Abstract
Purpose of review
The etiology of stroke remains unknown in roughly one third of patients despite extensive investigation. The prevalence of PFOs in the general population is around 25% but the prevalence in cryptogenic stroke patients is doubled. This suggests a causal relationship between PFO and CS. This has generally been attributed to paradoxical embolism. Regardless of mechanism, ~30,000 young patients each year have a cryptogenic stroke and PFO. Optimal management is uncertain.
Recent findings
Many physicians recommend PFO closure, an intuitively attractive mechanical solution for stroke prevention. Unfortunately, the benefit of PFO closure in patients with stroke has not been demonstrated. There are ongoing clinical trials comparing the safety and efficacy of PFO closure to medical therapy. Interpreting these trials will be complicated by two issues: first, it is unclear whether a patient's PFO is causally related to the event (“pathogenic”) or not (“incidental”); and second, recurrent strokes may be due to paradoxical embolism or another stroke mechanism.
Summary
Substantial heterogeneity of patients within trials along these two dimensions of risk may make overall trial results difficult to interpret. With appropriate analyses, the trials may be more informative than the overall data may suggest.
Keywords: Cryptogenic stroke, patent foramen ovale, risk heterogeneity, endovascular PFO closure, clinical trials
INTRODUCTION
The foramen ovale is a fetal conduit that allows maternal blood to gain access to the systemic circulation. It allows blood to bypass the fetal pulmonary arteries via a direct intracardiac shunt from the right atrium to the left atrium. The structure was first described in 1564 by Leonardi Botallo while working in the French royal court. He was unaware of its function. Paradoxical embolism was identified as a stroke mechanism by Connheim in 1877. Since then its relevance has been debated in the medical literature. Modern case control studies have repeatedly hinted at its pathogenic role in adult stroke patients. Modern interventional cardiology has progressed to the point that some have advocated screening for PFO and implanting closure devices as a form of “mechanical vaccination” against stroke [1]. Others support completing randomized trials before recommending invasive [2]. In this review, we discuss the evidence demonstrating an association between PFO and CS, review the risk of stroke recurrence in patients with PFO and CS and discuss some issues that affect the design and interpretation of randomized clinical trials for secondary stroke prevention in these patients.
PFO is Associated with CS
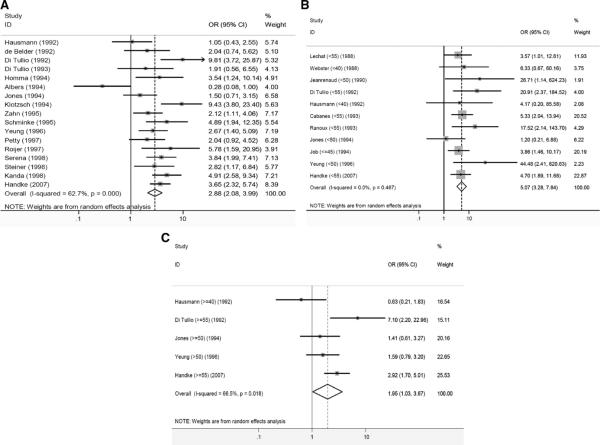
Since 1988, case control studies have demonstrated an increased prevalence of PFOs in patients with cryptogenic stroke compared to patients with stroke of known cause [3]. A strong association has been found in younger patients (OR, 5.1 [3.3 to 7.8]) and a weaker, but consistent, one in older patients (OR, 2.0 [>1.0 to 3.7]. The overall odds ratio for PFO being present in patients with cryptogenic stroke is 2.9 [2.1 to 4.0] (Figure 1).
Figure 1.
