: 10.1084/jem.181.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz GT, Altman JD, Doherty PC. Characteristics of virus-specific CD8(+) T cells in the liver during the control and resolution phases of influenza pneumonia. Proc Natl Acad Sci USA. 1998;95:13812–13817. doi: 10.1073/pnas.95.23.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino P, Heath WR, Hardy CL, Morahan G, Miller JF. Peripheral deletion of autoreactive CD8+ T cells in transgenic mice expressing H-2 Kb in the liver. Eur J Immunol. 1995;25:1932–1942. doi: 10.1002/eji.1830250721. [DOI] [PubMed] [Google Scholar]
- Cooper S, Erickson AL, Adams EJ, Kansopon J, Weiner AJ, Chien DY, Houghton M, Parham P, Walker CM. Analysis of a successful immune response against hepatitis C virus. Immunity. 1999;10:439–449. doi: 10.1016/s1074-7613(00)80044-8. [DOI] [PubMed] [Google Scholar]
- Crispe IN. Death and destruction of activated T lymphocytes. Immunol Res. 1999;19:143–157. doi: 10.1007/BF02786483. [DOI] [PubMed] [Google Scholar]
- Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- Depraetere V, Golstein P. Fas and other cell death signaling pathways. Semin Immunol. 1997 Apr;9:93–107. doi: 10.1006/smim.1997.0062. [DOI] [PubMed] [Google Scholar]
- Diepolder HM, Zachoval R, Hoffmann RM, Wierenga EA, Santantonio T, Jung MC, Eichenlaub D, Pape GR. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006–1007. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- Diepolder HM, Zachoval R, Hoffmann RM, Jung MC, Gerlach T, Pape GR. The role of hepatitis C virus specific CD4+ T lymphocytes in acute and chronic hepatitis C. J Mol Med. 1996;74:583–588. doi: 10.1007/s001090050062. [DOI] [PubMed] [Google Scholar]
- Emi K, Nakamura K, Yuh K, Sugyo S, Shijo H, Kuroki M, Tamura K. Magnitude of activity in chronic hepatitis C is influenced by apoptosis of T cells responsible for hepatitis C virus. J Gastroenterol Hepatol. 1999;14:1018–1024. doi: 10.1046/j.1440-1746.1999.01993.x. [DOI] [PubMed] [Google Scholar]
- Enat R, Jefferson D, Ruiz-Opazo N, Gatmaitan Z, Leinwand L, Reid L. Hepatocyte proliferation in vitro: its dependence on the use of serum-free hormonally defined medium and substrata of extracellular matrix. Proc Natl Acad Sci USA. 1984;81:1411–1415. doi: 10.1073/pnas.81.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eray M, Matto M, Kaartinen M, Andersson L, Pelkonen J. Flow cytometric analysis of apoptotic subpopulations with a combination of annexin V-FITC, propidium iodide, and SYTO 17. Cytometry. 2001;43:134–142. doi: 10.1002/1097-0320(20010201)43:2<134::aid-cyto1028>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Galle PR, Hofmann WJ, Walczak H, Schaller H, Otto G, Stremmel W, Krammer PH, Runkel L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, Murthy KK, Rice CM, Walker CM. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- Hahn YS, Soguero C, Cruise M. Towards a reliable parameter of liver damage in hepatitis C: TUNEL versus caspase activation. Hepatology. 2001;34:840–841. doi: 10.1053/jhep.2001.28765. [DOI] [PubMed] [Google Scholar]
- He XS, Rehermann B, Lopez-Labrador FX, Boisvert J, Cheung R, Mumm J, Wedemeyer H, Berenguer M, Wright TL, Davis MM, Greenberg HB. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci USA. 1999;96:5692–5697. doi: 10.1073/pnas.96.10.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu N, Hayashi N, Katayama K, Mochizuki K, Kawanishi Y, Kasahara A, Fusamoto H, Kamada T. Immunohistochemical detection of Fas antigen in liver tissue of patients with chronic hepatitis C. Hepatology. 1994;19:1354–1359. [PubMed] [Google Scholar]
- Huang L, Soldevila G, Leeker M, Flavell R, Crispe IN. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity. 1994;1:741–749. doi: 10.1016/s1074-7613(94)80016-2. [DOI] [PubMed] [Google Scholar]
