Abstract
Purpose
To prospectively investigate the prognostic significance of p21 and p53 expression in diffuse large B cell lymphoma (DLBCL) in the context of the US Intergroup trial comparing conventional cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy to rituximab (R)-CHOP induction, with or without maintenance rituximab (MR).
Experimental Design
Immunohistochemical staining of 197 paraffin-embedded biopsy specimens was scored by an independent panel of experts.
Results
The cyclin-dependent kinase inhibitor, p21, was expressed in 55% of cases examined. In a multivariable analysis adjusting for International Prognostic Index score and BCL2 status, p21 expression was a significant, independent, favorable predictive factor for failure free survival and overall survival (FFS: relative risk 0.3; P = 0.001; OS: relative risk 0.3; P = 0.003) for patients treated with R-CHOP. Expression of p21 was not predictive of outcome for CHOP-treated patients.
Only p21-positive cases benefited from the addition of rituximab to CHOP. Among p21-positive patients, treatment with R-CHOP was associated with a higher FFS rate at 5 years compared to CHOP (61% versus 24%; P = 0.01). In contrast, no significant differences were detected in FFS according to treatment arm for p21-negative patients. Expression of p53, alone or in combination with p21, did not predict for outcome in uni- or multivariable analyses.
Conclusions
In this study, p21 protein expression emerged as an important independent predictor of a favorable clinical outcome when rituximab was added to CHOP therapy. These data suggest that rituximab-related effects on lymphoma survival pathways may be functionally linked to p21 activity.
Introduction
The cyclin-dependent kinase inhibitor p21 negatively regulates cell cycle progression and inhibits cellular proliferation.1–3 In addition to its cell cycle regulatory function, p21 also has multiple other biologic functions including tumor suppressor and oncogenic activities.4 Although it is a downstream effector of the multifunctional tumor suppressor p53, its expression is controlled by both p53-dependent and -independent mechanisms.5,6 Expression of p21 quantified by immunohistochemistry has been associated with a favorable clinical outcome in solid tumors, including localized renal cell cancer, bladder cancer, ovarian cancer, and non-small cell lung cancer.7–10 In the hematologic malignancies, hypermethylation of the p21Waf1 promoter region resulting in decreased p21 expression has been correlated with adverse outcomes in acute lymphoblastic leukemia11 and deletion or loss of expression of the p21Waf1 gene has been associated with aggressive mantle cell lymphoma variants.12
Mutations of the TP53 gene have been identified in as many as 20% of all diffuse large B-cell lymphomas (DLBCL) and have been associated with poor prognosis in patients treated with conventional chemotherapy.13,14 Strong immunohistochemical nuclear staining for p53 with absent p21 staining has been associated with TP53 gene alterations, and has been used as an imperfect surrogate for mutated TP53 in some studies.14,15 When assessed by immunohistochemical staining, p53 alone is not a consistent predictor of clinical outcome in DLBCL.14,16–18
The addition of the anti-CD20 monoclonal antibody, rituximab (R), to standard chemotherapy such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) has improved clinical outcomes for both young and old patients with diffuse, large B-cell lymphoma (DLBCL).19–21 Studies have shown that R modulates the prognostic significance of some biomarkers reflecting a selective benefit for the addition of R to CHOP chemotherapy for some subsets of DLBCL and not others.22–25 In a companion trial to the US Intergroup phase III study comparing CHOP to R-CHOP, and maintenance R to observation in patients over age 60 with DLBCL, we prospectively investigated potential biologic markers of prognosis.20 In addition to a previously reported analysis of BCL6 and BCL2,25 we prospectively investigated the prognostic significance of p53 and p21. In this correlative study, we observed that p21 expression measured by immunohistochemical staining was a favorable independent prognostic indicator in older patients with DLBCL treated with R-CHOP but not among those treated with CHOP chemotherapy. Furthermore, our analysis showed that the addition of R to CHOP had differential effects on outcomes for p21-positive and p21-negative patients, benefiting the p21-positive patients selectively.
Materials and methods
Eligibility
Eastern Cooperative Oncology Group (ECOG) or Southwest Oncology Group (SWOG) patients enrolled in the phase III US Intergroup trial (E4494, C9793, S4494) comparing CHOP to R-CHOP followed by a second randomization to either maintenance R or observation were eligible for this study.20 Adequate pathologic material was required for participation as previously described.25 All participants signed informed consent forms as approved by the Institutional Review Boards of each participating institution in accordance with the Declaration of Helsinki. This analysis includes all cases for which material was submitted for p53 and p21 staining.
Clinical trial
Patients 60 years or older were stratified according to number of International Prognostic Index (IPI) risk factors and then randomized to treatment with either 6 – 8 cycles of CHOP alone or with R administered 7 and 3 days before the first cycle and then 2 days before cycles 3, 5, and 7 as previously described.20 Complete or partial responders then underwent a second randomization 3 weeks after induction chemotherapy to either observation or maintenance R administered as 4 weekly infusions (375 mg/m2) every 6 months for a total of 4 cycles.
