Abstract
8-Methyl-N-vanillyl-6-nonenamide (capsaicin) was locally applied in the tail of rhesus monkeys to evoke a nociceptive response, thermal allodynia, which was manifested as reduced tail-withdrawal latencies in normally innocuous 46°C water. Coadministration of three κ opioid ligands, U50,488 (3.2–100 μg), bremazocine (0.1–3.2 μg), and dynorphin A(1–13) (3.2–100 μg), with capsaicin in the tail dose-dependently inhibited capsaicin-induced allodynia. This local antinociception was antagonized by a small dose of an opioid antagonist, quadazocine; (0.32 mg), applied in the tail; however, this dose of quadazocine injected s.c. in the back did not antagonize local U50,488. Comparing the relative potency of either agonist or antagonist after local and systemic administration confirmed that the site of action of locally applied κ opioid agonists is in the tail. In addition, local nor-binaltorphimine (0.32 mg) and oxilorphan (0.1–10 μg) antagonist studies raised the possibility of κ opioid receptor subtypes in the periphery, which indicated that U50,488 produced local antinociception by acting on κ1 receptors, but bremazocine acted probably on non-κ1 receptors. These results provide functional evidence that activation of peripheral κ opioid receptors can diminish capsaicin-induced allodynia in primates. This experimental pain model is a useful tool for evaluating peripherally antinociceptive actions of κ agonists without central side effects and suggests new approaches for opioid pain management.
There is a growing literature concerning the antinociceptive effectiveness of exogenous opioids administered in the periphery (for reviews, see Barber and Gottschlich, 1992; Stein 1995). In particular, locally administered opioid agonists produced antinociceptive effects by interacting with peripheral opioid receptors in inflamed tissues (e.g., Stein et al., 1989; Nagasaka et al., 1996; Wilson et al., 1996). Such receptors are present on peripheral sensory nerves where they can modulate both afferent and efferent neuronal functions to eventually result in antinociception (Carlton and Coggeshall, 1998). The discovery that modulation of nociceptive processes can occur in the periphery has stimulated research toward treatments that minimize central side effects. One possibility is the application of small, systemically inactive doses of analgesics directly into the injured tissue (Stein et al., 1991; Joshi et al., 1993). This may be useful, particularly under localized pathologic conditions. Another approach is the use of peripherally selective compounds, which have a reduced ability to cross the blood-brain barrier (Junien and Riviere, 1995; Read et al., 1997). This provides the possibility of systemic administration and may be beneficial in painful conditions of a more dispersed origin. Many nociceptive conditions, including postoperative pain, cancer, and arthritis, are associated with inflammation. In establishing experimental pain models, it is valuable to explore potential antinociceptive agents and to investigate the mechanisms underlying various forms of inflammatory pain.
Capsaicin, the pungent ingredient in hot chili peppers, has been used to evoke nociceptive responses for evaluating an-tinociceptive agents in humans (Eisenach et al., 1997; Kinnman et al., 1997). Exposure of nociceptor terminals, such as C-fibers, to capsaicin initially leads to excitation of the neuron and, subsequently, to the painful perception and local release of inflammatory pain mediators such as Substance P and calcitonin gene-related peptide (CGRP; e.g., Holzer, 1991; Winter et al., 1995; Caterina et al., 1997). Capsaicin-sensitive nerve fibers play an important role in many types of nociceptive conditions, including arthritis and neuropathic pain (Barthó et al., 1990; Kim et al., 1995; Winter et al., 1995). It has been reported that topical or intradermal administration of capsaicin to human skin produces burning pain and allodynia/hyperalgesia responses (Simone et al., 1989; LaMotte et al., 1992). Previously, we have characterized capsaicin-induced nociception in nonhuman primates (Ko et al., 1998b). After capsaicin was s.c. administered in the tail of rhesus monkeys, it dose-dependently produced thermal allodynia, which was manifested as reduced tail-withdrawal latencies in normally innocuous warm water. More interestingly, when small, systemically inactive doses of μ opioid agonists were coadministered with capsaicin in the tail, they locally inhibited nociceptive responses. These findings support the feasibility of pharmacological studies of capsaicin-induced pain to investigate the function of peripheral opioid receptors (Eisenach et al., 1997; Kinnman et al., 1997).
κ opioid agonists are of particular clinical interest because of their ability to modulate antinociceptive functions without μ opioid-related side effects, which include constipation, pruritus, and respiratory depression. However, the centrally mediated effects of κ agonists, such as sedation and dysphoria, could also limit their usefulness (Pfeiffer et al., 1986; Walsh et al., 1998). Thus, it will be valuable to evaluate peripheral antinociception caused by κ agonists in different experimental pain models. To date, many studies indicate that activation of peripheral κ receptors contribute to relief of visceral pain (e.g., Junien and Riviere, 1995; Langlois et al., 1997; Burton and Gebhart, 1998). Although rodent studies have also investigated the functional ability of peripheral κ receptors against somatic pain (Stein et al., 1989; Nagasaka et al., 1996; Wilson et al., 1996), there are no primate studies exploring this possibility. Given the evidence that peripheral κ receptors may act on primary afferents to inhibit nociceptive transmission by C-fibers (Russell et al., 1987; Haley et al., 1990; Andreev et al., 1994), it is possible that activation of peripheral κ receptors may inhibit capsaicin-induced nociception in primates. In addition, the possibility of κ receptor subtypes has been raised. Based on pharmacological studies, antinociceptive effects of systemic κ agonists have been suggested to be mediated by κ receptor subtypes (e.g., Horan et al., 1991; Butelman et al., 1993, 1998; Ko et al., 1998a). It is interesting to further explore whether these κ agonists produce local antinociceptive effects through different receptor subtypes in the periphery.
Therefore, the aim of this study was to evaluate the hypothesis that local administration of κ opioid agonists can diminish capsaicin-induced nociception in rhesus monkeys. The antinociceptive effects of three κ ligands, U50,488, bremazocine, and dynorphin A(1–13) [DYN A(1–13)], were compared after local and systemic administration. In addition, further antagonism studies were performed to investigate the possible role of peripheral κ receptor subtypes in this procedure.