- Jeremias I, Herr I, Boehler T, Debatin KM. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur J Immunol. 1998;28:143–152. doi: 10.1002/(SICI)1521-4141(199801)28:01<143::AID-IMMU143>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- John B, Crispe IN. Passive and active mechanisms trap activated CD8+ T cells in the liver. J Immunol. 2004;172 (9):5222–5229. doi: 10.4049/jimmunol.172.9.5222. [DOI] [PubMed] [Google Scholar]
- Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Kamada N, Davies HS, Roser B. Reversal of transplantation immunity by liver grafting. Nature. 1981;292:840–842. doi: 10.1038/292840a0. [DOI] [PubMed] [Google Scholar]
- Kanto T, Hayashi N, Takehara T, Tatsumi T, Kuzushita N, Ito A, Sasaki Y, Kasahara A, Hori M. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–5591. [PubMed] [Google Scholar]
- Kawamura T, Furusaka A, Koziel MJ, Chung RT, Wang TC, Schmidt EV, Liang TJ. Transgenic expression of hepatitis C virus structural proteins in the mouse. Hepatology. 1997;25:1014–1021. doi: 10.1002/hep.510250437. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Goldblatt PJ, Hinton DE, Lipsky MM, Chacko J, Trump BF. Mouse liver cell culture: I. Hepatocyte isolation. In Vitro. 1981;17:913–925. doi: 10.1007/BF02618288. [DOI] [PubMed] [Google Scholar]
- Large MK, Kittlesen DJ, Hahn YS. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J Immunol. 1999;162:931–938. [PubMed] [Google Scholar]
- Lechner F, Gruener NH, Urbani S, Uggeri J, Santantonio T, Kammer AR, Cerny A, Phillips R, Ferrari C, Pape GR, Klenerman P. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000a;30:2479–2487. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000b;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- Liu ZX, Nishida H, He JW, Lai MM, Feng N, Dennert G. Hepatitis C virus genotype 1b core protein does not exert immunomodulatory effects on virus-induced cellular immunity. J Virol. 2002;76:990–997. doi: 10.1128/JVI.76.3.990-997.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Vargas-Cuero AL, Lauer GM, Barnes E, Willberg CB, Semmo N, Walker BD, Phillips R, Klenerman P. Pervasive influence of hepatitis C virus on the phenotype of antiviral CD8+ T cells. J Immunol. 2004;172:1744–1753. doi: 10.4049/jimmunol.172.3.1744. [DOI] [PubMed] [Google Scholar]
- McCloskey TW, Chavan S, Lakshmi Tamma SM, Pahwa S. Comparison of seven quantitative assays to assess lymphocyte cell death during HIV infection: measurement of induced apoptosis in anti-Fas-treated Jurkat cells and spontaneous apoptosis in peripheral blood mononuclear cells from children infected with HIV. AIDS Res Hum Retrovir. 1998;14:1413–1422. doi: 10.1089/aid.1998.14.1413. [DOI] [PubMed] [Google Scholar]
- Mehal WZ, Crispe IN. TCR ligation on CD8+ T cells creates double-negative cells in vivo. J Immunol. 1998;161:1686–1693. [PubMed] [Google Scholar]
- Mehal WZ, Juedes AE, Crispe IN. Selective retention of activated CD8+ T cells by the normal liver. J Immunol. 1999;163:3202–3210. [PubMed] [Google Scholar]
- Meyer D, Thorwarth M, Otto C, Gassel HJ, Timmermann W, Ulrichs K, Thiede A. Apoptosis of alloreactive T cells in liver allografts during tolerance induction. Transplant Proc. 1999;31:474. doi: 10.1016/s0041-1345(98)01714-x. [DOI] [PubMed] [Google Scholar]
- Missale G, Bertoni R, Lamonaca V, Valli A, Massari M, Mori C, Rumi MG, Houghton M, Fiaccadori F, Ferrari C. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J Clin Invest. 1996;98:706–714. doi: 10.1172/JCI118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita E, Hayashi N, Iio S, Takehara T, Hijioka T, Kasahara A, Fusamoto H, Kamada T. Role of Fas ligand in apoptosis induced by hepatitis C virus infection. Biochem Biophys Res Commun. 1994;204:468–474. doi: 10.1006/bbrc.1994.2483. [DOI] [PubMed] [Google Scholar]
- Murray DA, Crispe IN. TNF-alpha controls intrahepatic T cell apoptosis and peripheral T cell numbers. J Immunol. 2004;173 (4):2402–2409. doi: 10.4049/jimmunol.173.4.2402. [DOI] [PubMed] [Google Scholar]
- Nagata S. Fas ligand-induced apoptosis. Annu Rev Genet. 1999;33:29–55. doi: 10.1146/annurev.genet.33.1.29. [DOI] [PubMed] [Google Scholar]