Immunohistochemical studies
Sections (5 µm) from biopsy specimens were stained with hematoxylin and eosin to confirm the presence of lymphoma in the tissue to be studied. Immunohistochemical staining was performed using heat-induced antigen retrieval as previously described.26 Commercial mouse monoclonal antibodies to p53 (DAKO M7001/clone DO-7) and p21/WAF1 (Oncogene Research Products #0P64) were used in this study. Staining for BCL6 and BCL2 was described previously.25
Pathology review
All cases underwent tertiary review as previously described.20 Immunohistochemical staining was reviewed by at least 2 members of the expert panel with discordant cases resolved by a consensus review of the panel or a third reviewer. Cases were scored as entirely negative, 1% to 9%, 10% to 20%, 21% to 50%, and more than 50% positive. Consistent with previously published series, p53 and BCL2 positive cases were defined as those in which greater than 50% of the large neoplastic cells stained positively for the protein of interest.16,17,22,25,27,28
Previous studies of p21 staining in solid tumors have used a cut-off of either 2% or 5% to define positivity. Among series of mantle cell lymphomas, the mean number of positive cells has been in the low single digits.12,29 Similar to the largest series of NHL, we found that fewer than 20% of cases had more than 10% immunoreactive cells.15 Given the large number of cases in which fewer than 10% of cells were scored as positive and the difficulties inherent in distinguishing between cases with very low levels of staining, cases with any definitive staining of large, neoplastic cells for p21 were considered to be positive for purposes of the presented analysis. A similar definition of positivity was applied to the BCL6 analysis as previously reported.25 This cutpoint (no staining versus staining of any large, neoplastic cells for BCL6), has now been validated by the Lunenburg Lymphoma Biomarker Consortium.30
Statistical Plan
The Fisher exact test and Wilcoxon rank-sum test were used to compare patient characteristics between groups. Failure-free survival (FFS) was defined as the time from randomization to relapse, nonprotocol treatment, or death. Overall survival (OS) was measured as the time from randomization to death from any cause. The Kaplan Meier method was used to estimate FFS and OS.31,25 The prognostic significance of all biomarkers was evaluated for all patients and according to induction and maintenance therapy by using both univariable (Fisher exact test, log rank) and multivariable (Cox proportional hazard regression models) analyses. The multivariable analyses controlled for the effect of the IPI (low/low-intermediate versus high-intermediate/high), BCL2 expression status, and BCL6 expression status. Using similar methods, outcomes for CHOP versus R-CHOP according to p21 expression or p53 expression were compared to evaluate the predictive value of the biomarkers. All P values were based on two-sided tests and were adjusted for multiple comparisons.
Whereas maintenance R improved outcomes after CHOP but not R-CHOP, methods applied in the published analysis of the US Intergroup trial were employed to compare induction treatments without the confounding effect of MR.20,25 Rather than exclude all patients who received MR from the analysis which would increase the proportion of nonresponders relative to the whole population and underestimate the FFS and OS, an unbiased estimate was obtained by applying a weighted Cox regression model as previously described.32,33 This analysis provides prognostic information for each biomarker in the context of induction therapy without the potentially confounding effect of maintenance R. Accordingly, the effect of maintenance R has been removed in all analyses presented in this manuscript.
Results
P21 and p53 protein expression
The US Intergroup trial accrued 632 patients including 544 with complete IPI data. A total of 387 patients were enrolled from the participating cooperative groups (ECOG and SWOG). Within this subset of patients, there were 197 cases with adequate pathology material and interpretable p53 and p21 staining. The primary reason for exclusion of cases was insufficient pathology material. Either material was not submitted for this study or the submitted tissue was judged to be insufficient by the expert hematopathology panel.
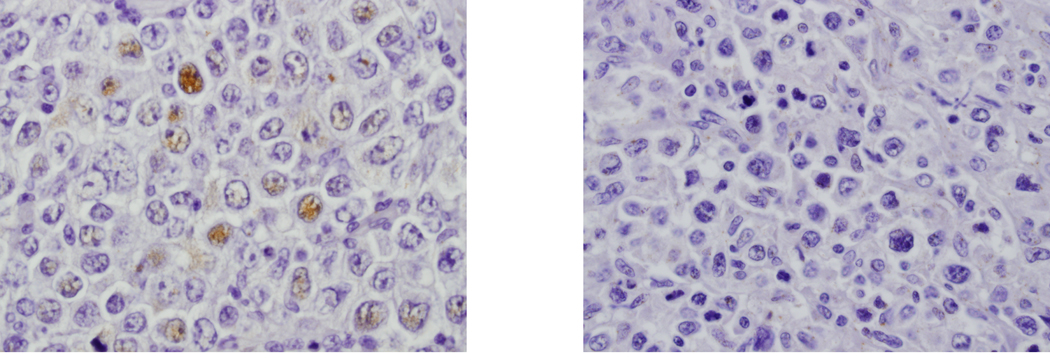
P21 positivity was defined as the presence of any large neoplastic cells that were immunoreactive for nuclear p21 by immunohistochemical staining. In just over half (108 or 55%) of the 197 cases, p21 was scored as positive. In the majority (67 or 62%) of cases scored as positive, less than 10% of the cells were immunoreactive (Figure 1). There were 22 (11%) cases with 10–20% immunoreactive neoplastic cells; in 16 (8%) cases, 21–50% neoplastic cells stained with the anti-p21 reagent. Only 3 (2%) cases had greater than 50% of the large neoplastic cells stain for p21. Consistent with the largest non-Hodgkin lymphoma series, only 20% of cases had more than 10% of the neoplastic cells stain positively for p21.15 The remaining 90 cases (46%) were completely negative for p21 expression.
Figure 1. Immunostaining of diffuse large B-cell lymphoma biopsy specimens with anti-p21 antibody.
A: Specimen showing less than 10% of neoplastic cells staining for p21. B: Case with no cells immunoreactive for p21.
Only 45 (23%) of cases were p53+. In additional analyses, p53 immunoreactivity associated with p21 negativity served as a possible surrogate for mutated TP53.