Forest plots of random-effects meta-analyses of case-control studies examining the prevalence of PFO in cases with CS versus controls with stroke of determined cause. Individual studies are represented on the left with first author and year of publication. Gray boxes with sizes corresponding to each study's weight in the analysis represent the point estimate of the odds ratio (OR) for PFO in cases with CS versus controls with stroke of determined cause with corresponding 95% confidence intervals represented with the black lines. The diamond in the last row represents the summary estimate of the OR. The bold black line in the middle of the panel represents an OR of 1 (i.e. no association between PFO and CS). Panel A: age-inclusive studies, Panel B: analyses in young patients (with age cutoff in parenthesis), and Panel C: analyses in old patients (with age cut-off in parenthesis). [from ref 3]
There are fewer studies that have included analyses based on other demographic data (e.g. the presence of conventional vascular risk factors) or on morphological characteristics of the PFO. Nevertheless, PFOs have been associated with CS when patients are younger and do not have hypertension, hyperlipidemia, diabetes, or a history of smoking [4]. The “atherosclerotic burden” is lower in CS patients with PFO than in those without [5]. Larger PFOs (both anatomically and physiologically) are more likely to be found in the CS population [6]. Finally, PFOs with a concomitant atrial septal aneurysm are more common in patients with CS. The odds ratio for this combination is 8.9 [1.2 to 64] when compared to patients with stroke of known cause [3].
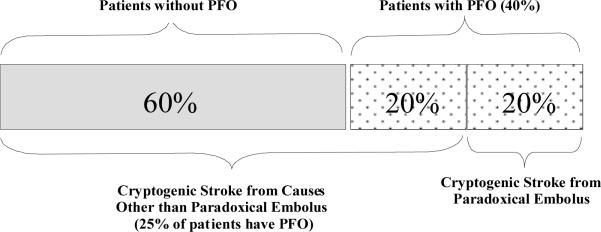
Assuming a 25% prevalence of PFO in the general population and a prevalence of PFO in patients with CS of 40% then it can be shown that the proportion of patients with PFO and CS in whom the PFO is incidental is 50%. The other half of patients represent excess risk attributable to the PFO via paradoxical embolism or other mechanisms (Figure 2). These rates are usually surprising to some clinicians, who presume that a PFO found in the setting of a CS must be etiologically related, or pathogenic.
Figure 2.
Likelihood of a found PFO being associated with paradoxical embolus. This figure assumes that 1) 40% of patients with cryptogenic stroke have a PFO and 2) 25% of the general population have a PFO (including patients with CS not due to paradoxical embolism).
By the same reasoning, factors found to strengthen the association between PFO and CS, can be shown to increase the likelihood that a PFO is pathogenic. Conversely, the absence of such factors increases the likelihood that a discovered PFO is incidental. Thus, the likelihood that a PFO is pathogenic may be high among well-selected patients under age 55 [7]. But some patients, even young ones, will harbor incidental PFOs, particularly those near the upper margin of the age category and with conventional stroke risk factors (HTN, high cholesterol, DM, smoking) and/or lower risk PFO features on TEE. Note also that the prevalence of pathogenic PFOs does not go to zero for older patients, especially ones who do not have conventional stroke risk factors and who do have high-risk features on TEE. A recent analysis confirmed this association in an older population [8].
Risk of Stroke Recurrence
The simple presence of a PFO has not been associated with stroke in population-based studies of people without a prior neurological event but the strong association between PFO and cryptogenic stroke in case control studies of stroke patients has led many to advocate PFO closure for secondary stroke prevention. This approach can only be of benefit if patients presenting with CS and PFO are at risk of recurrent paradoxical embolism. A recently published meta-analysis identified 15 studies that examined the risk of a recurrent events in 1,958 patients with CS and PFO [9]. Using a random effects model, the pooled rate of recurrent stroke or TIA was 4.0 events per 100 person-years (95% CI 3.0 to 5.1) and the pooled rate of recurrent stroke was 1.6 events per 100 person-years (95% CI 1.1 to 2.1). However, there was substantial between-study heterogeneity in the estimated risk of stroke recurrence, probably attributable to differences in study designs, including criteria for detecting PFO, investigations performed prior to classifying the event as cryptogenic, the treatments used, and outcome ascertainment. This underscores the need for a more standardized approach to this problem.
Among the 15 studies in the review, 4 compared the rate of stroke recurrence in CS patients with PFO to those without PFO. The relative risk (RR) of stroke or recurrent TIA in those with a PFO compared to those without ranged from 0.5 to 1.7, with a pooled (fixed effect) RR of 1.1 (95% CI 0.8 to 1.5). When considering only recurrent strokes, the RR ranged from 0.5 to 1.1 with a pooled (fixed effect) RR of 0.8 (95% CI 0.5 to 1.3). While much has been made of the fact that the risk of stroke recurrence is similar in CS patients with or without PFO, we would urge interpreting this null result with caution. Since the control groups in these studies all had cryptogenic strokes, they all, by definition, had occult stroke etiologies. Therefore, the findings of these studies may simply reflect the fact that the recurrence risk in patients with still-cryptogenic stroke (without PFO but with other occult stroke risks) is similar to that in patients with PFO. Even if the recurrence risk in both of these groups is similar, it may still be (unacceptably) higher than in the risk-factor adjusted population without a history of stroke or TIA.