- Nuti S, Rosa D, Valiante NM, Saletti G, Caratozzolo M, Dellabona P, Barnaba V, Abrignani S. Dynamics of intra-hepatic lymphocytes in chronic hepatitis C: enrichment for Valpha24+ T cells and rapid elimination of effector cells by apoptosis. Eur J Immunol. 1998;28:3448–3455. doi: 10.1002/(SICI)1521-4141(199811)28:11<3448::AID-IMMU3448>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, Segal JP, Cao Y, Rowland-Jones SL, Cerundolo V, Hurley A, Markowitz M, Ho DD, Nixon DF, McMichael AJ. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- Ogg GS, Kostense S, Klein MR, Jurriaans S, Hamann D, McMichael AJ, Miedema F. Longitudinal phenotypic analysis of human immunodeficiency virus type 1- specific cytotoxic T lymphocytes: correlation with disease progression. J Virol. 1999;73:9153–9160. doi: 10.1128/jvi.73.11.9153-9160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S, Lu L, Fu F, Li Y, Li W, Starzl TE, Fung JJ, Thomson AW. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J Immunol. 1997;158:4654–4661. [PMC free article] [PubMed] [Google Scholar]
- Rao AS, Starzl TE, Demetris AJ, Trucco M, Thomson A, Qian S, Murase N, Fung JJ. The two-way paradigm of transplantation immunology. Clin Immunol Immunopathol. 1996;80:S46–S51. doi: 10.1006/clin.1996.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri A, Murdolo M, Rapicetta M. Induction of FAS ligand expression in a human hepatoblastoma cell line by HCV core protein. Virus Res. 2003;97:103–110. doi: 10.1016/j.virusres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Veazey RS, Seth A, Taylor WM, Nickerson CE, Lifton MA, Dailey PJ, Forman MA, Racz P, Tenner-Racz K, Letvin NL. Simian immunodeficiency virus (SIV)-specific CTL are present in large numbers in livers of SIV-infected rhesus monkeys. J Immunol. 2000;164:6015–6019. doi: 10.4049/jimmunol.164.11.6015. [DOI] [PubMed] [Google Scholar]
- Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, Walker CM. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Bodola F, Fan X, Irshad H, Soong L, Lemon SM, Chan TS. Hepatitis C virus core and envelope proteins do not suppress the host’s ability to clear a hepatic viral infection. J Virol. 2001;75:11992–11998. doi: 10.1128/JVI.75.24.11992-11998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagashira M, Yamamoto K, Fujio K, Nagano T, Okamoto R, Ibuki N, Yabushita K, Matsumura S, Okano N, Tsuji T. Expression of perforin and Fas ligand mRNA in the liver of viral hepatitis. J Clin Immunol. 2000;20:347–353. doi: 10.1023/a:1006668013276. [DOI] [PubMed] [Google Scholar]
- Takaki A, Wiese M, Maertens G, Depla E, Seifert U, Liebetrau A, Miller JL, Manns MP, Rehermann B. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–582. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- Tan LC, Gudgeon N, Annels NE, Hansasuta P, O’Callaghan CA, Rowland-Jones S, McMichael AJ, Rickinson AB, Callan MF. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- Taya N, Torimoto Y, Shindo M, Hirai K, Hasebe C, Kohgo Y. Fas-mediated apoptosis of peripheral blood mononuclear cells in patients with hepatitis C. Br J Haematol. 2000;110:89–97. doi: 10.1046/j.1365-2141.2000.01945.x. [DOI] [PubMed] [Google Scholar]
- Toubi E, Kessel A, Goldstein L, Slobodin G, Sabo E, Shmuel Z, Zuckerman E. Enhanced peripheral T-cell apoptosis in chronic hepatitis C virus infection: association with liver disease severity. J Hepatol. 2001;35:774–780. doi: 10.1016/s0168-8278(01)00207-0. [DOI] [PubMed] [Google Scholar]
- Tussey L, Speller S, Gallimore A, Vessey R. Functionally distinct CD8+ memory T cell subsets in persistent EBV infection are differentiated by migratory receptor expression. Eur J Immunol. 2000;30:1823–1829. doi: 10.1002/1521-4141(200007)30:7<1823::AID-IMMU1823>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Wack A, Corbella P, Harker N, Crispe IN, Kioussis D. Multiple sites of post-activation CD8+ T cell disposal. Eur J Immunol. 1997;27:577–583. doi: 10.1002/eji.1830270302. [DOI] [PubMed] [Google Scholar]