Patient characteristics
Patient characteristics in this correlative study were representative of the larger U S Intergroup trial (Table 1). When the p21+ and p21− subgroups were compared, no significant differences were observed in the distribution of patient characteristics (all P > 0.4). A similar percentage of patients in the CHOP and R-CHOP arms were p21+ (56% versus 54%, respectively, P = 0.9), and patient characteristics were also balanced between treatment arms. Similarly, no significant differences in patient characteristics were observed according to treatment with R-CHOP or CHOP by p21 status.
Table 1.
Patient characteristics according to p21 expression and treatment
| Patient characteristic |
Intergroup Trial |
Total | p21+ | p21− | |||||
|---|---|---|---|---|---|---|---|---|---|
| R-CHOP | CHOP | R-CHOP | CHOP | P | RCHOP | CHOP | P | ||
| No. of patients | 544 | 102 | 95 | 55 | 53 | 47 | 42 | ||
| Median age (range) |
70 (60–92) |
70 (60, 83) |
71 (61, 87) |
70 (60,83) |
69 (60,87) |
0.6 | 68 (60,81) |
71 (60,85) |
0.6 |
| Male (%) | 272(50) | 55(53.9) | 47(49.5) | 31(56.4) | 24(45.3) | 0.3 | 23(48.9) | 23(54.8) | 0.7 |
| Elevated LDH (%) | 321(59) | 60(58.8) | 62(65.3) | 32(58.2) | 33(62.3) | 0.7 | 28(59.6) | 29(69.0) | 0.4 |
| Stage III–IV (%) | 403(74) | 78(76.5) | 73(76.8) | 43(78.2) | 40(75.5) | 0.8 | 35(74.5) | 33(78.6) | 0.8 |
| ECOG PS > 1 (%) | 78(14) | 17(16.7) | 12(12.6) | 8(14.6) | 6(11.3) | 0.8 | 9(19.2) | 6(14.3) | 0.6 |
| EN Site> 1 (%) | 162(30) | 31(30.4) | 28((29.5) | 16(29.1) | 13(24.5) | 0.7 | 15(31.9) | 15(35.7) | 0.8 |
| BM + (%) | 111(20) | 19(18.6) | 18(18.9) | 7(12.7) | 13(24.5) | 0.1 | 12(25.5) | 5(11.9) | 0.1 |
| HI/High IPI (%) | 327(60) | 62(60.8) | 61(64.2) | 32(58.2) | 32(60.4) | 0.8 | 30(63.8) | 29(69.0) | 0.7 |
| AA HI/High (%) | 280(51) | 55(53.9) | 52(54.7) | 29(52.7) | 27(50.9) | 0.9 | 26(55.3) | 25(59.5) | 0.8 |
Abbreviations: LDH, lactate dehydrogenase; IPI, International Prognostic Index; PS, performance status; EN, extranodal; BM+, bone marrow positive; HI, high-intermediated; AA, age-adjusted.
When analyzed according to p53 expression and treatment, patient characteristics were also similar between groups (Supplemental Table 1), with the exception of age; p53+ cases treated with R-CHOP were slightly older than those treated with CHOP (69 vs. 66, P = 0.04).
P21 expression and clinical outcome
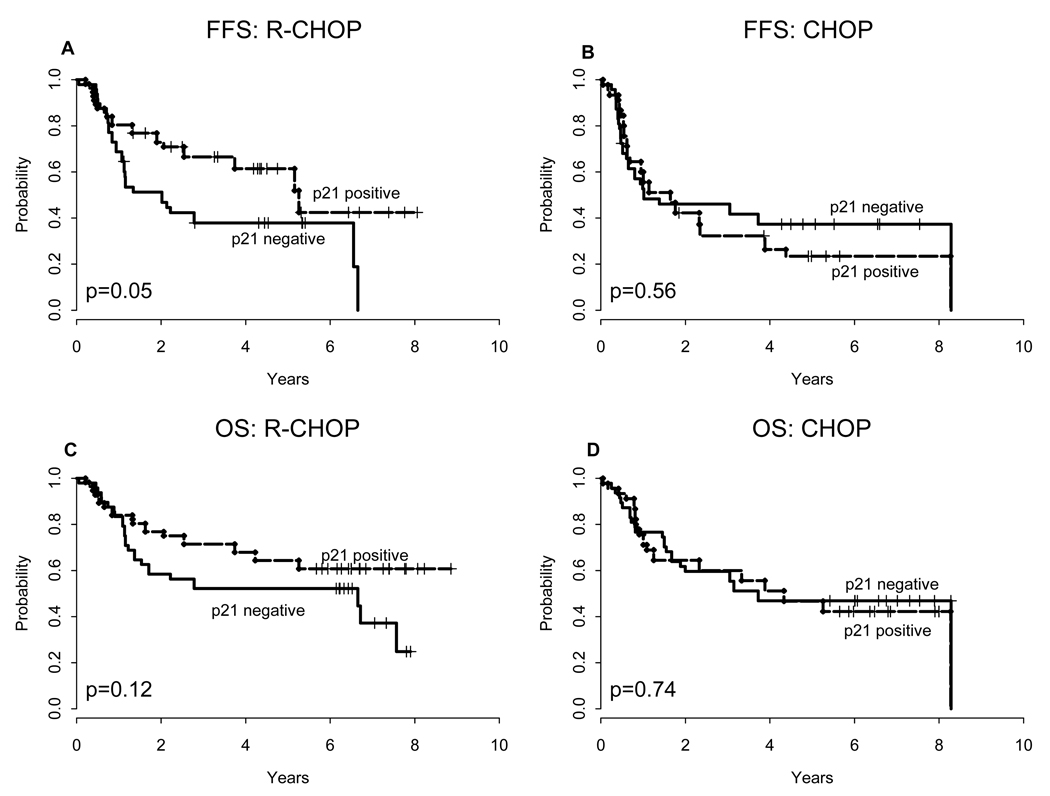
With a median follow-up of 6.85 years, p21 predicted FFS when patients were analyzed according to induction arm. For patients treated with R-CHOP, the five-year estimated FFS was superior for p21-positive patients relative to p21-negative patients (61% ± 6% versus 38% ± 7%, P = 0.05; Figure 2). In contrast, no significant differences in FFS were detected between outcomes for p21-positive and p21-negative cases treated with CHOP (FFS: 24% ± 7% versus 37% ± 7%; P = 0.6). Overall response rates (CR/PR) for p21-positive and p21-negative patients treated with CHOP or R-CHOP did not differ significantly (P = 0.4 and P = 0.8, respectively).