Materials and Methods
Subjects
Six male and female adult rhesus monkeys (Macaca mulatta) with body weights ranging between 7.6 and 11.9 kg (their mean weight during this study was 9.7 kg) were used. They were housed individually with free access to water and were fed approximately 25 to 30 biscuits (Purina Monkey Chow; Ralston Purina Co., St. Louis, MO) and fresh fruit daily. All monkeys had previous experience in the tail-withdrawal procedure and did not have exposure to capsaicin and opioids for 1 month before the present study. Animals used in this study were maintained in accordance with the University Committee on the Use and Care of Animals in the University of Michigan, and the Guide for the Care and Use of Laboratory Animals (7th ed) by the Institute of Laboratory Animal Resources (Natl Acad Press, Washington D.C., revised 1996).
Procedure
Thermal antinociception was measured by a warm-water tail-withdrawal procedure that has been described previously (Ko et al., 1998b). Briefly, the subjects were seated in restraint chairs and the lower part of the shaved tail (approximately 15 cm) was immersed into warm water maintained at temperatures of 42, 46, and 50°C. Tail-withdrawal latencies were recorded manually on a computerized timer. A maximum cutoff latency (20 s) was recorded if the subjects failed to remove their tails by this time. Each experimental session began with control determinations at each temperature. Subsequent tail-withdrawal latencies were determined at 5, 15, 30, 45, and 60 min after injection. The subjects were tested one to two times at three temperatures in a varying order, with approximately 1- to 2-min intervals between tests. In most cases, experimental sessions were conducted once per week, except in the time course study of nor-binaltorphimine (nor-BNI) antagonism. A single dosing procedure was used in all test sessions.
Experimental Design
Capsaicin was injected s.c. in the terminal 1 to 4 cm of the tail, in a constant 0.1-ml volume. In this procedure, the small amount of capsaicin dose-dependently produced transient allodynia (5–30 min; Ko et al., 1998b). Based on this former characterization, 0.1 mg of capsaicin was chosen as a standard noxious stimulus for the present studies in 46°C water.
Antinociceptive Effects of κ Agonists
U50,488 (3.2–100 μg), bremazocine (0.1–3.2 μg), or DYN A(1–13) (3.2–100 μg) was coadministered with capsaicin in the tail to assess local antinociceptive effects in 46°C water. Maximally effective local doses of the three agonists were also administered either in the back against capsaicin or in the tail against 50°C water in the absence of capsaicin. In addition, systemic antinociceptive effects of U50,488 (0.032–0.32 mg/kg) and DYN A(1–13) (0.1–3.2 mg/kg) were studied by s.c. administration in the midscapular region of the back immediately after capsaicin injection. As described below (see Data Analysis), comparisons were drawn between potency (ED50 values) of locally administered agents and potency of the same agent administered systemically based on the weight of each animal.
Antagonism of κ Agonist-Induced Antinociception
Given that onset and distribution factors may be minimized with local administration, opioid antagonists were coadministered with capsaicin and the κ agonists in the tail to investigate local antagonist effects. In addition, the highest effective doses of different antagonists were injected s.c. in the back to verify whether the antagonist effects were localized in the tail.
There were three substudies in this experiment. First, quadazocine (0.01–0.32 mg) was coadministered with capsaicin and U50,488 (100 μg) in the tail to evaluate local antagonist potency. Then, quadazocine (0.032–1 mg/kg) was given s.c. in the back 30 min before local injection of capsaicin and U50,488, to determine systemic antagonist potencies. Thus, the relative potency of quadazocine was compared by its ID50 values obtained following local and systemic routes.
Second, nor-BNI, a κ antagonist, was administered to determine whether it displayed differential antagonism against the local effects of U50,488 (100 μg) and bremazocine (3.2 μg) in the tail. The dose of nor-BNI (0.32 mg) was initially chosen based on an approximately 100-fold potency difference relative to systemically effective doses in antinociceptive studies in rhesus monkeys (e.g., Butelman et al., 1993). We previously found that systemically administered nor-BNI (3.2 mg/kg) produced inconsistent antinociceptive effects during the first hour after injection. In addition, nor-BNI does not have a clear κ selectivity during the first hour after injection (Endoh et al., 1992). To avoid the possible interference of early action of nor-BNI, we used a paradigm similar to one used in a previous study (Butelman et al., 1993). Thus, the data were obtained from separate experiments, in which nor-BNI was initially administered s.c. in the marked tail on day 0. After the nor-BNI injection, the tail-withdrawal responses were measured for 1 h. Then, the local antinociceptive effect of U50,488 or bremazocine against capsaicin was determined 1, 3, 7, 14, and 21 days after local nor-BNI administration by injecting in the marked location. The interval between two experimental sessions was at least 2 weeks. Multiple doses of nor-BNI antagonist were not given because frequent capsaicin exposure in the first week after nor-BNI pretreatment might desensitize the subject's nociceptive responses (Holzer, 1991).
Finally, oxilorphan was chosen to further evaluate the possibility of κ receptor subtypes in the periphery. Based on in vivo pA2 analysis, oxilorphan displayed a κ1 antagonist selectivity, approximately 7- to 10-fold, in antinociception (M.-C.K., M. D. Johnson, D. W. Tyson, J. R. Traynor and J.H.W., in preparation). Oxilorphan (0.1–10 μg) was coadministered with capsaicin and U50,488 (100 μg) or bremazocine (3.2 μg) in the tail to determine local antagonist potency. The differential potency of oxilorphan was compared with that of quadazocine, based on their local ID50 values.
There were two groups of subjects in this study. The first group (n = 3) was used in all experimental sessions. The second group (n = 3) was used to confirm selected experimental data from the first group; in particular, studies of the time course of local nor-BNI antagonism, locally and systemically effective doses of κ agonists against capsaicin, and locally effective doses of antagonists (e.g., quadazocine and oxilorphan) were replicated.