Several studies have sought to identify risk factors for stroke recurrence in patients with CS and PFO [10;11]. The largest, the French PFO/Atrial Septal Aneurysm (ASA) Study [4;12], included over 581 CS patients, 267 with PFO. Patients with PFO alone were no more likely than patients without to have another stroke but an ASA with a PFO increased the recurrence risk ~4-fold. Older age was also associated with a higher recurrence rate. While it seems intuitive that PFO size and degree of right-to-left shunting should also be risk factors for recurrence, this was not found. The study had extremely limited power to examine multiple variables or to detect risk factors of only moderate influence. A more recent study based on PFO screening with transcranial Doppler did not confirm the predictive value of large shunt size or concurrent atrial septal aneurysm for stroke recurrence [13].
The PFO in Cryptogenic Stroke Study (PICSS) [6] also sought to identify PFO features indicating a high risk of recurrence but yielded paradoxical results, which can be understood by considering the fact that patients with non-cryptogenic strokes were included in the analysis. In this population, there was no difference in the 2-year recurrence rate between those with PFO alone (14.5%) and those with both PFO and an ASA (15.9%). More surprising perhaps was the trend for higher 2-year recurrence rates in patients with small PFOs (18.5%) compared with larger ones (9.5%). The explanation for this counter-intuitive finding lies in the fact that patients who had PFOs with high-risk features (e.g. large size or ASA) were more likely to have paradoxical embolism and less likely to have conventional stroke risk factors and other stroke mechanisms. If the risk of recurrence in patients with paradoxical embolism is lower than the risk of recurrence in patients with other stroke subtypes, high risk PFO-features would appear to be paradoxically “protective”. Again, this study points to the need to carefully consider the control group to which PFO patients (or subsets of PFO patients) are being compared when evaluating recurrent stroke risk.
Further complicating the clinical epidemiology of PFO and CS is the fact that risk factors for stroke recurrence may also affect the likelihood that a PFO is pathogenic – but not always in a consistent direction. Some factors likely to be predictors of stroke recurrence even in patients with CS (e.g. age, HTN, DM.) are more likely to be associated with an incidental PFO and not paradoxical embolism. Other risk factors for recurrence (e.g. increased PFO shunting) identify patients more likely to have had a paradoxical embolus. The interaction of these two risk dimensions needs to be carefully considered, since for the purposes of selecting patients most likely to benefit from PFO closure what is at issue is not stroke recurrence per se, but recurrence of paradoxical embolism. Prior studies have not taken these confounding associations (Figure 3) into account in developing risk models.
Figure 3.
Treatment of Patients with PFOs
Options for secondary stroke prevention in patients with CS and PFO include antithrombotic medical therapy (antiplatelet drugs and warfarin), endovascular implantation of PFO closure devices, and direct surgical closure of the PFO. Some physicians argue against the relevance of the diagnosis of PFO altogether [14].
Currently the only randomized treatment data come from PICSS [6], which compared warfarin to aspirin. In patients with CS and PFO, anticoagulation prevented about twice as many strokes as did aspirin, but this difference did not reach statistical significance with regards to the primary endpoint (stroke or death). A meta-analysis of studies evaluating therapeutic options for preventing stroke recurrence in patients with PFO [15], included PICCS and other non-randomized data. The investigators found that warfarin was associated with fewer recurrent events than antiplatelet therapy, but was comparable to surgical closure. PFO closure was associated with a decreased risk of recurrent stroke or TIA compared with medical treatment in another meta-analysis [16]. However, the high degree of heterogeneity across studies in the estimation of recurrence risk even when therapy is similar, suggests that any non-randomized data should be viewed with extreme caution, since differences in patient characteristics are likely to be highly influential in determining outcome rates and the meta-analyses were unable to control for these characteristics.