Figure 2. FFS and OS according to p21 status.
Failure-free survival (A–B) and overall survival (C–D) according to p21 status for R-CHOP and CHOP treated patients. Analysis excludes patients who were randomized to receive maintenance R.
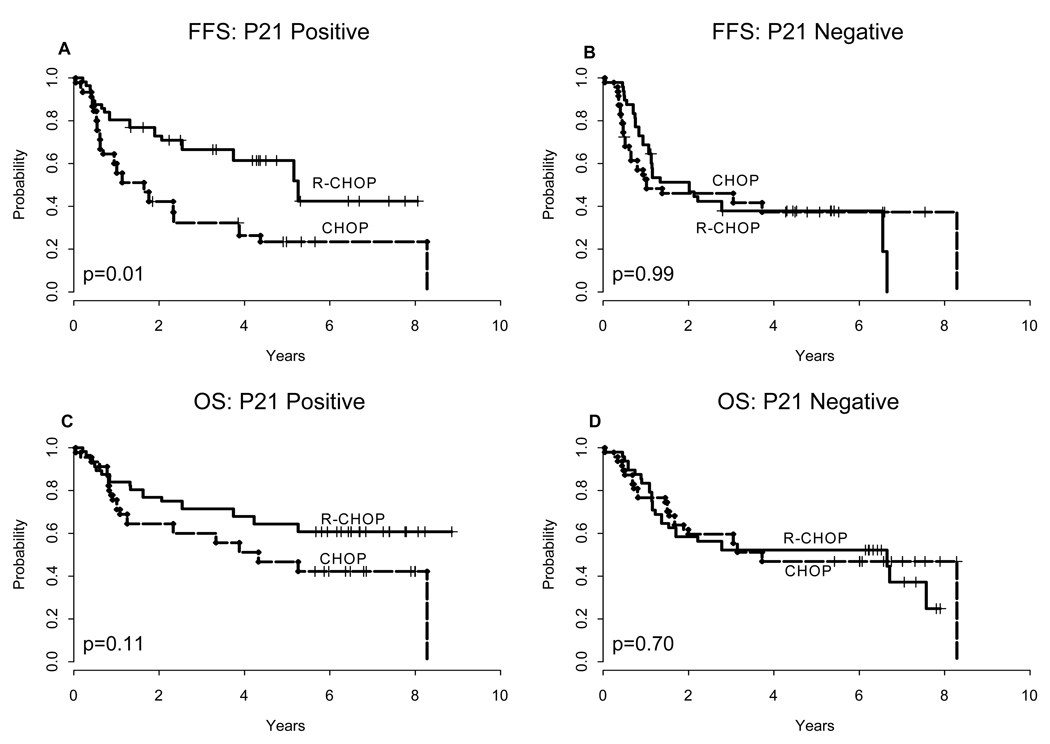
Figure 3 illustrates the impact of R on clinical outcomes according to p21 status. P21-positive but not p21-negative patients benefited from the addition of R to CHOP. The 5 year FFS for p21-positive patients treated with R-CHOP compared to CHOP induction was 61% ± 7% versus 24% ± 7% (P = 0.01). A trend was observed towards a 5 year OS benefit for p21-positive patients treated with R-CHOP compared to CHOP (64%± 6% versus 47% ± 7%, P = 0.1).
Figure 3. FFS and OS according to induction treatment.
Failure-free survival (A–B) and overall survival (C–D) according to induction treatment for p21-positive and p21-negative cases. Analysis excludes patients who were randomized to receive maintenance R.
Table 2 shows the relative risk estimates from the multivariable analysis by treatment. The multivariable analysis adjusted for the effects of IPI, BCL6 and BCL2 expression. Among patients treated with R-CHOP, p21 expression was an independent determinant of improved 5 year FFS and OS (RR 0.3, P = 0.001 and RR 0.3, P = 0.003 respectively). In contrast, p21 expression was not a determinant of clinical outcome in patients treated with CHOP. No significant interactions were detected between BCL2, BCL6 and p21 for either FFS or OS (p=0.55, p=0.40). Furthermore, no significant interaction was identified between p21 and IPI (P > 0.9 for both FFS and OS).
Table 2.