Data Analysis
The 15-min time point was used for analysis because this was the time of peak effects of both capsaicin and κ agonists (Ko et al., 1998b). Individual tail-withdrawal latencies were converted to percentage of maximum possible effect (% MPE) by the formula: % MPE = [(test latency − control latency)/(cutoff latency, 20 s − control latency)] × 100. Individual control latencies were averaged from two determinations after application of 0.1 mg of capsaicin in the tail in 46°C water. Mean ED50 values were obtained after log transformation of individual ED50 values, which were calculated by least-squares regression using the portion of the dose-effect curves spanning the 50% MPE, and 95% CL values were also determined. Mean ID50 values of antagonists were determined in the same manner by defining the dose that inhibited the 50% MPE induced by local κ agonists. Comparison of relative potencies of each compound administered locally or systemically was performed by converting the mg/kg units to total mg units based on the individual monkey's body weight (i.e., 0.1 mg/kg corresponds to 1 mg, assuming an approximate monkey weight of 10 kg). A significant difference was defined as a lack of overlap in the 95% CL of ED50 or ID50 values. In addition, the dose-dependent effects were analyzed with one-way ANOVA followed by the Newman-Keuls test (p < .01). In the nor-BNI antagonism study, a significant reduction in tail-withdrawal latency was also determined by the Newman-Keuls test (p < .01).
Drugs
U50,488 (Upjohn Co., Kalamazoo, MI), bremazocine methanesulfonate (Sandoz, Basel, Switzerland), DYN A(1–13) (National Institute on Drug Abuse, Bethesda, MD), quadazocine methanesulfonate (Sanofi, Malvern, PA), nor-BNI (obtained from Dr. H. I. Mosberg, Dept. of Medicinal Chemistry, University of Michigan), and oxilorphan tartrate (Bristol Myers Co., Wallingford, CT) were dissolved in sterile water. For systemic administration, all compounds were administered s.c. in the back, at a volume of 0.1 ml/kg. Capsaicin (Sigma Chemical Co., St. Louis, Mo) was dissolved in a solution of Tween 80/ethanol/saline in a ratio of 1:1:8. For local coadministration, all compounds were mixed in capsaicin solution and injected in a 0.1-ml volume in the tail.
Results
Nociceptive Effects of Capsaicin
Normally, the monkeys kept their tails in 42 and 46°C water until the cutoff time (20 s), which indicated that both temperatures were innocuous. In contrast, they would remove their tails from 50°C water rapidly, typically within 1 to 3 s. When 0.1 mg of capsaicin was injected into the tail, it evoked a nociceptive response, thermal allodynia, which was shown as reduced tail-withdrawal latencies. In particular, from 5 min after injection, capsaicin caused rapid tail-withdrawal latencies of approximately 2 s in 46°C water and this effect lasted for 30 min (Ko et al., 1998b). In the current study, administration of capsaicin caused erythema (redness) at the site of injection in only one monkey. This erythema could not be observed in all subjects due to dark pigmentation of the tail. Thus, a nociceptive response (reduced tail-withdrawal latencies) was used to examine the local effects of κ opioids in this preparation.
Antinociceptive Effects of κ Agonists
Figure 1 compares the local antinociceptive effects of three κ agonists, U50,488, bremazocine, and DYN A(1–13). Coadministration of U50,488 (3.2–100 μg) with capsaicin (0.1 mg) in the tail dose-dependently inhibited capsaicin-induced thermal allodynia in 46°C water (Fig. 1, top). However, when applied in the back, the high dose of U50,488 (100 μg) was not effective against capsaicin and it was not locally effective against a noxious stimulus, 50°C water, in the absence of capsaicin. Although the 15-min time point was used to analyze the data, it was worth noting that this ineffectiveness was observed throughout 1 h in the test session. Local administration of bremazocine (0.1–3.2 μg) and DYN A(1–13) (3.2–100 μg) also dose-dependently inhibited capsaicin-induced allodynia (Fig. 1, middle and bottom). Similarly, the antinociceptive effect of bremazocine and DYN A(1–13) was not observed when the high dose of bremazocine (3.2 μg) or DYN A(1–13) (100 μg) was applied in the back or when injected into the tail in 50°C water in the absence of capsaicin. The order of local antinociceptive potency (ED50) was bremazocine (1.0 μg) > U50,488 (26.2 μg) ≥ DYN A(1–13) (27.9 μg). As noted, the locally effective doses of these three κ agonists did not cause any behavioral changes, such as sedation, after injection.
Fig. 1.
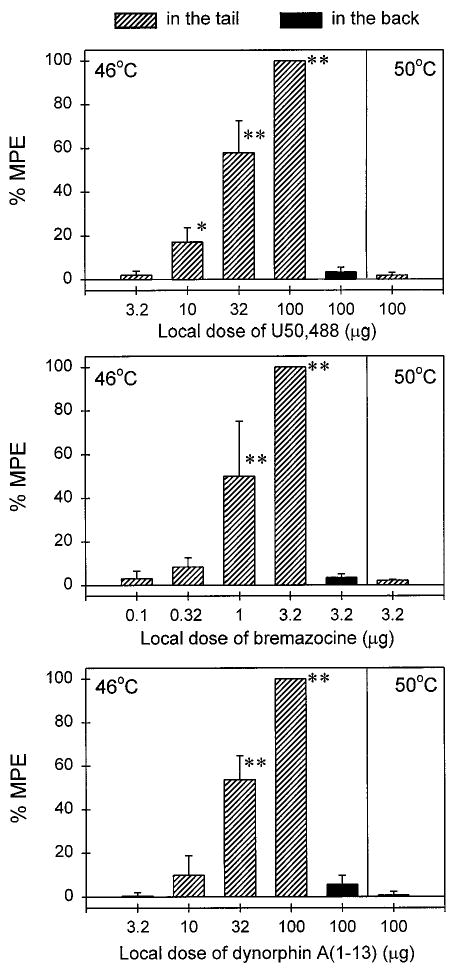
Local antinociceptive effects of U50,488 (top), bremazocine (middle), and DYN A(1–13) (bottom) administered in the tail (slashed columns) or in the back (solid columns) against 46°C water in the presence of capsaicin (0.1 mg) or 50°C water in the absence of capsaicin. Each value represents the mean ± S.E.M. (n = 3–6). Asterisks represent a significant difference (**p < .01; *p < .05) from control. Abscissae, agonist doses in μg. Ordinates, percentage of maximum possible effect (% MPE). Each data point was obtained 15 min after injection. See Materials and Methods for other details.