The non-randomized data and the compelling clinical logic behind PFO closure have led some to advocate this approach even before the results of randomized trials are available. Indeed, a recent review identified 46 uncontrolled case series of device or surgical closure of PFO in patients with stroke, including 4,519 patients after device closure and 233 patients after surgical closure, often touting the low stroke recurrence rate [9]. However, it should be appreciated that PFO closure devices are not without complications, including access site complications, cardiac tamponade, thrombus formation, device fracture, and atrial fibrillation [17]. In a migraine trial with randomized assignment to PFO closure or medical therapy, serious adverse events occurred in 11% of otherwise healthy young subjects [18]. Thus, particularly given the relatively benign natural history in many patients with CS and PFO, meticulous patient selection is likely to be critical. The risks of the treatment may outweigh its benefits for some, even if the device is shown to improve outcomes overall.
Pending the results of on-going trials (ref), the practice parameter from the American Academy of Neurology [19] indicates that there is insufficient evidence to make specific recommendations regarding therapy for patients with CS and PFO.
Risk Stratification and Clinical Trial Interpretation
The discussion above suggests that any trial that tests strategies for secondary prevention of paradoxical embolism will enroll patients that have different probabilities of a pathogenic versus an incidental stroke and different recurrence risk. Multiple recent investigations have shown that risk heterogeneity can give rise to treatment effect heterogeneity that might make the overall results of trials difficult to interpret [20–24]. Risk modeling can be a useful approach to applying such results to individual patients.
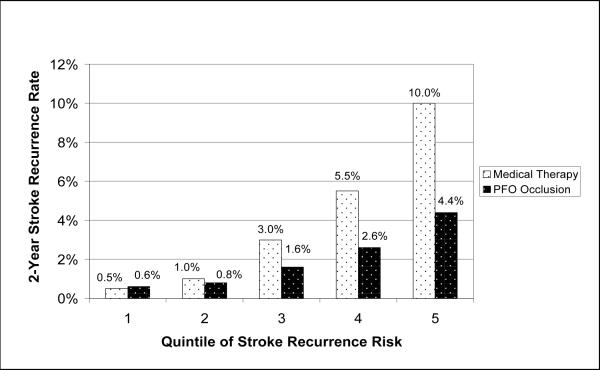
Ideally, to select appropriate patients for PFO closure, one would be able to predict the risk of recurrent paradoxical embolism. Figure 4 illustrates risk-based treatment-effect heterogeneity in CS and PFO. It demonstrates hypothetical outcome rates over two years with risk stratification by quintile of risk. The overall outcome rate would be 2% in the treatment group and 4% in the control group. However, with these assumptions, it can be seen that the overall benefit would be misleading since the degree of benefit from treatment will vary considerably depending on baseline recurrence risk, from net harm (though only slight) in the lowest quintile, to greater than 5% absolute benefit in the highest quintile. Thus, even with favorable assumptions, PFO-closure is associated with net harm in the low risk group; conversely, higher risk patients might get tremendous benefit obscured by the more modest overall trial results. In the two lowest quintiles, the number needed to treat (NNT) to prevent one recurrence in two years is 1000; in the highest-risk quintile, the 2-year NNT is only 18. Increasing the serious treatment-related harm to 1.5% (which may still be an underestimate for some devices [16;17]) would increase the treatment effect heterogeneity. Overall the trial may not show benefit because of the substantial net harm in the first two quintiles. However, the benefit in the two highest risk quintiles would still be substantial.
Figure 4.
This figure is based on the following assumptions: 1) annualized average stroke recurrence risk is 2%; 2) degree of risk heterogeneity is moderate (extreme quintile risk ratio is 20); 3) 60% of recurrent strokes are caused by paradoxical embolism; 4) PFO devices are 100% effective in preventing recurrent paradoxical embolism; and 5) PFO devices are associated with a small degree of treatment-related stroke risk (2 in 1000 per year).
The results of this hypothetical risk stratification are useful but over-simplified, since each stratum is assumed to be at the same risk of recurrent paradoxical embolism. However, as discussed above, the risk factors that determine stroke recurrence are also known to be predictive of an incidental (versus a pathogenic) PFO [4;6;8;25–29]. Thus, the ratio of patients with paradoxical embolism is likely to vary systematically across risk strata, depending on the factors used to risk stratify. For example, if non-specific risk factors for recurrent stroke (e.g. age, sex, presence/absence of diabetes, presence/absence of HTN, etc.) are used, PFO closure might be no more beneficial in high risk versus low risk patients; paradoxically, relative risk reduction would actually decrease substantially in higher risk patients. We illustrate this below by considering two patients.