Multivariable analysis of prognostic factors by induction treatment (CHOP, R-CHOP): relative risk estimates comparing p21-positive to p21-negative cases adjusting for IPI risk group, BCL6 and BCL2-expression
| CHOP | R-CHOP | ||||||
|---|---|---|---|---|---|---|---|
| Clinical outcome | |||||||
| RR | 95% CI | P | RR | 95% CI | P | ||
| FFS | |||||||
| P21+ | 0.7 | (0.3, 1.4) | 0.29 | 0.3 | (0.1, 0.6) | 0.001 | |
| BCL6+ | 0.2 | (0.1, 0.5) | <0.0001 | 1.6 | (0.7, 3.8) | 0.31 | |
| HI/high IPI | 1.4 | (0.6, 3.1) | 0.41 | 4.7 | (1.8, 12.2) | 0.002 | |
| BCL2+ | 1.2 | (0.5, 2.6) | 0.71 | 2.4 | (1.2, 4.7) | 0.010 | |
| OS | |||||||
| P21+ | 0.8 | (0.4, 1.7) | 0.64 | 0.3 | (0.2, 0.7) | 0.003 | |
| BCL6+ | 0.2 | (0.1, 0.4) | <0.0001 | 1.8 | (0.8, 4.3) | 0.17 | |
| HI/high IPI | 1.8 | (0.7, 4.8) | 0.21 | 5.8 | (2.0, 16.5) | 0.001 | |
| BCL2+ | 0.8 | (0.4, 1.7) | 0.59 | 3.7 | (1.6, 8.6) | 0.002 | |
Consistent with our previous report and with nearly seven years of follow-up, BCL6 expression retained its favorable prognostic significance among patients treated with CHOP, but was not an independent predictor of either FFS or OS among patients treated with R-CHOP. Although BCL6 has been reported to repress transcription of the gene which encodes p21, no correlation was found between p21 immunoreactivity and BCL6 staining (P = 0.7) in our clinical specimens.5 Among the BCL6-positive subset of cases studied, p21 status predicted clinical outcome among patients treated with R-CHOP (FFS: P = 0.01; OS: P = 0.05) but not CHOP (FFS: P = 0.9; OS: P = 0.9). There were too few BCL6-negative cases to perform a similar analysis.
Because this study was stratified by IPI and not by p21 status, a multivariable analysis was also performed to evaluate the effect of treatment, controlling for p21 expression. In a proportional hazards model, among p21-positive patients, the addition of R to CHOP improved FFS (RR 0.5, 95% CI 0.2, 1.0, P = 0.05) after adjusting for IPI, BCL6 and BCL2 expression. No such treatment effect was seen among p21-negative patients (RR 0.9, 95% CI 0.4, 1.7, P = 0.7).
P53 expression and clinical outcome
In the univariable analysis, strong p53 expression (>50% of large neoplastic cells; 24% of all cases) was not a significant prognostic indicator when all cases were considered (P > 0.9) or when cases were analyzed according to induction treatment (Supplemental Table 2). Whereas other groups have shown p53 over-expression to be associated with inferior outcomes,16,28 we also performed a multivariable analysis that controlled for the effect of IPI, BCL6 and BCL2 expression and found no prognostic significance for p53 expression on clinical outcomes for patients treated with CHOP or R-CHOP. No association between p53-positivity and p21 immunostaining was observed (P = 0.99).
P53-positive/P21-negative phenotype
In keeping with previous reports, the p53-positive/p21-negative phenotype was used as a surrogate marker of mutated TP53 forms to further investigate the prognostic significance of p53 in this trial.16,28 Only 20 patients (10%) had a p53-positive/p21-negative phenotype while the remaining 177 patients (90%) had alternate p53/p21 phenotypes, but there were no significant differences in clinical outcomes for these subgroups. Furthermore, when analyzed according to p53/p21 phenotype and induction treatment, no significant differences in clinical outcomes were demonstrated (Supplemental Data Table 2).
Discussion
Expression of p21 protein was a powerful predictor of clinical outcomes in patients with DLBCL treated with R-CHOP chemotherapy on the US Intergroup trial. This observation was independent of BCL6 or BCL2 expression and IPI risk group in multivariable analysis. In contrast, p21 expression was not predictive of clinical outcome among patients treated with CHOP alone. The addition of R to standard CHOP improved clinical outcomes in p21-immunoreactive cases selectively, suggesting that R has beneficial biologic effects on survival pathways active in p21-positive cases.
P21 is multifaceted, having many biologic functions that are context-dependent including tumor suppressor and oncogenic acitivities.4 P21 inhibits cell cycle progression through its inhibition of cyclin-dependent kinase (CDK)-cyclin complexes and proliferating cell nuclear antigen.34,35 Although expression of p21 is known to be under the regulatory control of p53, it is also regulated by p53-independent mechanisms. P21 modulates apoptosis, regulates transcription of genes important in cell cycle progression and senescence, and impacts DNA repair processes. In turn, a variety of transcription factors, ubiquitin ligases and protein kinases regulate transcription, stability and cellular localization of p21. Studies in mouse lymphoma models suggest that p21 may also function as an oncogene, possibly related to its antiapoptotic activity.36–38 Activation of multiple signaling pathways, including PI3/Akt and Raf/MEK/ERK has been shown to upregulate p21 in non-lymphoid model systems.39–43 Lenalidomide, a thalidomide analogue with activity in relapsed and refractory DLBCL,44 histone deacetylase inhibitors, and the proteasome inhibitor bortezomib have all been reported to upregulate p21 in lymphoma cell lines.45,46,47 Future studies will elucidate the precise role p21 plays in DLBCL.