Figure 2 illustrates the systemic antinociceptive effects of U50,488 and DYN A(1–13). After s.c. administration in the back, U50,488 (0.032–0.32 mg/kg) dose-dependently inhibited capsaicin-induced allodynia. Comparing the potency of both administration routes, local injection of U50,488 was approximately 70-fold more potent than systemic injection (Table 1). In contrast, s.c. administration of DYN A(1–13) (0.1–3.2 mg/kg) did not inhibit capsaicin-induced allodynia. This ineffectiveness was observed through the whole test session (5–60 min). Given that the mean weight of the monkeys was 9.7 kg during this study, 3.2 mg/kg DYN A(1–13) approximately corresponded to a 31-mg total dose per monkey. Thus, the antinociceptive potency of local DYN A(1–13) (ED50 = 27.9 μg) was at least 1000-fold higher than s.c. DYN A(1–13) (Table 1).
Fig. 2.
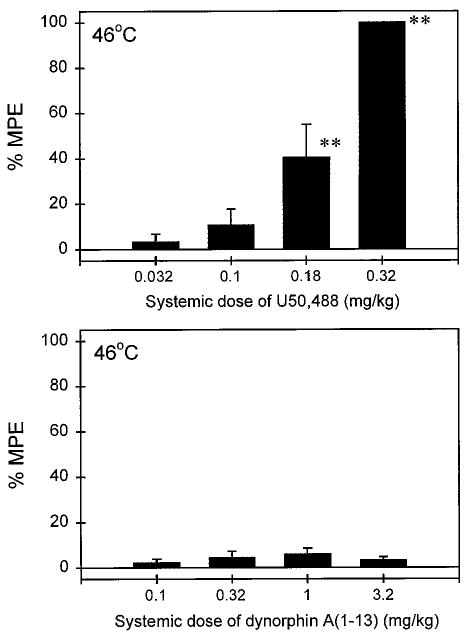
Systemic antinociceptive effects of U50,488 (top) and DYN A(1–13) (bottom) administered s.c. in the back against capsaicin in 46°C water. Other details are as in Fig. 1.
TABLE 1.
Comparison of potencies of κ opioid agonists after local and systemic administration
| Agonists | ED50a (95% CL) | Relative Potenciesb | |
|---|---|---|---|
| Local (mg): in the tail | Systemic (mg/kg): in the back | ||
| U50,488 | 0.026 (0.014–0.049) | 0.186 (0.100–0.350) | 68.7 |
| Dynorphin A(1–13) | 0.028 (0.010–0.081) | NAc | >1000 |
ED50 values were the mean of individual values (n = 3–6).
Values were the mean of individual relative potencies, which were obtained by converting mg/kg to total mg unit based on each monkey's body weight.
Inactive up to 3.2 mg/kg.
Antagonism of κ Agonist-Induced Antinociception
Local administration of quadazocine (0.01–0.32 mg) antagonized the local antinociceptive effects of U50,488 (100 μg) against capsaicin in a dose-dependent manner (ID50, 0.028 mg; 95% CL, 0.017–0.044 mg; Fig. 3, top). When the locally effective dose of quadazocine (0.32 mg) was applied in the back, it did not antagonize local U50,488. This locally effective dose of quadazocine also significantly antagonized local bremazocine (3.2 μg) and DYN A(1–13) (100 μg; data not shown). Although systemic administration of quadazocine (0.032–1 mg/kg) dose-dependently antagonized local U50,488 (ID50, 0.27 mg/kg; 95% CL, 0.11–0.64 mg/kg; Fig. 3, bottom), the relative antagonist potency of quadazocine was approximately 100-fold higher after local versus systemic injections.
Fig. 3.
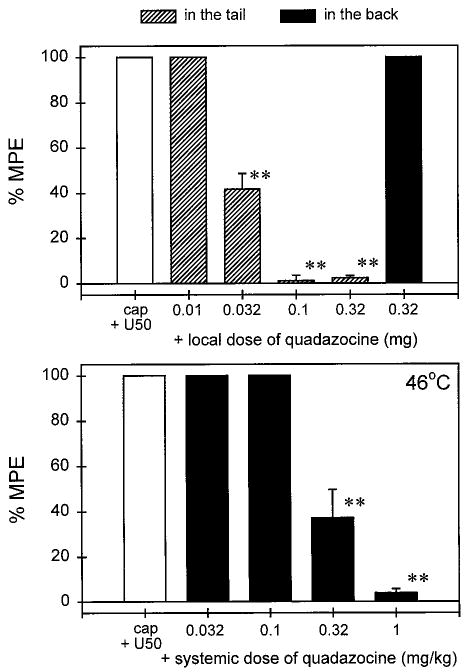
Antagonist effects of quadazocine administered locally in the tail and systemically in the back against local U50,488 in 46°C water in the presence of capsaicin. The label cap + U50 represents the effects of coadministration of 0.1 mg of capsaicin and 100 μg of U50,488 in the tail. Other details are as in Fig. 1.
Figure 4 illustrates the time course of local antagonist effects of nor-BNI (0.32 mg) against U50,488 and bremazocine. After s.c. injection of nor-BNI in the tail, there were no reduced or elevated tail-withdrawal latencies in 46 and 50°C water, respectively (data not shown). nor-BNI also did not change the monkeys' pain threshold from 24 h and beyond. This local nor-BNI pretreatment produced a significant antagonism against local U50,488 and the antagonist effect was observed up to 1 week after administration. The effects of local U50,488 then returned to control levels by 2 weeks after nor-BNI administration. In contrast, there were no significant antagonist effects of nor-BNI against local bremazocine through all test days (i.e., days 1–21) after nor-BNI pretreatment. In the same pretreatment paradigm, after 0.32 mg of nor-BNI was administered s.c. in the back, it did not antagonize local U50,488 one day after pretreatment (data not shown). The following time course experiment was not continued because we did not want to expose the subjects to frequent capsaicin administration to avoid possible desensitization (Holzer, 1991; Winter et al., 1995).