Ms. Cross and Mr. Small
Consider two patients presenting with CS and identical PFOs (similar size, shunting and septal wall motion mobility).
Ms. Cross is 25 years-old without conventional vascular risk factors. After returning from vacation (by intercontinental airplane) she became hemiplegic while lifting her suitcase in the airport. MRI shows a 3cm acute stroke in the territory of several adjacent lenticulostriate arteries, two other small areas of cortical encephalomalacia and normal vessels. She is found to have a PFO with an atrial septal aneurysm and shunting at rest (not requiring Valsalva).
Mr. Small is a 59 year-old man with 20 years of hypertension, obesity, obstructive sleep apnea, and intermittent smoking who wakes from sleep with hemiplegia. MRI shows a 3cm acute stroke in the territory of several adjacent lenticulostriate arteries, two other small areas of probable deep white matter infarcts, and slightly irregular intracranial arteries. He is also found to have a PFO with an atrial septal aneurysm and shunting at rest - like Ms. Cross.
Both of these patients would qualify for the ongoing clinical trials for patients with cryptogenic stroke and PFO. Ms. Cross has a very low risk of another stroke but is more likely to have another paradoxical embolism. Mr. Small has a higher risk of recurrence but it is more likely to be due to some other mechanism.
Stratification of patients for closure thus needs to consider the joint probability of 1) the likelihood of a PFO being pathogenic and 2) the likelihood of recurrent paradoxical embolism (rather than recurrent stroke in general).
Conclusion
Patients with cryptogenic stroke are a heterogeneous group some of whom have PFOs. A substantial proportion of those PFOs is likely to be incidental and would not benefit from PFO closure. By using clinical, echocardiographic, and radiological characteristics available at the time of the index stroke it should be possible to identify those patients who are both unlikely to have incidental PFOs and yet still be at high risk of stroke recurrence. These are the patients whose future strokes are most likely to be caused by paradoxical embolism and the ones most likely to benefit from closure. Indeed, whether the on-going clinical trials yield overall positive or null results, substantial risk-heterogeneity in the trial population would suggest that the overall results would not apply uniformly to individual patients and more sophisticated approaches for analyzing the trials may be needed.
Acknowledgements
We thank Jennifer Donovan for help with manuscript preparation.
DET is on the Steering Committee for the RESPECT Trial and is a consultant to AGA Medical Corp, Plymouth, MN
Partially supported by NINDS 1 R01 NS062153-01
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meier B. Closure of patent foramen ovale: technique, pitfalls, complications, and follow up. Heart. 2005;91:444–448. doi: 10.1136/hrt.2004.052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2**.O'Gara PT, Messe SR, Tuzcu EM, Catha G, Ring JC. Percutaneous device closure of patent foramen ovale for secondary stroke prevention: a call for completion of randomized clinical trials: a science advisory from the American Heart Association/American Stroke Association and the American College of Cardiology Foundation. Circulation. 2009;119:2743–2747. doi: 10.1161/CIRCULATIONAHA.109.192272. [DOI] [PubMed] [Google Scholar]; A joint position statement from several large physicians' organizations encouraging continued recruitment into the ongoing randomized clinical trials.
- 3*.Alsheikh-Ali AA, Thaler DE, Kent DM. Patent Foramen Ovale in Cryptogenic Stroke. Incidental or Pathogenic? Stroke. 2009 doi: 10.1161/STROKEAHA.109.547828. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bayesian analysis of case control studies that quantifies likelihood of pathogenic or incidental PFO based on age and PFO characteristics.
- 4.Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, Coste J, Patent Foramen Ovale and Atrial Septal Aneurysm Study Group Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. New England Journal of Medicine. 2001;345:1740–1746. doi: 10.1056/NEJMoa011503. [DOI] [PubMed] [Google Scholar]
- 5*.Rodes-Cabau J, Noel M, Marrero A, Rivest D, Mackey A, Houde C, Bedard E, Larose E, Verreault S, Peticlerc M, Pibarot P, Bogaty P, Bertrand OF. Atherosclerotic burden findings in young cryptogenic stroke patients with and without a patent foramen ovale. Stroke. 2009;40:419–425. doi: 10.1161/STROKEAHA.108.527507. [DOI] [PubMed] [Google Scholar]; Case control study confirming fewer atherosclerotic risk factors in CS patients with PFO compared with CS without PFO. This suggests a nonatherosclerotic mechanism (e.g. paradoxical embolism) is more common in CS patients with PFO than without.