Our finding that p21-expression identifies a subgroup that benefits from R raises new questions as to how the anti-CD20 antibody facilitates cell death in p21-expressing DLBCL. Recent studies show that R downregulates p38 MAPK, NFkappa B, ERK1/2, and PI3/Akt survival pathways contributing to the sensitization of drug resistant lymphoma cells to chemotherapy-induced apoptosis.48 Rituximab’s selective effect on p21 expressing cases may relate - at least, in part - to its effects on the MAP kinase and/or Akt kinase pathways.48,49 Expression of p21 may be a marker of hyperactivation of these pathways,39–43 which may predict sensitivity to R. Alternatively, high levels of p21 may sensitize lymphomas to R more directly, in an as yet unknown fashion. This effect does not appear to relate to proliferative activity, as there was no significant association between p21 and Ki-67 staining in this study (P = 0.67; data not shown).
The small percentage of positive cells scored in this series and in other lymphoma and solid tumor studies is not inconsistent with the significant biologic role being postulated for p21. Immunohistochemistry likely underestimates p21 expression as evidenced by Western blotting in a series of mantle cell lymphomas.12 In actively proliferating cells, p21 is short-lived, also contributing to the relatively small number of immunostaining cells detected in paraffin-embedded tissue.
In contrast to p21, p53 expression did not emerge as a significant prognostic indicator in this study. Previous studies examining the prognostic significance of p53 in DLBCL have yielded variable results.14,16,17,28 Mutations of TP53, particularly in the DNA-binding regions of the molecule, have been associated with adverse outcomes in DLBCL patients treated with CHOP-like chemotherapy.13,14 Some studies have demonstrated concordance between the presence of TP53 mutations and the p53-positive/p21-negative phenotype by immunohistochemistry suggesting that it may be used as a surrogate, albeit an imperfect one, for mutated TP53 status.14,15 Despite this association, the p53-positive/p21-negative phenotype has not predicted clinical outcome in the majority of studies including this series.14–16 Differences between immunohistochemical studies may be attributable to differences in patient characteristics, cutoffs used for scoring, and immunohistochemical techniques.50 Additionally, the sample size of the p53-positive/p21-negative phenotype (about 10% of all cases) may have been too small to detect a difference in our study.
Changes in the prognostic significance of immunohistochemical markers in DLBCL treated with R-CHOP rather than CHOP likely reflect the impact of R on growth and survival mechanisms including those that relate to p21, BCL6 and BCL2. Data presented in this study coupled with published laboratory studies show p21 to be at the intersection of cell cycle regulation, proliferation and apoptotic pathways, and suggest that R-related effects on clinical outcome may be functionally linked to p21 activity for a subset of cases. Confirmation that p21 status is predictive of clinical outcome among R-CHOP-treated patients in larger datasets is needed. Similarly, the apparent selective benefit of R therapy to p21-expressing cases treated with CHOP chemotherapy requires validation. Although the cell of origin classification is an important biomarker for distinct biologic subsets of DLBCL, other biologic subgroups dependent on specific growth and survival pathways have been identified and more will likely follow. Therapy that is tailored specifically to the unique biology of these individual subcategories of DLBCL is likely to have the greatest impact.
Translational Relevance
The cyclin-dependent kinase inhibitor, p21, is multifaceted, having many biological functions that are context dependent, including tumor suppressor and oncogenic activities. We now identify p21 expression as a new, powerful and independent predictive marker among older patients with DLBCL treated with R-CHOP, but NOT CHOP. The clinical benefit associated with the addition of rituximab to CHOP appears to derive from its effect on the p21-positive cases selectively, likely representing its impact on survival pathways active in this subgroup of DLBCL. These findings will require confirmation, but provide rationale for new therapeutic approaches that focus on p21, and for further investigation into the impact of rituximab on p21-related pathways.
Supplementary Material
Acknowledgments
We thank Alex Minella, M.D. for helpful discussion, Martin Bast for administrative assistance and Ren-Wei Guo, Adekunle Raji and the staff of the ECOG Pathology Coordinating Office for technical assistance. In addition, we are grateful to the investigators, nurses, and data managers of ECOG and SWOG for their participation. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by grants CA23318, CA66636, CA21115, CA38926, CA32102, CA04919, CA11083, CA13650, CA17145, CA81534, CA39229, CA81534 from the Public Health Service, the National Cancer Institute of the National Institutes of Health, and the Department of Health and Human Services and Genentech.
Footnotes
Previously presented in a poster discussion session, 43rd Annual Meeting, ASCO, 2007, Chicago, IL, and in the Plenary Session on Diffuse Large B-cell Lymphoma, 9th International Conference on Malignant Lymphoma, 2008, Lugano, Switzerland.