Fig. 4.
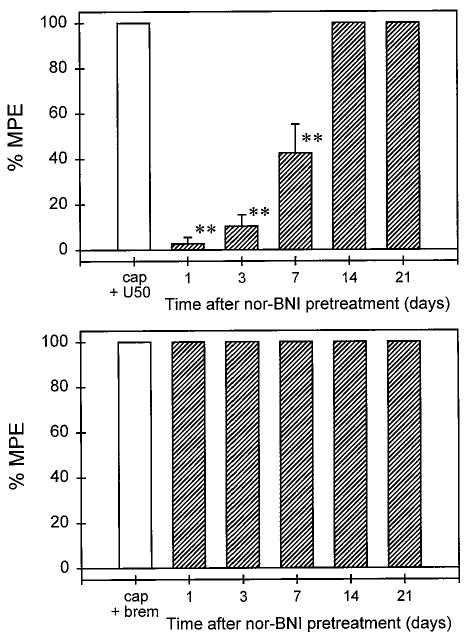
Time course of antagonist effects of nor-BNI (0.32 mg) administered locally in the tail against local U50,488 (top) and bremazocine (bottom) in 46°C water in the presence of capsaicin. Asterisks represent a significant difference (p < .01) from cap + U50. The label cap + brem represents the effects of coadministration of 0.1 mg of capsaicin and 3.2 μg of bremazocine in the tail. Other details are as in Fig. 3.
When oxilorphan was coadministered with capsaicin and U50,488 or bremazocine in the tail, it dose-dependently antagonized local antinociception of both κ agonists (Fig. 5). However, oxilorphan displayed a different antagonist potency against U50,488 and bremazocine in that it was approximately 7-fold more potent in antagonizing local U50,488 than bremazocine (Table 2). Similar to nor-BNI, the high dose of oxilorphan (10 μg) alone did not change the monkey's nociceptive responses in this procedure (data not shown). However, quadazocine (0.01–0.32 mg) was coadministered with capsaicin and bremazocine in the tail. It also dose-dependently antagonized local bremazocine, although there was no significant differentiation in the antagonist potency of local quadazocine against U50,488 and bremazocine (Table 2).
Fig. 5.
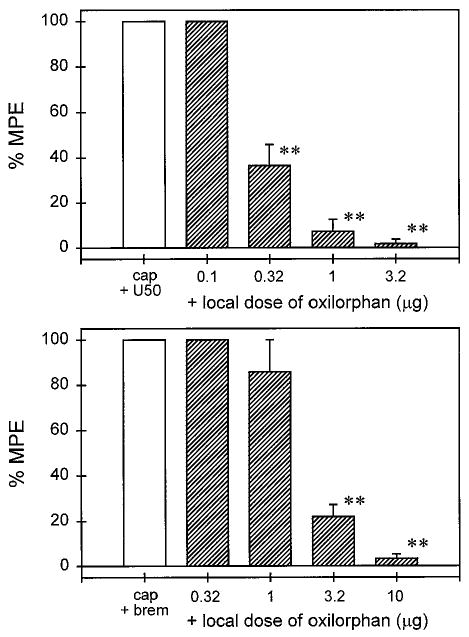
Local antagonist effects of oxilorphan against local U50,488 (top) and bremazocine (bottom) in 46°C water in the presence of capsaicin. The labels cap + U50 and cap + brem represent the effects of coadministration of capsaicin (0.1 mg) with U50,488 (100 μg) and bremazocine (3.2 μg) in the tail, respectively. Other details are as in Fig. 1.
TABLE 2.
Comparison of potencies of opioid antagonists administered locally in the tail against U50,488 and bremazocine
| Antagonists (μg) | ID a (95% CL) | Relative Potenciesb | |
|---|---|---|---|
| versus U50,488 | versus bremazocine | ||
| Quadazocine | 27.8 (17.4–44.4) | 53.6 (13.2–218.1) | 2.2 |
| Oxilorphan | 0.27 (0.12–0.59) | 1.82 (0.88–3.87) | 7.0* |
ID50 values were the mean of individual values (n = 3).
Values were the mean of individual relative potencies, which were obtained by dividing the ID50 value versus bremazocine by the ID50 value versus U50,488.
Indicates that the ID50 of oxilorphan antagonism of bremazocine is significantly different from that for U50,488.
Discussion
The present study illustrated that local administration of κ opioid agonists significantly diminished capsaicin-induced nociception in a dose-dependent manner. The antagonist study confirmed that this local antinociception was in the tail and could be mediated by κ opioid receptor subtypes. These results support the hypothesis that activation of peripheral κ opioid receptors can relieve nociception caused by capsaicin, which is thought to be mediated by stimulating primary afferent C-fibers (Holzer, 1991; Winter et al., 1995; Caterina et al., 1997).
Antinociceptive Effects of κ Agonists
This is the first demonstration that local administration of selective κ agonists U50,488, bremazocine, and DYN A(1–13) can inhibit capsaicin-induced thermal allodynia in nonhuman primates. The locally effective doses of three κ agonists, when applied in the back, did not inhibit capsaicin-induced allodynia. This indicates that the site of κ agonist-induced antinociception against capsaicin may be located in the tail. In particular, comparing the antinociceptive potency of U50,488 and DYN A(1–13) after local and systemic administration, DYN A(1–13), a κ opioid peptide, displayed a large difference in relative potency. Coadministration of DYN A(1–13) with capsaicin in the tail was at least 1000-fold more potent than s.c. administration of DYN A(1–13) in the back. At a dose up to 3.2 mg/kg, DYN A(1–13) did not produce any overt behavioral changes. In contrast, although 0.32 mg/kg U50,488 produced antinociception, this systemic dose also induced a moderate sedation in monkeys (Dykstra et al., 1987; the present study). This observation strengthens the notion that peripheral antinociception can be achieved by local administration of compounds into the injured tissue without producing central side effects (Barber and Gottschlich, 1992; Stein 1995; Wilson et al., 1996).