- 6.Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002;105:2625–2631. doi: 10.1161/01.cir.0000017498.88393.44. [DOI] [PubMed] [Google Scholar]
- 7*.Ozdemir AO, Tamayo A, Munoz C, Spence JD. Cryptogenic stroke and patent foramen ovale: clinical clues to paradoxical embolism. J Neurol Sci. 2008;275:121–127. doi: 10.1016/j.jns.2008.08.018. [DOI] [PubMed] [Google Scholar]; A case control study that suggested that a history of DVT, PE, migraine, recent prolonged travel, sleep apnea, symptoms on waking, and Valsalva at onset are associated with CS and PFO, and so may be supportive of a paradoxical embolism mechanism.
- 8**.Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;357:2262–2268. doi: 10.1056/NEJMoa071422. [DOI] [PubMed] [Google Scholar]; Demonstration of a continued increased risk of finding a PFO in CS patients when patients are older than a “young” stroke patient.
- 9*.Almekhlafi MA, Wilton SB, Rabi DM, Ghali WA, Lorenzetti DL, Hill MD. Recurrent cerebral ischemia in medically treated patent foramen ovale: a meta-analysis. Neurology. 2009;73:89–97. doi: 10.1212/WNL.0b013e3181aa2a19. [DOI] [PubMed] [Google Scholar]; Detailed meta-analysis of medically treated patients that demonstrated a wide variability in recurrence rates. This argues against using “historical controls” as a comparator to newer treatments. Lack of uniformity of inclusion criteria, outcome ascertainment and so forth makes individual studies potentially ungeneralizable.
- 10.Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, Coste J. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740–1746. doi: 10.1056/NEJMoa011503. [DOI] [PubMed] [Google Scholar]
- 11.De CS, Cartoni D, Fiorelli M, Rasura M, Anzini A, Zanette EM, Beccia M, Colonnese C, Fedele F, Fieschi C, Pandian NG. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke. 2000;31:2407–2413. doi: 10.1161/01.str.31.10.2407. [DOI] [PubMed] [Google Scholar]
- 12.Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation. 2005;112:1063–1072. doi: 10.1161/CIRCULATIONAHA.104.524371. [DOI] [PubMed] [Google Scholar]
- 13**.Serena J, Marti-Fabregas J, Santamarina E, Rodriguez JJ, Perez-Ayuso MJ, Masjuan J, Segura T, Gallego J, Davalos A. Recurrent stroke and massive right-to-left shunt: Results from the prospective Spanish multicenter (CODICIA) study. Stroke. 2008;39:3131–3136. doi: 10.1161/STROKEAHA.108.521427. [DOI] [PubMed] [Google Scholar]; An important contribution to the natural history literature for patients with PFO and CS. This was a large prospective multicenter study that used TCD to screen for right-to-left shunt and to grade the shunts when found. A non-random sample of 61% of subjects underwent TEE which limits the power of the observation that ASA did not contribute to stroke recurrence.
- 14.Meissner I, Khandheria BK, Heit JA, Petty GW, Sheps SG, Schwartz GL, Whisnant JP, Wiebers DO, Covalt JL, Petterson TM, Christianson TJ, Agmon Y. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol. 2006;47:440–445. doi: 10.1016/j.jacc.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 15.Orgera MA, O'Malley PG, Taylor AJ. Secondary prevention of cerebral ischemia in patent foramen ovale: systematic review and meta-analysis. South.Med J. 2001;94:699–703. [PubMed] [Google Scholar]
- 16.Khairy P, O'Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Annals of Internal Medicine. 2003;139:753–760. doi: 10.7326/0003-4819-139-9-200311040-00010. [DOI] [PubMed] [Google Scholar]
- 17.Dorenbeck U, Simon B, Skowasch D, Stusser C, Gockel A, Schild HH, Urbach H, Bauriedel G. Cerebral embolism with interventional closure of symptomatic patent foramen ovale: An MRI-based study using diffusion-weighted imaging. European Journal of Neurology. 2007;14:451–454. doi: 10.1111/j.1468-1331.2007.01689.x. [DOI] [PubMed] [Google Scholar]
- 18**.Dowson A, Mullen MJ, Peatfield R, Muir K, Khan AA, Wells C, Lipscombe SL, Rees T, De Giovanni JV, Morrison WL, Hildick-Smith D, Elrington G, Hillis WS, Malik IS, Rickards A. Migraine Intervention With STARFlex Technology (MIST) trial: a prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation. 2008;117:1397–1404. doi: 10.1161/CIRCULATIONAHA.107.727271. [DOI] [PubMed] [Google Scholar]; Randomized trial of PFO closure vs. sham closure for migraine prevention. The high rate of procedural adverse events was surprising and the only data available for procedural complications in a randomized trial. Uncontrolled case-series reporting of adverse events may be less reliable.