References
- 1.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 2.el-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 3.Gartel AL, Tyner AL. Transcriptional regulation of the p21((WAF1/CIP1)) gene. Exp Cell Res. 1999;246:280–289. doi: 10.1006/excr.1998.4319. [DOI] [PubMed] [Google Scholar]
- 4.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phan RT, Saito M, Basso K, Niu H, Dalla-Favera R. BCL6 interacts with the transcription factor Miz-1 to suppress the cyclin-dependent kinase inhibitor p21 and cell cycle arrest in germinal center B cells. Nat Immunol. 2005;6:1054–1060. doi: 10.1038/ni1245. [DOI] [PubMed] [Google Scholar]
- 6.Cerchietti LC, Polo JM, Da Silva GF, et al. Sequential transcription factor targeting for diffuse large B-cell lymphomas. Cancer Res. 2008;68:3361–3369. doi: 10.1158/0008-5472.CAN-07-5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss RH, Borowsky AD, Seligson D, et al. p21 is a prognostic marker for renal cell carcinoma: implications for novel therapeutic approaches. J Urol. 2007;177:63–68. doi: 10.1016/j.juro.2006.08.073. discussion 68-9. [DOI] [PubMed] [Google Scholar]
- 8.Stein JP, Ginsberg DA, Grossfeld GD, et al. Effect of p21WAF1/CIP1 expression on tumor progression in bladder cancer. J Natl Cancer Inst. 1998;90:1072–1079. doi: 10.1093/jnci/90.14.1072. [DOI] [PubMed] [Google Scholar]
- 9.Shoji T, Tanaka F, Takata T, et al. Clinical significance of p21 expression in non-small-cell lung cancer. J Clin Oncol. 2002;20:3865–3871. doi: 10.1200/JCO.2002.09.147. [DOI] [PubMed] [Google Scholar]
- 10.Rose SL, Goodheart MJ, DeYoung BR, Smith BJ, Buller RE. p21 expression predicts outcome in p53-null ovarian carcinoma. Clin Cancer Res. 2003;9:1028–1032. [PubMed] [Google Scholar]
- 11.Roman-Gomez J, Castillejo JA, Jimenez A, et al. 5' CpG island hypermethylation is associated with transcriptional silencing of the p21(CIP1/WAF1/SDI1) gene and confers poor prognosis in acute lymphoblastic leukemia. Blood. 2002;99:2291–2296. doi: 10.1182/blood.v99.7.2291. [DOI] [PubMed] [Google Scholar]
- 12.Pinyol M, Hernandez L, Cazorla M, et al. Deletions and loss of expression of p16INK4a and p21Waf1 genes are associated with aggressive variants of mantle cell lymphomas. Blood. 1997;89:272–280. [PubMed] [Google Scholar]
- 13.Ichikawa A, Kinoshita T, Watanabe T, et al. Mutations of the p53 gene as a prognostic factor in aggressive B-cell lymphoma. N Engl J Med. 1997;337:529–534. doi: 10.1056/NEJM199708213370804. [DOI] [PubMed] [Google Scholar]
- 14.Young KH, Weisenburger DD, Dave BJ, et al. Mutations in the DNA-binding codons of TP53, which are associated with decreased expression of TRAILreceptor-2, predict for poor survival in diffuse large B-cell lymphoma. Blood. 2007;110:4396–4405. doi: 10.1182/blood-2007-02-072082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chilosi M, Doglioni C, Magalini A, et al. p21/WAF1 cyclin-kinase inhibitor expression in non-Hodgkin's lymphomas: a potential marker of p53 tumor-suppressor gene function. Blood. 1996;88:4012–4020. [PubMed] [Google Scholar]
- 16.Visco C, Canal F, Parolini C, et al. The impact of P53 and P21(waf1) expression on the survival of patients with the germinal center phenotype of diffuse large B-cell lymphoma. Haematologica. 2006;91:687–690. [PubMed] [Google Scholar]
- 17.Kramer MH, Hermans J, Parker J, et al. Clinical significance of bcl2 and p53 protein expression in diffuse large B-cell lymphoma: a population-based study. J Clin Oncol. 1996;14:2131–2138. doi: 10.1200/JCO.1996.14.7.2131. [DOI] [PubMed] [Google Scholar]
- 18.Leroy K, Haioun C, Lepage E, et al. p53 gene mutations are associated with poor survival in low and low-intermediate risk diffuse large B-cell lymphomas. Ann Oncol. 2002;13:1108–1115. doi: 10.1093/annonc/mdf185. [DOI] [PubMed] [Google Scholar]
- 19.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 20.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 21.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 22.Mounier N, Briere J, Gisselbrecht C, et al. Rituximab plus CHOP (R-CHOP) overcomes BCL2--associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL) Blood. 2003;101:4279–4284. doi: 10.1182/blood-2002-11-3442. [DOI] [PubMed] [Google Scholar]
- 23.Liu YY, Leboeuf C, Shi JY, et al. Rituximab plus CHOP (R-CHOP) overcomes PRDM1-associated resistance to chemotherapy in patients with diffuse large B-cell lymphoma. Blood. 2007;110:339–344. doi: 10.1182/blood-2006-09-049189. [DOI] [PubMed] [Google Scholar]
- 24.Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26:2717–2724. doi: 10.1200/JCO.2007.13.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winter JN, Weller EA, Horning SJ, et al. Prognostic significance of BCL6 protein expression in DLBCL treated with CHOP or R-CHOP: a prospective correlative study. Blood. 2006;107:4207–4213. doi: 10.1182/blood-2005-10-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krajewski S, Bodrug S, Gascoyne R, Berean K, Krajewska M, Reed JC. Immunohistochemical analysis of Mcl-1 and BCL2 proteins in normal and neoplastic lymph nodes. Am J Pathol. 1994;145:515–525. [PMC free article] [PubMed] [Google Scholar]
- 27.Barrans SL, Carter I, Owen RG, et al. Germinal center phenotype and BCL2 expression combined with the International Prognostic Index improves patient risk stratification in diffuse large B-cell lymphoma. Blood. 2002;99:1136–1143. doi: 10.1182/blood.v99.4.1136. [DOI] [PubMed] [Google Scholar]