When a noxious thermal stimulus, 50°C water, was assessed in the absence of capsaicin, local application of κ agonists did not produce antinociception. These observations were similar to findings in rodents, in which the antinociceptive potency of opioid agonists is enhanced on the peripheral terminals of nociceptive primary afferents innervating inflamed tissue (Stein et al., 1989; Andreev et al., 1994; Nagasaka et al., 1996). There are several factors that may account for the increased effectiveness of locally administered opioids in a variety of nociceptive origins. For instance, opioid agonists may have easier access to neuronal opioid receptors due to perineurial disruption (Antonijevic et al., 1995). The activity of peripheral sensory fibers is dynamically regulated by the products of tissue injury and inflammation, as well as by a number of exogenous irritant chemicals (Levine et al., 1993; Dray 1997; Carlton and Coggeshall, 1998). The observation that local administration of κ agonists is more effective in inflamed tissue could, therefore, have therapeutic value, considering that many painful conditions are associated with tissue injury and inflammation.
Capsaicin evokes pain sensations by activating C-fiber nociceptors and stimulating the release of neuropeptides such as Substance P and CGRP from primary nociceptive afferents (Holzer 1991; Winter et al., 1995; Caterina et al., 1997; Kilo et al., 1997). Both Substance P and CGRP play an important role in neurogenic inflammation and contribute to the transmission of nociceptive information (McDonald et al., 1996; Kilo et al., 1997; Cao et al., 1998). It will be interesting to investigate whether Substance P or CGRP antagonists can relieve capsaicin-induced nociception in this procedure. In contrast, activation of peripheral κ receptors has been shown to inhibit the excitability of nociceptive neurons (Russell et al., 1987; Haley et al., 1990; Andreev et al., 1994) and to reduce the release of Substance P from peripheral sensory endings (Yonehara et al., 1992). In addition, in vitro evidence also supports the inhibitory effects of κ opioid receptors (e.g., Levine et al., 1993; Schafer et al., 1994; Minami et al., 1995). Capsaicin-sensitive nerve fibers are involved in a variety of nociceptive conditions (Barthó et al., 1990; Kim et al., 1995; Winter et al., 1995). Given the observation that activation of peripheral κ receptors can produce antinociception in different experimental pain models (Stein et al., 1989; Nagasaka et al., 1996; Wilson et al., 1996; the present study), clinical studies should explore the possibility of local administration of κ opioids for painful conditions with various inflammatory components.
Antagonism of κ Agonist-Induced Antinociception
Local administration of quadazocine, an opioid antagonist, dose-dependently antagonized the local inhibition of U50,488 against capsaicin-induced allodynia. However, the locally effective dose of quadazocine, when applied in the back, did not antagonize local U50,488. This observation confirms the local agonist study, indicating that the site of action of locally applied κ opioids is in the tail. Similarly, a greater relative potency of quadazocine after local versus systemic routes was observed. Compared with a previous study (Ko et al., 1998b), quadazocine is approximately 7-fold more potent in antagonizing fentanyl than U50,488. This different antagonist potency of quadazocine against μ versus κ agonists is expected because quadazocine has a higher affinity for μ receptors than κ receptors in binding preparations (Negus et al., 1993). Quadazocine has been used previously to differentiate μ receptor- and κ receptor-mediated effects in vivo (e.g., Negus et al., 1993).
κ selective actions of locally applied κ agonists were further investigated. The local nor-BNI antagonist study raised the possibility of κ receptor subtypes in the periphery. As previously demonstrated by nor-BNI and naltrexone antagonist studies, systemic U50,488 and bremazocine produced antinociception probably through κ1 and non-κ1 receptors, respectively, in rhesus monkeys (Butelman et al., 1993, 1998; Ko et al., 1998a). In the present preparation, lack of antagonism by nor-BNI against local bremazocine is consistent with results of a previous study using systemic administration of nor-BNI in this species (Butelman et al., 1993). Local nor-BNI displayed a shorter duration of antagonism against local U50,488. This could be due to the site of action (i.e., central versus peripheral) or the smaller local dose that was applied. A larger dose of nor-BNI, applied locally in the tail, could therefore have a longer duration of antagonism against U50,488.
In addition to the nor-BNI antagonist study, oxilorphan was used to evaluate the possibility of κ1 opioid receptor subtypes. Similar to naltrexone in vivo pA2 analysis (Ko et al., 1998a), oxilorphan has a 7- to 10-fold selectivity for κ1 over non-κ1 effects (Ko, 1998). In the present study, local administration of oxilorphan significantly differentiated U50,488- and bremazocine-induced antinociception. In contrast, local quadazocine displayed similar antagonist potency against both κ agonists. These results suggested that U50,488 produced local antinociception by acting on κ1 receptors, but bremazocine might produce local antinociception on non-κ1 receptors. Although bremazocine displayed a high affinity for μ receptor sites, it is unlikely that bremazocine produced antinociception mainly through μ opioid receptors in rhesus monkeys. This can be explained by two functional observations. One is that systemic bremazocine-induced antinociception was not influenced by clocinnamox, a functionally irreversible μ antagonist (Ko et al., 1998a). The other finding is that quadazocine displayed similar potency against U50,488 and bremazocine, but it was more potent in antagonizing μ agonists, such as fentanyl and [D-Ala2,N-Me-Phe4,Gly-ol5]-enkephalin, in this procedure (Ko et al., 1998b). Furthermore, bremazocine has been characterized to be a μ antagonist by in vitro preparations (e.g., Corbett and Kosterlitz, 1986).
Several studies in rodents have suggested that κ opioids (κ1 versus κ2) have distinctive cardiovascular and neurophysiological profiles (Herrero and Headley, 1993; Schoffelmeer et al., 1997). Also, κ2 receptors may represent an important route to the modulation of N-methyl-d-aspartate receptor function on certain neuropathologies (Caudle et al., 1994). Other studies have emphasized that κ agonists are promising analgesics for treating visceral pain because they are effective on irritable bowel syndrome mainly through peripheral actions, but μ and δ agonists have direct inhibitory effects on motility and transit, which makes their use deleterious (Junien and Riviere, 1995; Langlois et al., 1997; Read et al., 1997; Burton and Gebhart, 1998). The present study only suggests the functional possibility of κ receptor subtypes in the periphery against somatic pain. More studies would be needed to determine whether κ subtype agonists have different analgesic efficacy against a variety of pain modalities in primates. To date, due to lack of κ2 subtype-selective antagonists and lack of identification of separate clones, there have been difficulties in clarifying the exact nature of proposed κ receptor subtypes. The development of κ subtype-selective agonists and antagonists would facilitate the pharmacological characterization of these putative receptor subtypes and allow exploration of their potential therapeutic profiles.