- 19.Messe SR, Silverman IE, Kizer JR, Homma S, Zahn C, Gronseth G, Kasner SE. Practice parameter: recurrent stroke with patent foramen ovale and atrial septal aneurysm: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2004;62:1042–1050. doi: 10.1212/01.wnl.0000119173.15878.f3. [DOI] [PubMed] [Google Scholar]
- 20*.Kent DM, Hayward RA. When averages hide individual differences in clinical trials. American Scientist. 2009;95:60–68. [Google Scholar]; A review of heterogeneity in populations in clinical trials and how to interpret future trial data.
- 21.Rothwell PM, Mehta Z, Howard SC, Gutnikov SA, Warlow CP. Treating individuals 3: from subgroups to individuals: general principles and the example of carotid endarterectomy. Lancet. 2005;365:256–265. doi: 10.1016/S0140-6736(05)17746-0. [DOI] [PubMed] [Google Scholar]
- 22.Hayward RA, Kent DM, Vijan S, Hofer TP. Multivariable risk prediction can greatly enhance the statistical power of clinical trial subgroup analysis. BMC.Med Res.Methodol. 2006;6:18. doi: 10.1186/1471-2288-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007;298:1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 24.Rothwell PM, Warlow CP. Prediction of benefit from carotid endarterectomy in individual patients: a risk-modelling study. European Carotid Surgery Trialists' Collaborative Group. Lancet. 1999;353:2105–2110. doi: 10.1016/s0140-6736(98)11415-0. [DOI] [PubMed] [Google Scholar]
- 25.Homma S, Di Tullio MR, Sacco RL, Mihalatos D, Li MG, Mohr JP. Characteristics of patent foramen ovale associated with cryptogenic stroke. A biplane transesophageal echocardiographic study. Stroke. 1994;25:582–586. doi: 10.1161/01.str.25.3.582. [DOI] [PubMed] [Google Scholar]
- 26.Steiner MM, Di Tullio MR, Rundek T, Gan R, Chen X, Liguori C, Brainin M, Homma S, Sacco RL. Patent foramen ovale size and embolic brain imaging findings among patients with ischemic stroke. Stroke. 1998;29:944–948. doi: 10.1161/01.str.29.5.944. [DOI] [PubMed] [Google Scholar]
- 27.Serena J, Segura T, Perez-Ayuso MJ, Bassaganyas J, Molins A, Davalos A. The need to quantify right-to-left shunt in acute ischemic stroke: a case-control study. Stroke. 1998;29:1322–1328. doi: 10.1161/01.str.29.7.1322. [DOI] [PubMed] [Google Scholar]
- 28.DeCastro S, Cartoni D, Fiorelli M, Rasura M, Anzini A, Zanette EM, Beccia M, Colonnese C, Fedele F, Fieschi C, Pandian NG. Morphological and functional characteristics of patent foramen ovale and their embolic implications. Stroke. 2000;31:2407–2413. doi: 10.1161/01.str.31.10.2407. [DOI] [PubMed] [Google Scholar]
- 29.Anzola GP, Zavarize P, Morandi E, Rozzini L, Parrinello G. Transcranial Doppler and risk of recurrence in patients with stroke and patent foramen ovale. European Journal of Neurology. 2003;10:129–135. doi: 10.1046/j.1468-1331.2003.00561.x. [DOI] [PubMed] [Google Scholar]