- 28.Farinha P SL, Skinnider B, Wu L, Patten N, Truong S, Connors JM, Gascoyne RD. Strong p53 Expression Is an Independent Predictor of Outcome in De Novo Diffuse Large B Cell Lymphoma (DLBCL) Treated with Either CHOP or CHOP-R. Blood. 2006;Volume 108 abstract 812. [Google Scholar]
- 29.Izban KF, Alkan S, Singleton TP, Hsi ED. Multiparameter immunohistochemical analysis of the cell cycle proteins cyclin D1, Ki-67, p21WAF1, p27KIP1, and p53 in mantle cell lymphoma. Arch Pathol Lab Med. 2000;124:1457–1462. doi: 10.5858/2000-124-1457-MIAOTC. [DOI] [PubMed] [Google Scholar]
- 30.Lunenberg Lymphoma Biomarker Consortium: First results of an international study to establish a new clinico-biological prognostic index for diffuse large B-cell lymphoma (DLBCL) Ann Oncol. 2008;19:iv100. [Google Scholar]
- 31.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 32.Lunceford JK, Davidian M, Tsiatis AA. Estimation of survival distributions of treatment policies in two-stage randomization designs in clinical trials. Biometrics. 2002;58:48–57. doi: 10.1111/j.0006-341x.2002.00048.x. [DOI] [PubMed] [Google Scholar]
- 33.Wahed AS, Tsiatis AA. Optimal estimator for the survival distribution and related quantities for treatment policies in two-stage randomization designs in clinical trials. Biometrics. 2004;60:124–133. doi: 10.1111/j.0006-341X.2004.00160.x. [DOI] [PubMed] [Google Scholar]
- 34.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Jackson PK, Kirschner MW, Dutta A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 1995;374:386–388. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- 36.De la Cueva E, Garcia-Cao I, Herranz M, et al. Tumorigenic activity of p21Waf1/Cip1 in thymic lymphoma. Oncogene. 2006;25:4128–4132. doi: 10.1038/sj.onc.1209432. [DOI] [PubMed] [Google Scholar]
- 37.Roninson IB. Oncogenic functions of tumour suppressor p21(Waf1/Cip1/Sdi1): association with cell senescence and tumour-promoting activities of stromal fibroblasts. Cancer Lett. 2002;179:1–14. doi: 10.1016/s0304-3835(01)00847-3. [DOI] [PubMed] [Google Scholar]
- 38.Wang YA, Elson A, Leder P. Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc Natl Acad Sci U S A. 1997;94:14590–14595. doi: 10.1073/pnas.94.26.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitsuuchi Y, Johnson SW, Selvakumaran M, Williams SJ, Hamilton TC, Testa JR. The phosphatidylinositol 3-kinase/AKT signal transduction pathway plays a critical role in the expression of p21WAF1/CIP1/SDI1 induced by cisplatin and paclitaxel. Cancer Res. 2000;60:5390–5394. [PubMed] [Google Scholar]
- 40.Li Y, Dowbenko D, Lasky LA. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem. 2002;277:11352–11361. doi: 10.1074/jbc.M109062200. [DOI] [PubMed] [Google Scholar]
- 41.Rossig L, Badorff C, Holzmann Y, Zeiher AM, Dimmeler S. Glycogen synthase kinase-3 couples AKT-dependent signaling to the regulation of p21Cip1 degradation. J Biol Chem. 2002;277:9684–9689. doi: 10.1074/jbc.M106157200. [DOI] [PubMed] [Google Scholar]
- 42.Coleman ML, Marshall CJ, Olson MF. Ras promotes p21(Waf1/Cip1) protein stability via a cyclin D1-imposed block in proteasome-mediated degradation. Embo J. 2003;22:2036–2046. doi: 10.1093/emboj/cdg189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson MF, Paterson HF, Marshall CJ. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature. 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- 44.Wiernik PH, Lossos IS, Tuscano JM, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:4952–4957. doi: 10.1200/JCO.2007.15.3429. [DOI] [PubMed] [Google Scholar]
- 45.Verhelle D, Corral LG, Wong K, et al. Lenalidomide and CC-4047 inhibit the proliferation of malignant B cells while expanding normal CD34+ progenitor cells. Cancer Res. 2007;67:746–755. doi: 10.1158/0008-5472.CAN-06-2317. [DOI] [PubMed] [Google Scholar]
- 46.Piekarz RL, Robey RW, Zhan Z, et al. T-cell lymphoma as a model for the use of histone deacetylase inhibitors in cancer therapy: impact of depsipeptide on molecular markers, therapeutic targets, and mechanisms of resistance. Blood. 2004;103:4636–4643. doi: 10.1182/blood-2003-09-3068. [DOI] [PubMed] [Google Scholar]
- 47.Matta H, Chaudhary PM. The proteasome inhibitor bortezomib (PS-341) inhibits growth and induces apoptosis in primary effusion lymphoma cells. Cancer Biol Ther. 2005;4:77–82. doi: 10.4161/cbt.4.1.1379. [DOI] [PubMed] [Google Scholar]
- 48.Bonavida B. Rituximab-induced inhibition of antiapoptotic cell survival pathways: implications in chemo/immunoresistance, rituximab unresponsiveness, prognostic and novel therapeutic interventions. Oncogene. 2007;26:3629–3636. doi: 10.1038/sj.onc.1210365. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki E, Umezawa K, Bonavida B. Rituximab inhibits the constitutively activated PI3K-Akt pathway in B-NHL cell lines: involvement in chemosensitization to drug-induced apoptosis. Oncogene. 2007;26:6184–6193. doi: 10.1038/sj.onc.1210448. [DOI] [PubMed] [Google Scholar]
- 50.de Jong D, Rosenwald A, Chhanabhai M, et al. Immunohistochemical prognostic markers in diffuse large B-cell lymphoma: validation of tissue microarray as a prerequisite for broad clinical applications--a study from the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol. 2007;25:805–812. doi: 10.1200/JCO.2006.09.4490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.