In summary, this report demonstrates that local administration of κ opioid agonists diminishes capsaicin-induced thermal nociception in nonhuman primates. This experimental pain model could provide a useful tool for evaluating newly developed κ agonists and expand new approaches for opioid pain management, such as local administration of κ agonists or the use of peripherally selective κ agonists.
Acknowledgments
The authors express their gratitude to Beck McLaughlin for help in preparing the manuscript and Mark Johnson and John Bussenbark for technical assistance.
Abbreviations
- Capsaican
8-methyl-N-vanillyl-6-nonenamide
- DYN A(1–13)
dynorphin A(1–13)
- nor-BNI
nor-binaltorphimine
- % MPE
percentage of maximum possible effect
- CGRP
calcitonin gene-related peptide
- U50,488
(trans)-3,4-dichloro-N-methyl-N[2-(1-pyrrolidinyl)-cyclohexyl]benzeneacetamide
Footnotes
Support for this research was provided by United States Public Health Services Grant DA00254. Preliminary results were presented at the 60th annual meeting of College on Problems of Drug Dependence, Scottsdale, AZ, June 13–18, 1998.
This paper is available online at http://www.jpet.org
References
- Andreev N, Urban L, Dray A. Opioids suppress spontaneous activity of polymodal nociceptors in rat paw skin induced by ultraviolet irradiation. Neuro-science. 1994;58:793–798. doi: 10.1016/0306-4522(94)90456-1. [DOI] [PubMed] [Google Scholar]
- Antonijevic I, Mousa SA, Schäfer M, Stein C. Perineurial defect and peripheral opioid analgesia in inflammation. J Neurosci. 1995;15:165–172. doi: 10.1523/JNEUROSCI.15-01-00165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A, Gottschlich R. Opioid agonists and antagonists: An evaluation of their peripheral actions in inflammation. Med Res Rev. 1992;12:525–562. doi: 10.1002/med.2610120505. [DOI] [PubMed] [Google Scholar]
- Barthó L, Stein C, Herz A. Involvement of capsaicin-sensitive neurones in hyperalgesia and enhanced opioid antinociception in inflammation. Naunyn-Schmiedeberg's Arch Pharmacol. 1990;342:666–670. doi: 10.1007/BF00175710. [DOI] [PubMed] [Google Scholar]
- Burton MB, Gebhart GF. Effects of kappa-opioid receptor agonists on responses to colorectal distension in rats with and without acute colonic inflammation. J Pharmacol Exp Ther. 1998;285:707–715. [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther. 1993;267:1269–1276. [PubMed] [Google Scholar]
- Butelman ER, Ko MC, Sobczyk-Kojiro K, Mosberg HI, Van Bemmel B, Zernig G, Woods JH. Kappa-opioid receptor binding populations in rhesus monkey brain: Relationship to an assay of thermal antinociception. J Pharmacol Exp Ther. 1998;285:595–601. [PubMed] [Google Scholar]
- Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Coggeshall RE. Nociceptive integration: Does it have a peripheral component? Pain Forum. 1998;7:71–78. [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen T, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature (London) 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caudle RM, Chavkin C, Dubner R. K2 opioid receptors inhibit NMDA receptor-mediated synaptic currents in guinea pig CA3 pyramidal cells. J Neurosci. 1994;14:5580–5589. doi: 10.1523/JNEUROSCI.14-09-05580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett AD, Kosterlitz HW. Bremazocine is an agonist at κ-opioid receptors and an antagonist at μ-opioid receptors in the guinea-pig myenteric plexus. Br J Pharmacol. 1986;89:245–249. doi: 10.1111/j.1476-5381.1986.tb11141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A. Peripheral mediators of pain. In: Dickenson A, Besson JM, editors. The Pharmacology of Pain. Springer-Verlag; Berlin: 1997. pp. 21–41. [Google Scholar]
- Dykstra LA, Gmerek DE, Winger G, Woods JH. Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther. 1987;242:413–420. [PubMed] [Google Scholar]
- Eisenach JC, Hood DD, Curry R, Tong C. Alfentanil, but not amitriptyline, reduces pain, hyperalgesia, and allodynia from intradermal injection of capsaicin in humans. Anesthesiology. 1997;86:1279–1287. doi: 10.1097/00000542-199706000-00008. [DOI] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C, Nagase H. Nor-binaltorphimine: A potent and selective κ-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn. 1992;316:30–42. [PubMed] [Google Scholar]
- Haley J, Ketchum S, Dickenson A. Peripheral k-opioid modulation of the formalin response: An electrophysiological study in the rat. Eur J Pharmacol. 1990;191:437–446. doi: 10.1016/0014-2999(90)94178-z. [DOI] [PubMed] [Google Scholar]
- Herrero JF, Headley PM. Functional evidence for multiple receptor activation by k-ligands in the inhibition of spinal nociceptive reflexes in the rat. Br J Pharmacol. 1993;110:303–309. doi: 10.1111/j.1476-5381.1993.tb13809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: Cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Horan PJ, de Costa BR, Rice KC, Porreca F. Differential antagonism of U69,593- and bremazocine-induced antinociception by (–)-UPHIT: Evidence of kappa opioid receptor multiplicity in mice. J Pharmacol Exp Ther. 1991;257:1154–1161. [PubMed] [Google Scholar]
- Joshi GP, McCarroll SM, O'Brien TM, Lenane P. Intraarticular analgesia following knee arthroscopy. Anesth Analg. 1993;76:333–336. [PubMed] [Google Scholar]
- Junien JL, Riviere P. Review article: The hypersensitive gut - peripheral kappa agonists as a new pharmacological approach. Aliment Pharmacol Ther. 1995;9:117–126. doi: 10.1111/j.1365-2036.1995.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Kilo S, Harding-Rose C, Hargreaves KM, Flores CM. Peripheral CGRP release as a marker for neurogenic inflammation: A model system for the study of neuropeptide secretion in rat paw skin. Pain. 1997;73:201–207. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- Kim YI, Na HS, Han JS, Hong SK. Critical role of the capsaicin-sensitive nerve fibers in the development of the causalgic symptoms produced by transecting some but not all of the nerves innervating the rat tail. J Neurosci. 1995;15:4133–4139. doi: 10.1523/JNEUROSCI.15-06-04133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnman E, Nygårds EB, Hansson P. Peripherally administrated morphine attenuates capsaicin-induced mechanical hypersensitivity in humans. Anesth Analg. 1997;84:595–599. doi: 10.1097/00000539-199703000-00024. [DOI] [PubMed] [Google Scholar]
- Ko MC. Pharmacological assessment of thermal antinociception mediated by kappa opioid receptors in rhesus monkeys. The University of Michigan; Ann Arbor: 1998. p. 119. (Doctoral Dissertation) [Google Scholar]
- Ko MC, Butelman ER, Traynor JR, Woods JH. Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. J Pharmacol Exp Ther. 1998a;285:518–526. [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Woods JH. The role of peripheral μ opioid receptors in the modulation of capsaicin-induced thermal nociception in rhesus monkeys. J Pharmacol Exp Ther. 1998b;286:150–156. [PMC free article] [PubMed] [Google Scholar]
- Langlois A, Diop L, Friese N, Pascaud X, Junien JL, Dahl SG, Riviere PJM. Fedotozine blocks hypersensitive visceral pain in conscious rats: Action at peripheral κ-opioid receptors. Eur J Pharmacol. 1997;324:211–217. doi: 10.1016/s0014-2999(97)00089-7. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LER, Torebjörk HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Fields HL, Basbaum AI. Peptides and the primary afferent nociceptor. J Neurosci. 1993;13:2273–2286. doi: 10.1523/JNEUROSCI.13-06-02273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DM, Bowden JJ, Baluk P, Bunnett NW. Neurogenic inflammation. A model for studying efferent actions of sensory nerves. Adv Exp Med Biol. 1996;410:453–462. [PubMed] [Google Scholar]
- Minami M, Maekawa K, Yabuuchi K, Satoh M. Double in situ hybridization study on coexistence of μ-, δ- and κ-opioid receptor mRNAs with preprotachy-kinin A mRNA in the rat dorsal root ganglia. Mol Brain Res. 1995;30:203–210. doi: 10.1016/0169-328x(94)00290-u. [DOI] [PubMed] [Google Scholar]
- Nagasaka H, Awad H, Yaksh TL. Peripheral and spinal actions of opioids in the blockade of the autonomic response evoked by compression of the inflamed knee joint. Anesthesiology. 1996;85:808–816. doi: 10.1097/00000542-199610000-00016. [DOI] [PubMed] [Google Scholar]
- Negus SS, Burke TF, Medzihradsky F, Woods JH. Effects of opioid agonists selective for μ, κ, and δ opioid receptors on schedule-controlled responding in rhesus monkeys: Antagonism by quadazocine. J Pharmacol Exp Ther. 1993;267:896–903. [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by κ opiate receptors. Science (Wash DC) 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Read NW, Abitbol JL, Bardhan KD, Whorwell PJ, Fraitag B. Efficacy and safety of the peripheral kappa agonist fedotozine versus placebo in the treatment of functional dyspepsia. Gut. 1997;41:664–668. doi: 10.1136/gut.41.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell NJW, Schaible HG, Schmidt RF. Opiates inhibit the discharges of fine afferent units from inflamed knee joint of the cat. Neurosci Lett. 1987;76:107–112. doi: 10.1016/0304-3940(87)90201-1. [DOI] [PubMed] [Google Scholar]
- Schafer MKH, Bette M, Romeo H, Schwaeble W, Weihe E. Localization of k-opioid receptor mRNA in neuronal subpopulations of rat sensory ganglia and spinal cord. Neurosci Lett. 1994;167:137–140. doi: 10.1016/0304-3940(94)91046-4. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer ANM, Hogenboom F, Mulder AH. κ1- and κ2-opioid receptors mediating presynaptic inhibition of dopamine and acetylcholine release in rat neostriatum. Br J Pharmacol. 1997;122:520–524. doi: 10.1038/sj.bjp.0701394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Stein C, Millan MJ, Shippenberg TS, Peter K, Herz A. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther. 1989;248:1269–1275. [PubMed] [Google Scholar]
- Stein C, Comisel K, Haimerl E, Yassouridis A, Lehrberger K, Herz A, Peter K. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N Engl J Med. 1991;325:1123–1126. doi: 10.1056/NEJM199110173251602. [DOI] [PubMed] [Google Scholar]
- Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685–1690. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Abreu ME, Bigelow GE. Pharmacodynamic profile of enadoline, a selective kappa agonist, in humans. 60th Annual Meeting of College on Problems of Drug Dependence; June 13-18, 1998; Scottsdale, AZ. 1998. [Google Scholar]
- Wilson JL, Nayanar V, Walker JS. The site of anti-arthritic action of the k-opioid, U-50,488H, in adjuvant arthritis: Importance of local administration. Br J Pharmacol. 1996;118:1754–1760. doi: 10.1111/j.1476-5381.1996.tb15601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Bevan S, Campbell EA. Capsaicin and pain mechanisms. Br J Anaesth. 1995;75:157–168. doi: 10.1093/bja/75.2.157. [DOI] [PubMed] [Google Scholar]
- Yonehara N, Imai Y, Chen JQ, Takiuchi S, Inoki R. Influence of opioids on substance P release evoked by antidromic stimulation of primary afferent fibers in the hind instep of rats. Regul Pept. 1992;38:13–22. doi: 10.1016/0167-0115(92)90068-6. [DOI] [PubMed] [Google Scholar]


